化工学报 ›› 2021, Vol. 72 ›› Issue (7): 3686-3695.DOI: 10.11949/0438-1157.20210033
王结祥1,2( ),李洪国2,叶松寿1,郑进保1(
),李洪国2,叶松寿1,郑进保1( ),陈秉辉1
),陈秉辉1
收稿日期:2021-01-08
修回日期:2021-05-13
出版日期:2021-07-05
发布日期:2021-07-05
通讯作者:
郑进保
作者简介:王结祥(1985—),男,博士,基金资助:
WANG Jiexiang1,2( ),LI Hongguo2,YE Songshou1,ZHENG Jinbao1(
),LI Hongguo2,YE Songshou1,ZHENG Jinbao1( ),CHEN Binghui1
),CHEN Binghui1
Received:2021-01-08
Revised:2021-05-13
Online:2021-07-05
Published:2021-07-05
Contact:
ZHENG Jinbao
摘要:
富氮型金属有机骨架材料(MOFs)具有良好的CO2捕集性能,但其CO2催化性能常需添加带氢键或亲核基团的助催化剂。以硝酸锌-腺嘌呤-异烟酸[Zn(NO3)2-Ad-Int-DMF]构建稳健的骨架材料,发现其催化合成碳酸丙烯酯(PC)收率不足2%;尝试选型锌盐前体引入亲核卤素,在DMF溶剂中部分卤代锌盐不能络合结晶成MOFs,而形成的ZnI2-Ad-Int-DMF收率提升至19.5%;溶剂由DMF调变为H2O-DMF混合溶剂,H2O的引入避免了卤素对金属和配体间络合的影响,使得以ZnCl2、ZnBr2和ZnI2为前体均能晶化成MOFs,其热分解起始温度(Tonset,434℃)明显高于ZnI2-Ad-Int-DMF(280℃),而比表面积(<14 m2/g)明显小于后者(571 m2/g),造成活性低于后者。通过CO2吸附脉冲和UV-Vis漫反射推测I-由于与Zn碱位的相互作用而均匀吸附于骨架上。进而,在无助剂、无溶剂工况下对CO2环加成反应进行活性评价。温度升高显著提升PC产率,140℃下ZnI2-Ad-Int-DMF催化产率可达98.5%,但由于吸附在骨架上的卤素在高温、溶剂环境下会脱落,造成重复性实验中活性下降,而该类MOFs在反应前后骨架保持稳定,未造成坍塌或明显孔堵塞现象。后续如能强化卤素的吸附或温和化反应条件,则具有良好的应用潜力。
中图分类号:
王结祥, 李洪国, 叶松寿, 郑进保, 陈秉辉. 卤素负载锌-腺嘌呤骨架材料的构建及无助剂催化CO2环加成反应[J]. 化工学报, 2021, 72(7): 3686-3695.
WANG Jiexiang, LI Hongguo, YE Songshou, ZHENG Jinbao, CHEN Binghui. Halogen-rich zinc-adeninate framework construction and its catalytic performance on CO2 cycloaddition without cocatalyst[J]. CIESC Journal, 2021, 72(7): 3686-3695.

图2 不同阴离子锌盐前体合成Zn-Ad-Int-DMF用于CO2环加成反应,其中NO3-+KI指Zn(NO3)2-Ad-Int-DMF中添加20%的KI[反应条件:PO 30 ml,PCO2 4 MPa(初始压力),催化剂 0.5 g,KI 0.1 g(如有添加),T=100℃,t=12 h]
Fig.2 Zn-Ad-Int-DMF prepared by different zinc salts and their catalytic activity comparison. Herein, NO3-+KI meant Zn(NO3)2-Ad-Int-DMF with KI (20% MOF dosage) added [Reaction conditions: PO 30 ml, initial PCO2 4 MPa, catalyst dosage 0.5 g, KI 0.1 g (if added), T=100℃, t=12 h]

图3 不同生长条件下ZnX2-Ad-Int在显微镜下的形态(a)、粉末XRD结构对比(b)及单晶XRD结构精修(c)
Fig.3 The morphology through microscope (a), structure comparison through PXRD for framework materials under different growth conditions (b) and X-ray single crystal refining for ZnI2-Ad-Int-DMF structure (c)
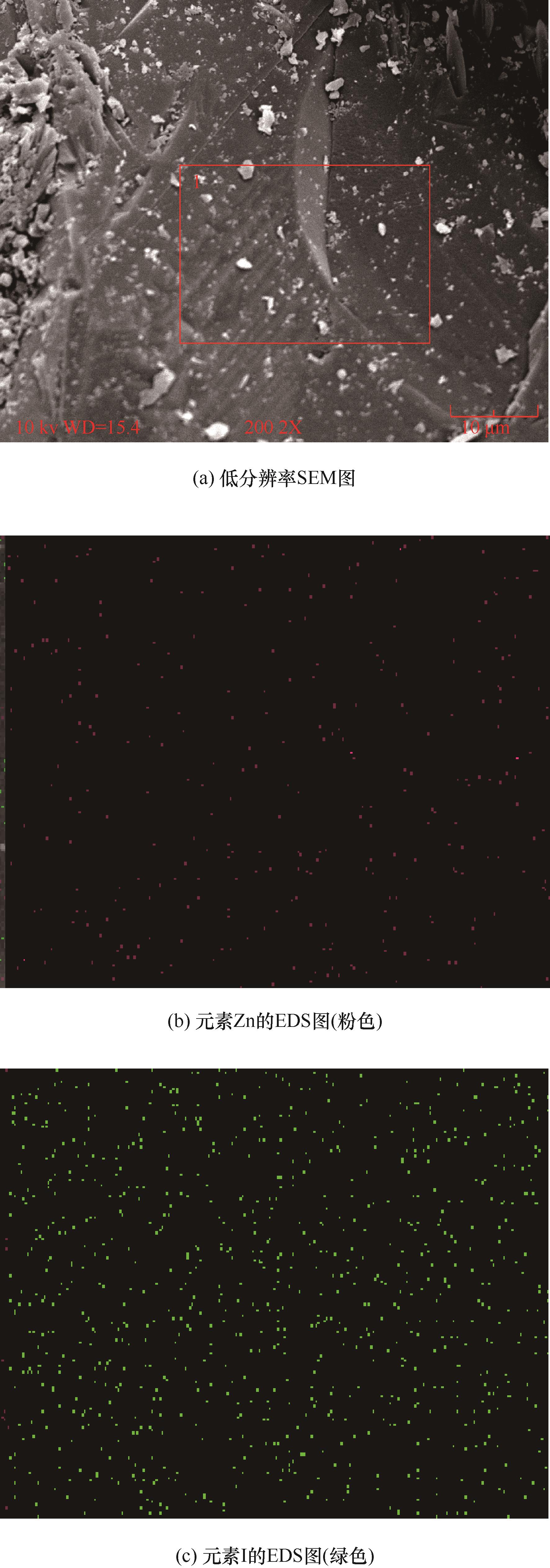
图4 ZnI2-Ad-Int-DMF低分辨率SEM图及相应的元素EDS图
Fig.4 Low-resolution scanning electron microscope image for ZnI2-Ad-Int-DMF and corresponding EDS elemental mapping images

图6 ZnX2-Ad-Int-DMF(X=NO3-, I-)的CO2脉冲吸附与UV-Vis漫反射光谱差异
Fig.6 The difference for ZnX2-Ad-Int (X=NO3-, I-) in CO2 pulse adsorption and UV-Vis diffuse reflectance spectra

图7 ZnX2-Ad-Int及反应温度对CO2催化性能影响[反应条件:PO 30 ml,PCO2 4 MPa(初始压力),催化剂 0.5 g,t=24 h;TON/TOF计算过程视I离子为活性位点]
Fig.7 Catalytic activity comparison for different ZnX2-Ad-Int at different reaction temperature[Reaction conditions: PO 30 ml, initial PCO2 4 MPa, catalyst dosage 0.5g, t=24 h. In TON/TOF calculation process, I- was regarded as active site]
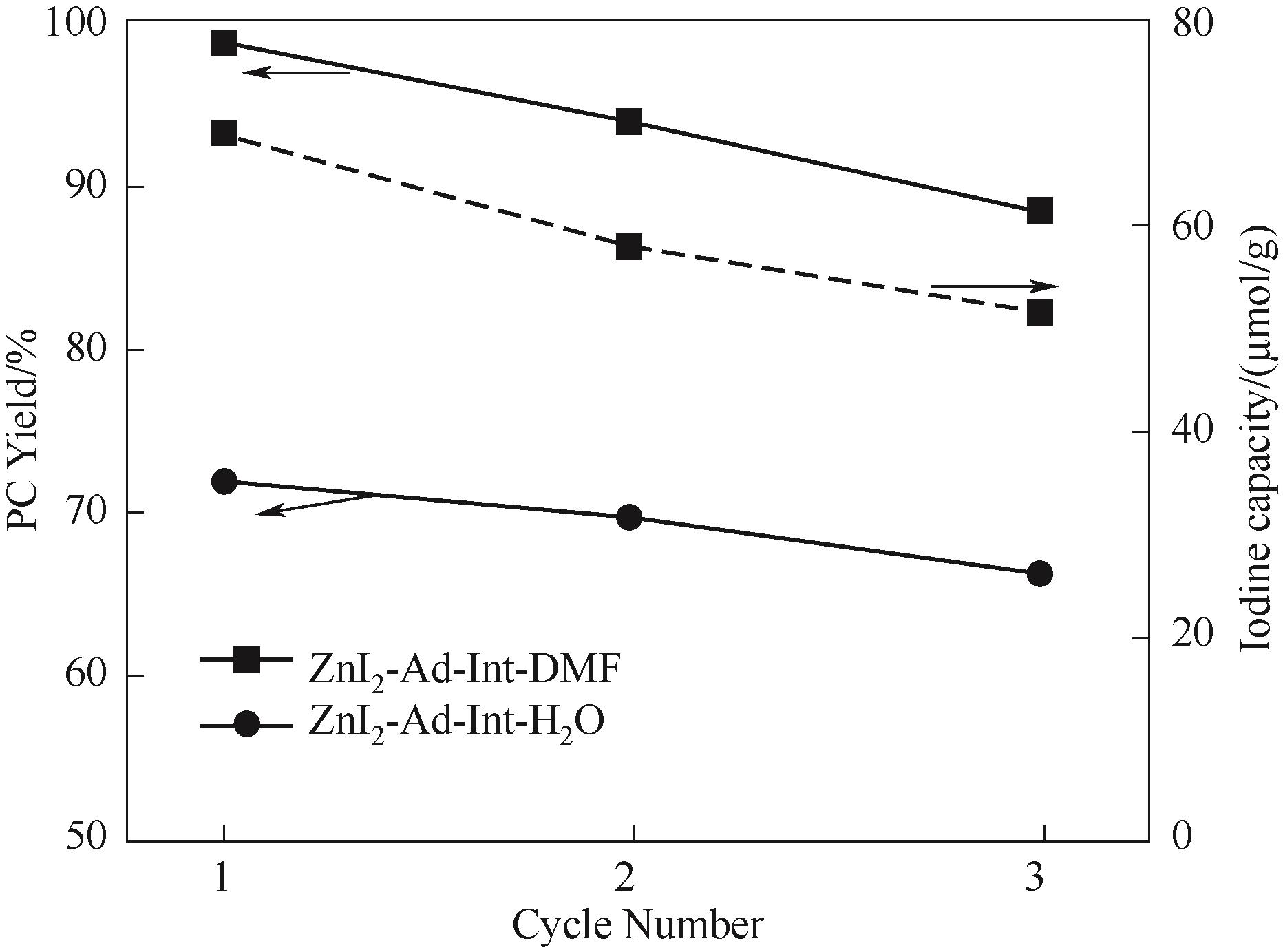
图8 两种ZnI2-Ad-Int的重复性实验,以及通过XRF测定重复性催化剂的碘含量变化[反应条件:PO 30 ml,PCO24 MPa(初始压力),催化剂 0.5 g,t=24 h,T=140℃]
Fig.8 Recycling test for two kinds of ZnI2-Ad-Int and iodine capacity test for recycling catalyst through XRF[Reaction conditions: PO 30 ml, initialPCO2 4 MPa, catalyst dosage 0.5 g, t=24 h,T=140℃]
| Sample | C/N ratio | Content/%(mass) | ||
|---|---|---|---|---|
| N | C | H | ||
| ZnI2-Ad-Int-H2O | 1.86 | 28.38 | 52.74 | 6.29 |
| ZnI2-Ad-Int-H2O-reacted | 2.06 | 25.84 | 53.08 | 5.88 |
| ZnI2-Ad-Int-DMF | 1.63 | 24.93 | 40.66 | 4.84 |
| ZnI2-Ad-Int-DMF-reacted | 1.82 | 23.61 | 42.97 | 4.01 |
表1 反应前后ZnI2-Ad-Int的元素分析
Table 1 Elemental analysis of ZnI2-Ad-Int
| Sample | C/N ratio | Content/%(mass) | ||
|---|---|---|---|---|
| N | C | H | ||
| ZnI2-Ad-Int-H2O | 1.86 | 28.38 | 52.74 | 6.29 |
| ZnI2-Ad-Int-H2O-reacted | 2.06 | 25.84 | 53.08 | 5.88 |
| ZnI2-Ad-Int-DMF | 1.63 | 24.93 | 40.66 | 4.84 |
| ZnI2-Ad-Int-DMF-reacted | 1.82 | 23.61 | 42.97 | 4.01 |

图10 ZnX2-Ad-Int的构建及其对CO2从吸附到活化的转变过程示意图
Fig.10 Schematic illustration for the construction of ZnX2-Ad-Int and the transformation process from CO2 adsorption to activation
| 1 | 罗晓菲,支云飞,陕绍云, 等. 多孔材料在催化CO2与环氧化物环加成反应中的研究进展[J]. 精细化工,2020,37(12): 2415-2425. |
| Luo X F, Zhi Y F, Shan S Y, et al. Research progress of porous materials in the cycloaddition of CO2 and epoxides[J]. Fine Chemicals, 2020, 37(12): 2415-2425. | |
| 2 | Müller P, Bucior B, Tuci G, et al. Computational screening, synthesis and testing of metal-organic frameworks with a bithiazole linker for carbon dioxide capture and its green conversion into cyclic carbonates[J]. Molecular Systems Design & Engineering, 2019, 4(5): 1000-1013. |
| 3 | Dhakshinamoorthy A, Garcia H. Catalysis by metal nanoparticles embedded on metal-organic frameworks[J]. Chemical Society Reviews, 2012, 41(15): 5262-5284. |
| 4 | Liang J, Huang Y B, Cao R. Metal–organic frameworks and porous organic polymers for sustainable fixation of carbon dioxide into cyclic carbonates[J]. Coordination Chemistry Reviews, 2019, 378: 32-65. |
| 5 | Wu Y F, Song X H, Zhang J H, et al. Mn-based MOFs as efficient catalysts for catalytic conversion of carbon dioxide into cyclic carbonates and DFT studies[J]. Chemical Engineering Science, 2019, 201: 288-297. |
| 6 | Wu X, Chen C T, Guo Z Y, et al. Metal- and halide-free catalyst for the synthesis of cyclic carbonates from epoxides and carbon dioxide[J]. ACS Catalysis, 2019, 9(3): 1895-1906. |
| 7 | 罗荣昌,周贤太,杨智, 等. 均相体系中酸碱协同催化二氧化碳与环氧化物的环加成反应[J]. 化工学报, 2016, 67(1): 258-276. |
| Luo R C, Zhou X T, Yang Z, et al. Acid-base synergistic effect promoted cycloaddition reaction from CO2 with epoxide in homogenous catalysis systems[J]. CIESC Journal, 2016, 67(1): 258-276. | |
| 8 | Shao P, Yi L C, Chen S M, et al. Metal-organic frameworks for electrochemical reduction of carbon dioxide: the role of metal centers[J]. Journal of Energy Chemistry, 2020, 40: 156-170. |
| 9 | Francke R, Schille B, Roemelt M. Homogeneously catalyzed electroreduction of carbon dioxide—methods, mechanisms, and catalysts[J]. Chemical Reviews, 2018, 118(9): 4631-4701. |
| 10 | 刘洋洋, 孙超,Singh M H, 等. 载体对铁基催化剂结构及CO2加氢制烯烃反应性能的影响特性[J]. 化工学报, 2020, 71(10): 4652-4662. |
| Liu Y Y, Sun C, Singh M H, et al. Effects of identities of supports on Fe-based catalyst and their consequences on activities of CO2 hydrogenation to olefins[J]. CIESC Journal, 2020, 71(10): 4652-4662. | |
| 11 | 杨金曼, 朱兴旺, 周固礼, 等. MOFs诱导中空Co3O4/CdIn2S4合成及光催化CO2还原性能研究[J]. 化工学报, 2020, 71(6): 2780-2787. |
| Yang J M, Zhu X W, Zhou G L, et al. Preparation of MOFs-derived hollow Co3O4/CdIn2S4 heterojunction with enhanced photocatalytic carbon dioxide reduction activity[J]. CIESC Journal, 2020, 71(6): 2780-2787. | |
| 12 | 任静, 谭玲, 赵宇飞, 等. 超薄二维材料光/电催化CO2还原的最新进展[J]. 化工学报, 2021, 72(1): 398-424. |
| Ren J, Tan L, Zhao Y F, et al. Latest development of ultrathin two-dimensional materials for photocatalytic and electrocatalytic CO2 reduction[J]. CIESC Journal, 2021, 72(1): 398-424. | |
| 13 | Tortajada A, Juliá-Hernández F, Börjesson M, et al. Transition-metal-catalyzed carboxylation reactions with carbon dioxide[J]. Angewandte Chemie International Edition, 2018, 57(49): 15948-15982. |
| 14 | Luo R C, Liu X Y, Chen M, et al. Recent advances on imidazolium-functionalized organic cationic polymers for CO2 adsorption and simultaneous conversion into cyclic carbonates[J]. ChemSusChem, 2020, 13(16): 3945-3966. |
| 15 | Luo R C, Chen M, Liu X Y, et al. Recent advances in CO2 capture and simultaneous conversion into cyclic carbonates over porous organic polymers having accessible metal sites[J]. Journal of Materials Chemistry A, 2020, 8(36): 18408-18424. |
| 16 | MacGillivray L R. Metal-Organic Frameworks: Design and Application[M]. New York: John Wiley & Sons, Inc., 2010: 37-90. |
| 17 | Farrusseng D. Metal-Organic Frameworks: Applications from Catalysis to Gas Storage[M]. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA, 2011: 191-210. |
| 18 | Song J L, Zhang B B, Jiang T, et al. Synthesis of cyclic carbonates and dimethyl carbonate using CO2 as a building block catalyzed by MOF-5/KI and MOF-5/KI/ K2CO3[J]. Frontiers of Chemistry in China, 2011, 6(1): 21–30. |
| 19 | Fei F, Dou Y, Hao X Y, et al. Construction of a porous Cu(Ⅱ)-coordinated framework for the catalytic properties of cycloaddition of carbon dioxide to epoxides[J]. Inorganic Chemistry Communications, 2019, 106: 22-26. |
| 20 | Miralda C M, Macias E E, Zhu M Q, et al. Zeolitic imidazole framework-8 catalysts in the conversion of CO2 to chloropropene carbonate[J]. ACS Catalysis, 2012, 2(1): 180-183. |
| 21 | Patel P, Parmar B, Pillai R S, et al. CO2 fixation by cycloaddition of mono/disubstituted epoxides using acyl amide decorated Co(Ⅱ) MOF as a synergistic heterogeneous catalyst[J]. Applied Catalysis A, General, 2020, 590: 117375-117382. |
| 22 | Hu L H, Chen L, Peng X, et al. Bifunctional metal-doped ZIF-8: a highly efficient catalyst for the synthesis of cyclic carbonates from CO2 cycloaddition[J]. Microporous and Mesoporous Materials, 2020, 299: 110123-110131. |
| 23 | 刘宁, 陈飞, 陶晟. 氢键给体促进有机催化的CO2与环氧化物的环加成反应[J]. 科学通报, 2020, 65(31): 3373–3388. |
| Liu N, Chen F, Tao S. Hydrogen bond donors promoted organocatalyzed cycloaddition of CO2 with epoxides[J]. Chinese Science Bulletin, 2020, 65(31): 3373–3388. | |
| 24 | Hu T D, Jiang Y, Ding Y H. Computational screening of metal-substituted HKUST-1 catalysts for chemical fixation of carbon dioxide into epoxides[J]. Journal of Materials Chemistry A, 2019, 7(24): 14825-14834. |
| 25 | An J. Design, synthesis and characterization of porous materials made from nucleobases and metals[D]. Pittsburgh: University of Pittsburgh. 2008. |
| 26 | Vogiatzis K D, Mavrandonakis A, Klopper W, et al. Ab initio study of the interactions between CO2 and N-containing organic heterocycles[J]. ChemPhysChem, 2009, 10(2): 374-383. |
| 27 | Wang F, Tan Y X, Yang H, et al. A new approach towards tetrahedral imidazolate frameworks for high and selective CO2 uptake[J]. Chemical Communications, 2011, 47(20): 5828–5830. |
| 28 | Doskocil E J, Bordawekar S V, Kaye B G, et al. UV-vis spectroscopy of iodine adsorbed on alkali-metal-modified zeolite catalysts for addition of carbon dioxide to ethylene oxide[J]. The Journal of Physical Chemistry B, 1999, 103(30): 6277-6282. |
| 29 | Xin B J, Zeng G, Gao L, et al. An unusual copper(Ⅰ) halide-based metal-organic framework with a cationic framework exhibiting the release/adsorption of iodine, ion-exchange and luminescent properties[J]. Dalton Transactions, 2013, 42(21): 7562-7570. |
| 30 | An J, Farha O K, Hupp J T, et al. Metal-adeninate vertices for the construction of an exceptionally porous metal-organic framework[J]. Nature Communications, 2012, 3: 604-610. |
| [1] | 杨天阳, 邹慧明, 周晖, 王春磊, 田长青. -30℃电动汽车补气式CO2热泵制热性能实验研究[J]. 化工学报, 2023, 74(S1): 272-279. |
| [2] | 代宝民, 王启龙, 刘圣春, 张佳宁, 李鑫海, 宗凡迪. 非共沸工质辅助过冷CO2冷热联供系统的热力学性能分析[J]. 化工学报, 2023, 74(S1): 64-73. |
| [3] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [4] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [5] | 杨灿, 孙雪琦, 尚明华, 张建, 张香平, 曾少娟. 相变离子液体体系吸收分离CO2的研究现状及展望[J]. 化工学报, 2023, 74(4): 1419-1432. |
| [6] | 何万媛, 陈一宇, 朱春英, 付涛涛, 高习群, 马友光. 阵列凸起微通道内气液两相传质特性研究[J]. 化工学报, 2023, 74(2): 690-697. |
| [7] | 王峰, 张顺鑫, 余方博, 刘亚, 郭烈锦. 光催化CO2还原制碳氢燃料系统优化策略研究[J]. 化工学报, 2023, 74(1): 29-44. |
| [8] | 党迎喜, 谈朋, 刘晓勤, 孙林兵. 辐射冷却和太阳能加热驱动的CO2变温捕获[J]. 化工学报, 2023, 74(1): 469-478. |
| [9] | 鲁军辉, 李俊明. H2O-CO2、H2O-N2、H2O-He水平管外自然对流凝结换热特性研究[J]. 化工学报, 2022, 73(9): 3870-3879. |
| [10] | 裴仁花, 王永洪, 张新儒, 李晋平. 碳纳米管/环糊精金属有机骨架协同强化混合基质膜的CO2分离[J]. 化工学报, 2022, 73(9): 3904-3914. |
| [11] | 郭丹, 方雨洁, 许一寒, 李致远, 黄守莹, 王胜平, 马新宾. 乙烷和二氧化碳催化转化的研究进展[J]. 化工学报, 2022, 73(8): 3406-3416. |
| [12] | 王立维, 王娟娟, 王永洪, 张新儒, 李晋平. 聚乙烯胺/Cu3(BTC)2-MMT-NH2混合基质膜的制备及气体传递性能[J]. 化工学报, 2022, 73(7): 3068-3077. |
| [13] | 王毅, 熊启钊, 陈杨, 杨江峰, 李立博, 李晋平. 锆基金属有机骨架材料用于氨吸附性能的研究[J]. 化工学报, 2022, 73(4): 1772-1780. |
| [14] | 吴子睿, 孙瑞, 石凌峰, 田华, 王轩, 舒歌群. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| [15] | 闫帅, 杨家宝, 龚岩, 郭庆华, 于广锁. CO2稀释对甲烷反扩散火焰结构的影响研究[J]. 化工学报, 2022, 73(3): 1335-1342. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号