化工学报 ›› 2020, Vol. 71 ›› Issue (9): 3866-3875.DOI: 10.11949/0438-1157.20200339
文国宇1( ),汪伟1,2,谢锐1,2,巨晓洁1,2,刘壮1,2,褚良银1,2(
),汪伟1,2,谢锐1,2,巨晓洁1,2,刘壮1,2,褚良银1,2( )
)
收稿日期:2020-03-31
修回日期:2020-05-27
出版日期:2020-09-05
发布日期:2020-09-05
通讯作者:
褚良银
作者简介:文国宇(1995—),男,博士研究生,基金资助:
Guoyu WEN1( ),Wei WANG1,2,Rui XIE1,2,Xiaojie JU1,2,Zhuang LIU1,2,Liangyin CHU1,2(
),Wei WANG1,2,Rui XIE1,2,Xiaojie JU1,2,Zhuang LIU1,2,Liangyin CHU1,2( )
)
Received:2020-03-31
Revised:2020-05-27
Online:2020-09-05
Published:2020-09-05
Contact:
Liangyin CHU
摘要:
水凝胶作为一种极具前景的吸附剂材料,由于具有高效、易操作且能耗低的特性,被广泛应用于工业废水和环境污水中金属离子的富集与分离。近年来,随着主-客体识别作用与金属离子配位作用研究的不断深入,利用水凝胶材料选择性分离和富集金属离子成为研究热点。本文综述了水凝胶材料在富集和分离特定金属离子领域的研究新进展,重点介绍了利用水凝胶材料特异性分离和富集放射性金属离子、稀土金属离子、贵重金属离子和重金属离子的研究现状。
中图分类号:
文国宇, 汪伟, 谢锐, 巨晓洁, 刘壮, 褚良银. 水凝胶材料在金属离子富集与分离领域的研究进展[J]. 化工学报, 2020, 71(9): 3866-3875.
Guoyu WEN, Wei WANG, Rui XIE, Xiaojie JU, Zhuang LIU, Liangyin CHU. Recent progress of hydrogel materials in the field of enrichment and separation of metal ions[J]. CIESC Journal, 2020, 71(9): 3866-3875.

图1 Zn2+-PAO超分子水凝胶离子交联与选择性吸附铀的机理[31]
Fig.1 The ionic crosslinking and selective uranium-adsorption mechanism of the Zn2+-PAO supramolecular hydrogel[31]
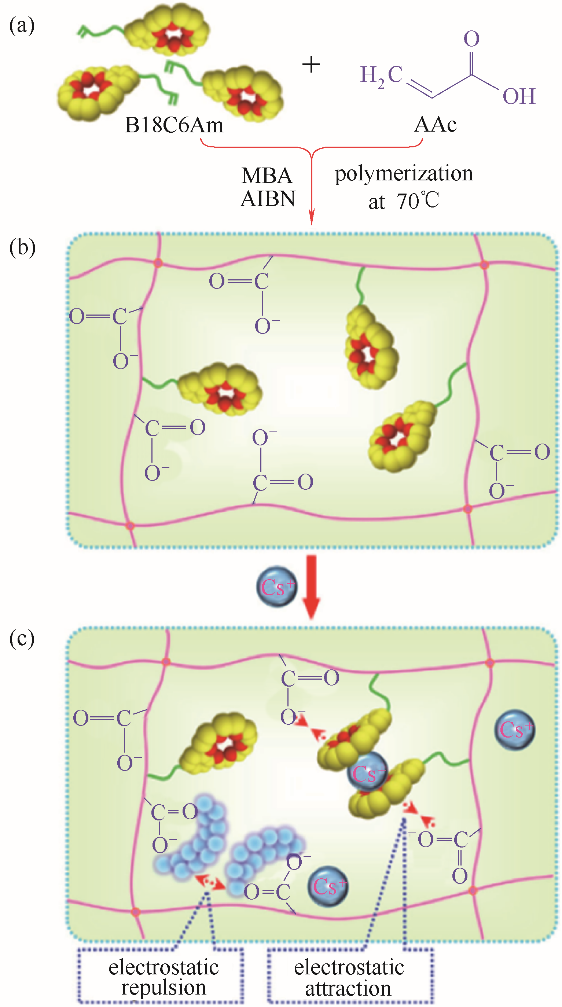
图2 P(AAc-co-B18C6Am)水凝胶的制备示意图(a)及其识别吸附铯离子的示意图[(b)、(c)][39]
Fig.2 Schematic illustration of the preparation process (a) and cesium recognition and adsorption property [(b), (c)] of the P(AAc-co-B18C6Am) hydrogel[39]
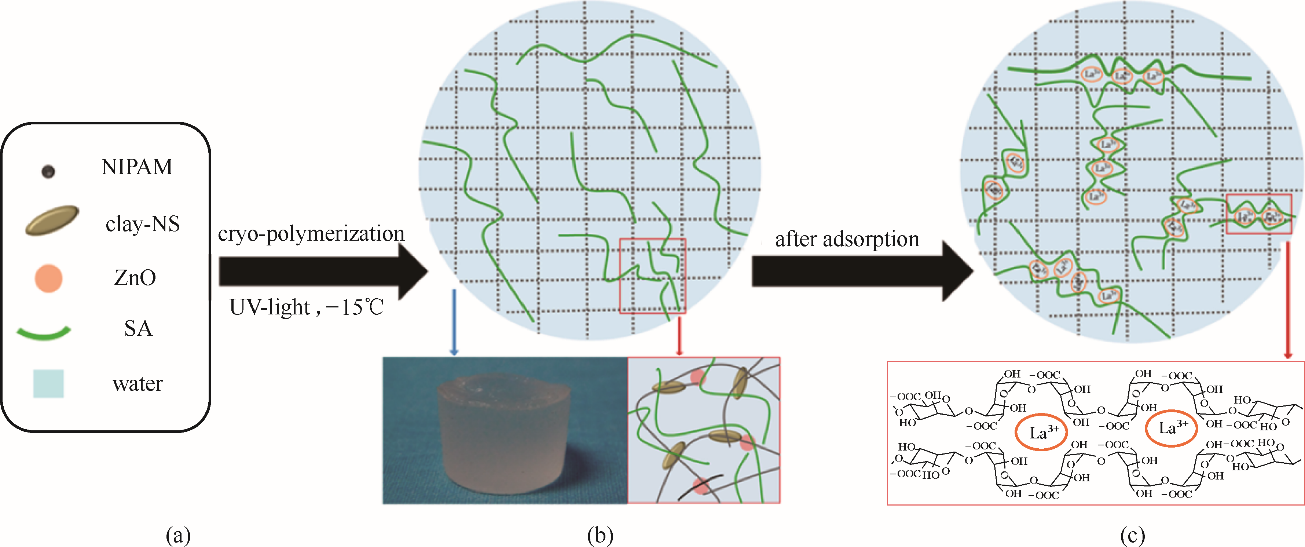
图3 PNIPAm-clay复合水凝胶制备过程和La3+吸附过程(a)预聚液中的所有成分(SA代表海藻酸钠);(b)PNIPAm-clay纳米复合晶胶的形成(-15℃),自立式晶胶(-15℃)的光学照片以及半互穿网络;(c)吸附后,由于La3+与海藻酸钠高分子链中的G组分的强螯合作用,两种聚合物网络相互交织,半互穿网络转变为互穿聚合物网络[50]
Fig.3 Preparation process and La3+ adsorption of PNIPAm-clay nanocomposite hydrogel(a) all of the components of the precursor solution (SA denotes sodium alginate); (b) formation of the PNIPAm-clay nanocomposite cryogel (-15℃), a photograph of a self-standing cryogel (-15℃), and a schematic diagram of the semi-IPN; (c) after adsorption, the two types of polymer networks are intertwined, semi-IPN changes to be an interpenetrating polymer network; demonstrated is a scheme of the strong chelating action between La3+ and G blocks in the alginate chains[50]

图4 充分利用多功能MoS2实现从水凝胶的制备到近红外光控制吸附-脱附循环的示意图[62]
Fig.4 Schematic illustration of the utilization of multi-functional MoS2 from fabrication of the hydrogel to the NIR manipulated adsorption-desorption cycle[62]
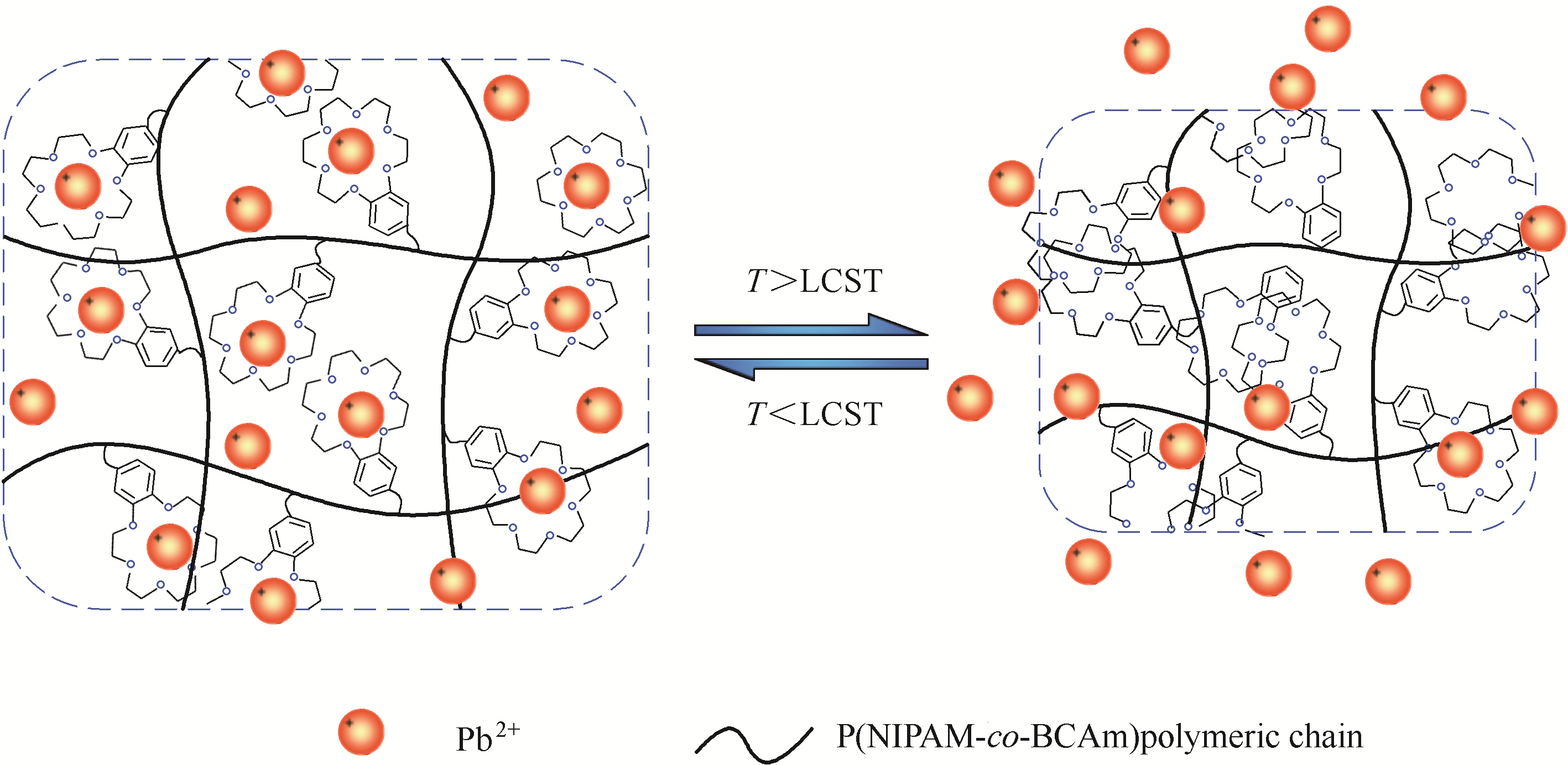
图5 P(NIPAM-co-BCAm)水凝胶针对Pb2+的温敏性吸附/解吸示意图:当温度低于LCST时吸附,当温度高于LCST时解吸[68]
Fig.5 Schematic illustration of the thermo-responsive adsorption/desorption behavior of P(NIPAM-co-BCAm) hydrogel towards Pb2+ ions, which exhibits adsorption at temperature lower than the LCST and desorption at temperature higher than the LCST[68]
| 吸附剂 | 金属离子 | 最大吸附容量/(mg·g-1) | pH |
|---|---|---|---|
| PCNS | 451.118 | 4 | |
| SUP | 9.2×10-3 | 8 | |
| Zn2+-PAO | 1188 | 7 | |
| β-CD(AN-co-AA) | Th4+ | 692 | 2.95 |
| P(AAc-co-B18C6Am) | Cs+ | 74.6 | 6 |
| alginate-clay-PNIPAm | La3+ | 182 | 5 |
| P(Penta3MP4/PEGDA/HEMA) | Au3+ | 45.19 | 0.5 |
| MNH | Ag+ | 40.5 | 6 |
| P(NIPAM-co-B18C6Am) | Pb2+ | 142 | 5 |
| MoS2-rGO | Hg2+ | 340 | 3.5 |
表1 水凝胶吸附剂对不同金属离子吸附容量及其吸附条件
Table 1 Adsorption capacity and adsorption conditions of hydrogel adsorbent on different metal ions
| 吸附剂 | 金属离子 | 最大吸附容量/(mg·g-1) | pH |
|---|---|---|---|
| PCNS | 451.118 | 4 | |
| SUP | 9.2×10-3 | 8 | |
| Zn2+-PAO | 1188 | 7 | |
| β-CD(AN-co-AA) | Th4+ | 692 | 2.95 |
| P(AAc-co-B18C6Am) | Cs+ | 74.6 | 6 |
| alginate-clay-PNIPAm | La3+ | 182 | 5 |
| P(Penta3MP4/PEGDA/HEMA) | Au3+ | 45.19 | 0.5 |
| MNH | Ag+ | 40.5 | 6 |
| P(NIPAM-co-B18C6Am) | Pb2+ | 142 | 5 |
| MoS2-rGO | Hg2+ | 340 | 3.5 |
| 1 | Yetisen A K, Butt H, Volpatti L R, et al. Photonic hydrogel sensors [J]. Biotechnology Advances, 2016, 34(3): 250-271. |
| 2 | Yin M J, Yao M, Gao S, et al. Rapid 3D patterning of poly(acrylic acid) ionic hydrogel for miniature pH sensors [J]. Advanced Materials, 2016, 28(7): 1394-1399. |
| 3 | Bailey S E, Olin T J, Bricka R M, et al. A review of potentially low-cost sorbents for heavy metals [J]. Water Research, 1999, 33(11): 2469-2479. |
| 4 | Hong H, Seo Y B, Kim D Y, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering [J]. Biomaterials, 2020, 232: 119679. |
| 5 | Wang J, Zhang F, Tsang W P, et al. Fabrication of injectable high strength hydrogel based on 4-arm star PEG for cartilage tissue engineering [J]. Biomaterials, 2017, 120: 11-21. |
| 6 | Roshanbinfar K, Vogt L, Greber B, et al. Electroconductive biohybrid hydrogel for enhanced maturation and beating properties of engineered cardiac tissues [J]. Advanced Functional Materials, 2018, 28(42): 1803951. |
| 7 | An Y H, Lee J, Son D U, et al. Facilitated transdermal drug delivery using nanocarriers-embedded electroconductive hydrogel coupled with reverse electrodialysis-driven iontophoresis [J]. ACS Nano, 2020, 14(4): 4523-4535. |
| 8 | Wei Z, Volkova E, Blatchley M R, et al. Hydrogel vehicles for sequential delivery of protein drugs to promote vascular regeneration [J]. Advanced Drug Delivery Reviews, 2019, 149: 95-106. |
| 9 | Zhao X, Liang Y, Huang Y, et al. Physical double‐network hydrogel adhesives with rapid shape adaptability, fast self-healing, antioxidant and NIR/pH stimulus-responsiveness for multidrug‐resistant bacterial infection and removable wound dressing [J]. Advanced Functional Materials, 2020, 30(17): 1910748. |
| 10 | Wang K, Wang J, Li L, et al. Novel nonreleasing antibacterial hydrogel dressing by a one-pot method [J]. ACS Biomaterials Science & Engineering, 2020, 6(2): 1259-1268. |
| 11 | Chen C S, Zeng F, Xiao X, et al. Three-dimensionally printed silk-sericin-based hydrogel scaffold: a promising visualized dressing material for real-time monitoring of wounds [J]. ACS Applied Materials & Interfaces, 2018, 10(40): 33879-33890. |
| 12 | Holtz J H, Asher S A. Polymerized colloidal crystal hydrogel films as intelligent chemical sensing materials [J]. Nature, 1997, 389(6653): 829-832. |
| 13 | Li W, Zhao H, Teasdale P R, et al. Synthesis and characterisation of a polyacrylamide-polyacrylic acid copolymer hydrogel for environmental analysis of Cu and Cd [J]. Reactive and Functional Polymers, 2002, 52(1): 31-41. |
| 14 | Zhang Y, Lin S, Qiao J, et al. Malic acid-enhanced chitosan hydrogel beads (mCHBs) for the removal of Cr(Ⅵ) and Cu(Ⅱ) from aqueous solution [J]. Chemical Engineering Journal, 2018, 353: 225-236. |
| 15 | Zhou G, Luo J, Liu C, et al. A highly efficient polyampholyte hydrogel sorbent based fixed-bed process for heavy metal removal in actual industrial effluent [J]. Water Research, 2016, 89: 151-160. |
| 16 | Ehlken S, Kirchner G. Environmental processes affecting plant root uptake of radioactive trace elements and variability of transfer factor data: a review [J]. Journal of Environmental Radioactivity, 2002, 58(2/3): 97-112. |
| 17 | Ewing R C, Weber W J, Lian J. Nuclear waste disposal—pyrochlore (A2B2O7): nuclear waste form for the immobilization of plutonium and “minor” actinides [J]. Journal of Applied Physics, 2004, 95(11): 5949-5971. |
| 18 | Wang J, Wan Z. Treatment and disposal of spent radioactive ion-exchange resins produced in the nuclear industry [J]. Progress in Nuclear Energy, 2015, 78: 47-55. |
| 19 | Shi C, Fernandez-Jimenez A. Stabilization/solidification of hazardous and radioactive wastes with alkali-activated cements [J]. Journal of Hazardous Materials, 2006, 137(3): 1656-1663. |
| 20 | Righi S, Lucialli P, Bruzzi L. Health and environmental impacts of a fertilizer plant (I): Assessment of radioactive pollution [J]. Journal of Environmental Radioactivity, 2005, 82(2): 167-182. |
| 21 | Koenig K L, Goans R E, Hatchett R J, et al. Medical treatment of radiological casualties: current concepts [J]. Annals of Emergency Medicine, 2005, 45(6): 643-652. |
| 22 | Atsuumi R, Endo Y, Suzuki A, et al. Radioactive substances in tap water [J]. Fukushima Journal of Medical Science, 2014, 60(1): 101-105. |
| 23 | Nair R N, Sunny F, Chopra M, et al. Estimation of radioactive leakages into the Pacific Ocean due to Fukushima nuclear accident [J]. Environmental Earth Sciences, 2013, 71(3): 1007-1019. |
| 24 | Kersting A B, Efurd D W, Finnegan D L, et al. Migration of plutonium in ground water at the Nevada Test Site [J]. Nature, 1999, 397(6714): 56-59. |
| 25 | Yasunari T J, Stohl A, Hayano R S, et al. Cesium-137 deposition and contamination of Japanese soils due to the Fukushima nuclear accident [J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(49): 19530-19534. |
| 26 | Jacobson M Z. Review of solutions to global warming, air pollution, and energy security [J]. Energy & Environmental Science, 2009, 2(2): 148-173. |
| 27 | Zinkle S J, Was G S. Materials challenges in nuclear energy [J]. Acta Materialia, 2013, 61(3): 735-758. |
| 28 | Lindner H, Schneider E. Review of cost estimates for uranium recovery from seawater [J]. Energy Economics, 2015, 49: 9-22. |
| 29 | He J, Jin J, Wang Z, et al. Encapsulating nanosilica into polyacrylic acid and chitosan interpenetrating network hydrogel for preconcentration of uranium from aqueous solutions [J]. Journal of Radioanalytical and Nuclear Chemistry, 2018, 317(3): 1299-1309. |
| 30 | Kou S, Yang Z, Sun F. Protein hydrogel microbeads for selective uranium mining from seawater [J]. ACS Applied Materials & Interfaces, 2017, 9(3): 2035-2039. |
| 31 | Yan B, Ma C, Gao J, et al. An ion-crosslinked supramolecular hydrogel for ultrahigh and fast uranium recovery from seawater [J]. Advanced Materials, 2020, 32(10): e1906615. |
| 32 | Liu P, Qi W, Du Y, et al. Adsorption of thorium(Ⅳ) on magnetic multi-walled carbon nanotubes [J]. Science China Chemistry, 2014, 57(11): 1483-1490. |
| 33 | Hu Y, Giret S, Meinusch R, et al. Selective separation and preconcentration of Th(Ⅳ) using organo-functionalized, hierarchically porous silica monoliths [J]. Journal of Materials Chemistry A, 2019, 7(1): 289-302. |
| 34 | Allahyari S A, Ahmadi S J, Minuchehr A, et al. Th(Ⅳ) recovery from aqueous waste via hollow fiber renewal liquid membrane (HFRLM) in recycling mode: modelling and experimental validation [J]. RSC Advances, 2017, 7(12): 7413-7423. |
| 35 | Duan G, Zhong Q, Bi L, et al. The poly(acrylonitrule-co-acrylic acid)-graft-beta-cyclodextrin hydrogel for thorium(Ⅳ) adsorption [J]. Polymers (Basel), 2017, 9(6): 201. |
| 36 | Kosaka K, Asami M, Kobashigawa N, et al. Removal of radioactive iodine and cesium in water purification processes after an explosion at a nuclear power plant due to the Great East Japan Earthquake [J]. Water Research, 2012, 46(14): 4397-4404. |
| 37 | Zhang N, Chen S, Hu J, et al. Robust and recyclable sodium carboxymethyl cellulose-ammonium phosphomolybdate composites for cesium removal from wastewater [J]. RSC Advances, 2020, 10(11): 6139-6145. |
| 38 | Moon S, Jang S W, Kim Y, et al. Prussian blue decorated hydrogel particles for effective removal of cesium ion from aqueous media [J]. Polymer, 2020, 186: 122029. |
| 39 | Yu H R, Hu J Q, Liu Z, et al. Ion-recognizable hydrogels for efficient removal of cesium ions from aqueous environment [J]. Journal of Hazardous Materials, 2017, 323(Pt B): 632-640. |
| 40 | Chen Z. Global rare earth resources and scenarios of future rare earth industry [J]. Journal of Rare Earths, 2011, 29(1): 1-6. |
| 41 | Eliseeva S V, Bünzli J C G. Rare earths: jewels for functional materials of the future [J]. New Journal of Chemistry, 2011, 35(6): 1165-1176. |
| 42 | Rabl S, Haas A, Santi D, et al. Ring opening of cis-decalin on bifunctional Ir/- and Pt/La-X zeolite catalysts [J]. Applied Catalysis A: General, 2011, 400(1/2): 131-141. |
| 43 | Diniz V, Volesky B. Biosorption of La, Eu and Yb using Sargassum biomass [J]. Water Research, 2005, 39(1): 239-247. |
| 44 | Brioschi L, Steinmann M, Lucot E, et al. Transfer of rare earth elements (REE) from natural soil to plant systems: implications for the environmental availability of anthropogenic REE [J]. Plant and Soil, 2012, 366(1/2): 143-163. |
| 45 | Ming L, Zhang B, Wang C, et al. La-doping and carbon-coating collaboratively enhance the cycling and rate properties of LiFeBO3 for Li-ion battery [J]. Chemical Physics Letters, 2020, 741: 137090. |
| 46 | Manikandan A, Manikandan E, Meenatchi B, et al. Rare earth element (REE) lanthanum doped zinc oxide (La: ZnO) nanomaterials: synthesis structural optical and antibacterial studies [J]. Journal of Alloys and Compounds, 2017, 723: 1155-1161. |
| 47 | Hammouda S B, Zhao F, Safaei Z, et al. Degradation and mineralization of phenol in aqueous medium by heterogeneous monopersulfate activation on nanostructured cobalt based-perovskite catalysts ACoO3 (A = La, Ba, Sr and Ce): characterization, kinetics and mechanism study [J]. Applied Catalysis B: Environmental, 2017, 215: 60-73. |
| 48 | Rahman M M, Khan S B, Marwani H M, et al. SnO2-TiO2 nanocomposites as new adsorbent for efficient removal of La(Ⅲ) ions from aqueous solutions [J]. Journal of the Taiwan Institute of Chemical Engineers, 2014, 45(4): 1964-1974. |
| 49 | Zhu B, Wu D, Yang Y, et al. Selective removal of La(Ⅲ) ions using super-paramagnetic nanosorbent coated by saponified sec-octylphenoxy acetic acid [J]. Journal of Chemical & Engineering Data, 2011, 57(2): 553-560. |
| 50 | Wu D, Gao Y, Li W, et al. Selective adsorption of La3+ using a tough alginate-clay-poly(n-isopropylacrylamide) hydrogel with hierarchical pores and reversible re-deswelling/swelling cycles [J]. ACS Sustainable Chemistry & Engineering, 2016, 4(12): 6732-6743. |
| 51 | Gasteiger H A, Kocha S S, Sompalli B, et al. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs [J]. Applied Catalysis B: Environmental, 2005, 56(1/2): 9-35. |
| 52 | Huang X, Wang Y, Liao X, et al. Adsorptive recovery of Au3+ from aqueous solutions using bayberry tannin-immobilized mesoporous silica [J]. Journal of Hazardous Materials, 2010, 183(1/2/3): 793-798. |
| 53 | Xiong Y, Wan L, Xuan J, et al. Selective recovery of Ag(I) coordination anion from simulate nickel electrolyte using corn stalk based adsorbent modified by ammonia-thiosemicarbazide [J]. Journal of Hazardous Materials, 2016, 301: 277-285. |
| 54 | Lee H W, Schmidt M A, Russell R F, et al. Pressure-assisted melt-filling and optical characterization of Au nano-wires in microstructured fibers [J]. Optics Express, 2011, 19(13): 12180-12189. |
| 55 | Fu Q, Saltsburg H, Flytzani-Stephanopoulos M. Active nonmetallic Au and Pt species on ceria-based water-gas shift catalysts [J]. Science, 2003, 301(5635): 935-938. |
| 56 | Varcoe J R, Slade R C, Wright G L, et al. Steady-state DC and impedance investigations of H2/O2 alkaline membrane fuel cells with commercial Pt/C, Ag/C, and Au/C cathodes [J]. Journal of Physical Chemistry B, 2006, 110(42): 21041-21049. |
| 57 | Firlak M, Kahraman M V, Yetimoğlu E K. Preparation and characterization of photocured thiol-ene hydrogel: adsorption of Au(Ⅲ) ions from aqueous solutions [J]. Journal of Applied Polymer Science, 2012, 126(1): 322-332. |
| 58 | Tedsree K, Li T, Jones S, et al. Hydrogen production from formic acid decomposition at room temperature using a Ag-Pd core-shell nanocatalyst [J]. Nature Nanotechnology, 2011, 6(5): 302-307. |
| 59 | Zhao Q, Liu Y, Wang C. Development and evaluation of electroless Ag-PTFE composite coatings with anti-microbial and anti-corrosion properties [J]. Applied Surface Science, 2005, 252(5): 1620-1627. |
| 60 | Mai L, Wang D, Zhang S, et al. Synthesis and bactericidal ability of Ag/TiO2 composite films deposited on titanium plate [J]. Applied Surface Science, 2010, 257(3): 974-978. |
| 61 | Zhang B, Wang X, Zhao Y, et al. Highly photosensitive colorimetric immunoassay for tumor marker detection based on Cu2+ doped Ag-AgI nanocomposite [J]. Talanta, 2017, 167: 111-117. |
| 62 | Wei G, Wei J, Zhou J, et al. MoS2 nanosheet initiated smart polymeric hydrogel for NIR-driven Ag(I) enrichment [J]. Chemical Engineering Journal, 2020, 382: 123018. |
| 63 | Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review [J]. Journal of Environmental Management, 2011, 92(3): 407-418. |
| 64 | Kim H N, Ren W X, Kim J S, et al. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions [J]. Chemical Society Reviews, 2012, 41(8): 3210-3244. |
| 65 | Li J, Lu Y. A highly sensitive and selective catalytic DNA biosensor for lead ions [J]. Journal of the American Chemical Society, 2000, 122(42): 10466-10467. |
| 66 | Abdel-Halim S H, Shehata A M A, El-Shahat M F. Removal of lead ions from industrial waste water by different types of natural materials [J]. Water Research, 2003, 37(7): 1678-1683. |
| 67 | Rafatullah M, Sulaiman O, Hashim R, et al. Adsorption of copper (Ⅱ), chromium (Ⅲ), nickel (Ⅱ) and lead (Ⅱ) ions from aqueous solutions by meranti sawdust [J]. Journal of Hazardous Materials, 2009, 170(2/3): 969-977. |
| 68 | Ju X J, Zhang S B, Zhou M Y, et al. Novel heavy-metal adsorption material: ion-recognition P(NIPAM-co-BCAm) hydrogels for removal of lead(Ⅱ) ions [J]. Journal of Hazardous Materials, 2009, 167(1/2/3): 114-118. |
| 69 | Yan G, Oliver S P. Adsorption of Hg(Ⅱ) from aqueous solution using functionalized hydrogel loaded with hydrous manganese dioxide particles [J]. Water Science & Technology, 2017, 76(3/4): 747-753. |
| 70 | Mercier L, Pinnavaia T J. Heavy metal ion adsorbents formed by the grafting of a thiol functionality to mesoporous silica molecular sieves: factors affecting Hg(Ⅱ) uptake [J]. Environmental Science & Technology, 1998, 32(18): 2749-2754. |
| 71 | Zhuang Y T, Zhang X, Wang D H, et al. Three-dimensional molybdenum disulfide/graphene hydrogel with tunable heterointerfaces for high selective Hg(Ⅱ) scavenging [J]. Journal of Colloid and Interface Science, 2018, 514: 715-722. |
| [1] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [2] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [3] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| [4] | 潘煜, 王子航, 王佳韵, 王如竹, 张华. 基于可得然-氯化锂复合吸附剂的除湿换热器热湿性能研究[J]. 化工学报, 2023, 74(3): 1352-1359. |
| [5] | 李敏, 阎雪茹, 刘新磊. 苯并咪唑连接聚合物吸附剂和膜研究进展[J]. 化工学报, 2023, 74(2): 599-616. |
| [6] | 钟国栋, 邓超和, 王洋, 王佳韵, 王如竹. 蜂窝状水凝胶吸附床传热传质特性数值模拟及验证[J]. 化工学报, 2022, 73(3): 1083-1092. |
| [7] | 王玉杰, 李申辉, 赵之平. M-MOF-74吸附分离H2/He混合物的分子模拟研究[J]. 化工学报, 2022, 73(10): 4507-4517. |
| [8] | 吴俊晔, 葛天舒, 吴宣楠, 代彦军, 王如竹. 基于吸附剂/木浆纤维纸耦合材料的空气净化[J]. 化工学报, 2021, 72(S1): 520-529. |
| [9] | 罗伟莉, 王雯雯, 潘权稳, 葛天舒, 王如竹. 基于活性碳纤维毡复合吸附剂的储热性能[J]. 化工学报, 2021, 72(S1): 554-559. |
| [10] | 冯宇, 张鑫, 张曼, 王建成, 阎智锋, 李甫, 费鹏飞, 卢建军, 米杰. 静电纺丝纤维对煤基气体污染物脱除研究进展[J]. 化工学报, 2021, 72(8): 3933-3945. |
| [11] | 邓超和, 王佳韵, 李金凤, 刘业凤, 王如竹. 可低温驱动的凝胶复合吸附剂的制备及吸/脱附性能研究[J]. 化工学报, 2021, 72(8): 4401-4409. |
| [12] | 肖弦, 徐文昊, 沈亮, 王远鹏, 卢英华. 氧化石墨烯与剩余活性污泥聚合制备多孔碳材料及其电化学性能[J]. 化工学报, 2021, 72(7): 3869-3879. |
| [13] | 梁苏卓成, 姬国勋, 孙新利, 王波, 张仕通, 代星. 硅杂原子提升冠醚对锂离子络合能力的机理理论研究[J]. 化工学报, 2021, 72(6): 3149-3159. |
| [14] | 王琪, 赵有璟, 刘洋, 王云昊, 王敏, 项顼. 高镁锂比盐湖镁锂分离与锂提取技术研究进展[J]. 化工学报, 2021, 72(6): 2905-2921. |
| [15] | 张瑞, 陆旗玮, 林森, 于建国. 铝系成型锂吸附剂性能测试评价与对比[J]. 化工学报, 2021, 72(6): 3053-3062. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号