化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4730-4739.DOI: 10.11949/0438-1157.20210289
胡小龙( ),公文学,彭艺,胡阳,汤颖,何辉,李文愿,赵钟兴,赵祯霞(
),公文学,彭艺,胡阳,汤颖,何辉,李文愿,赵钟兴,赵祯霞( )
)
收稿日期:2021-02-25
修回日期:2021-05-17
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
赵祯霞
作者简介:胡小龙(1995—),男,硕士研究生,基金资助:
Xiaolong HU( ),Wenxue GONG,Yi PENG,Yang HU,Ying TANG,Hui HE,Wenyuan LI,Zhongxing ZHAO,Zhenxia ZHAO(
),Wenxue GONG,Yi PENG,Yang HU,Ying TANG,Hui HE,Wenyuan LI,Zhongxing ZHAO,Zhenxia ZHAO( )
)
Received:2021-02-25
Revised:2021-05-17
Online:2021-09-05
Published:2021-09-05
Contact:
Zhenxia ZHAO
摘要:
基于配体诱导界面生长策略,制备了具有优异光芬顿降解性能与稳定性的NM88(D)/COF-OMe复合材料,并对该材料的结构形貌与光电性能进行表征。表征结果说明:DMTP的锚定能够诱导复合材料内部形成紧密连接的异质界面,并提高COF-OMe在NM88(D)表面的分散度,提升该材料的比表面积、可见光吸收能力和光生载流子分离能力,从而增强复合材料的光催化能力。光芬顿降解性能测试表明:NM88(D)/COF-OMe对磺胺甲嘧啶(SMR)的降解速率常数为0.0658 min-1,明显高于原始材料和其他已报道的催化剂。经循环使用10次后复合材料的降解率仍能保持初始态的95%以上,表明NM88(D)/COF-OMe具有良好的结构稳定性。最后,对复合材料光芬顿降解SMR的催化机理进行推导,NM88(D)/COF-OMe优异的催化能力归因于异质界面的存在提升了光生载流子分离效率并促使·OH高效生成。
中图分类号:
胡小龙, 公文学, 彭艺, 胡阳, 汤颖, 何辉, 李文愿, 赵钟兴, 赵祯霞. 配体诱导制备NM88(D)/COF-OMe复合材料及可见光芬顿联合降解抗生素磺胺甲嘧啶研究[J]. 化工学报, 2021, 72(9): 4730-4739.
Xiaolong HU, Wenxue GONG, Yi PENG, Yang HU, Ying TANG, Hui HE, Wenyuan LI, Zhongxing ZHAO, Zhenxia ZHAO. Construction of NM88(D)/COF-OMe composite via ligand-induced interfacial growth strategy for highly efficient photo-Fenton degradation of antibiotic sulfamerazine under visible light[J]. CIESC Journal, 2021, 72(9): 4730-4739.
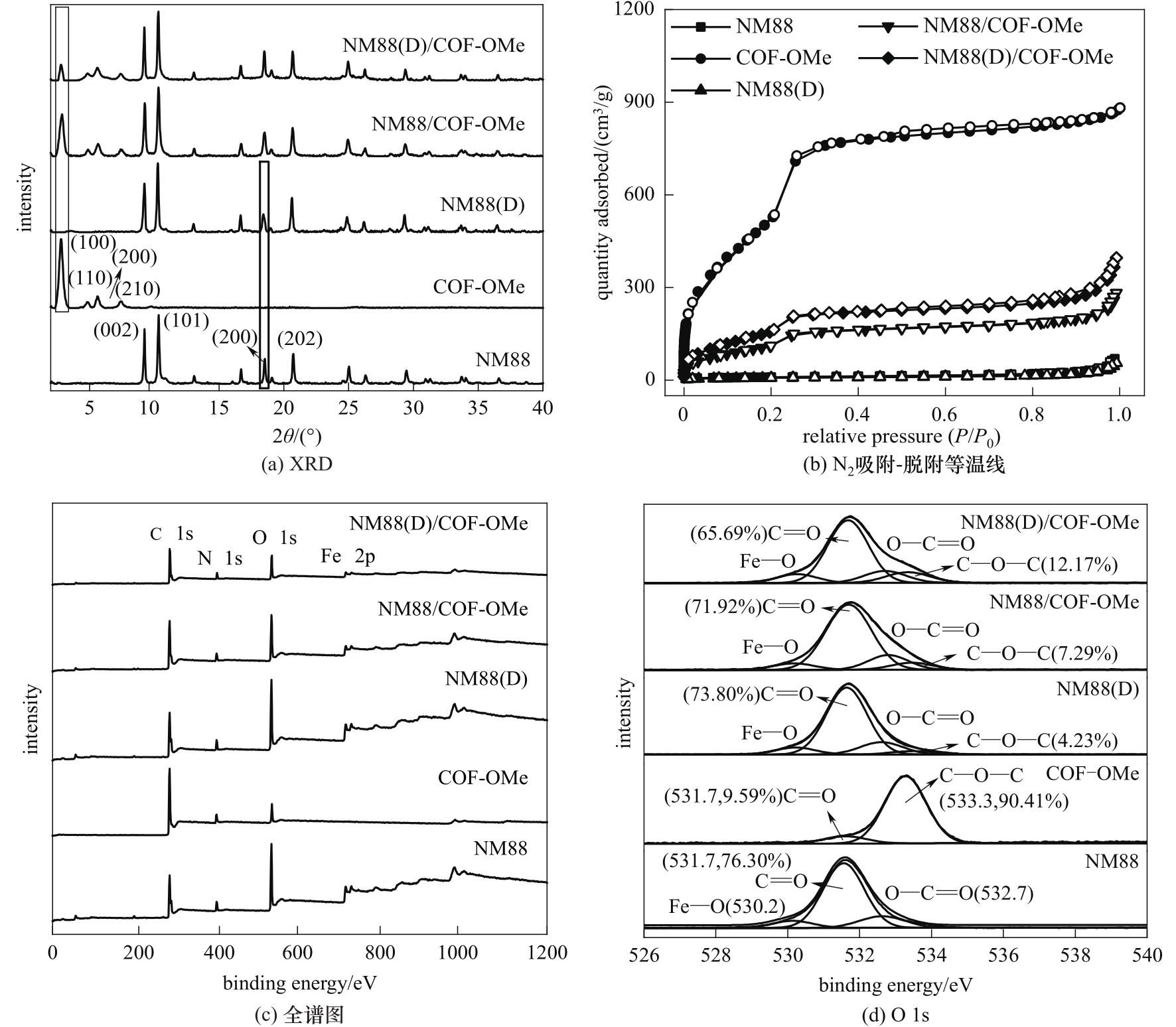
图2 不同材料的XRD谱图(a)、N2吸附-脱附等温线(b)和XPS谱图[(c)、(d)]
Fig.2 XRD patterns(a), N2 adsorption-desorption isotherms(b) and XPS spectra[(c), (d)] of different materials
| Sample | SBET/ (m2/g) | Smic/ (m2/g) | Smeso/ (m2/g) | Smeso/Smic | Vt/ (cm3/g) | Vmic/ (cm3/g) |
|---|---|---|---|---|---|---|
| NM88 | 35.3 | 9.5 | 25.8 | 2.7 | 0.106 | 0.028 |
| COF-OMe | 2166.7 | 698.9 | 1467.8 | 2.1 | 1.364 | 0.443 |
| NM88(D) | 28.6 | 7.3 | 21.3 | 2.9 | 0.087 | 0.022 |
| NM88/COF-OMe | 442.9 | 138.4 | 304.5 | 2.2 | 0.437 | 0.136 |
| NM88(D)/COF-OMe | 639.9 | 159.9 | 480.0 | 3.0 | 0.613 | 0.153 |
表1 不同材料的孔隙结构参数
Table 1 The pore structure parameters of different materials
| Sample | SBET/ (m2/g) | Smic/ (m2/g) | Smeso/ (m2/g) | Smeso/Smic | Vt/ (cm3/g) | Vmic/ (cm3/g) |
|---|---|---|---|---|---|---|
| NM88 | 35.3 | 9.5 | 25.8 | 2.7 | 0.106 | 0.028 |
| COF-OMe | 2166.7 | 698.9 | 1467.8 | 2.1 | 1.364 | 0.443 |
| NM88(D) | 28.6 | 7.3 | 21.3 | 2.9 | 0.087 | 0.022 |
| NM88/COF-OMe | 442.9 | 138.4 | 304.5 | 2.2 | 0.437 | 0.136 |
| NM88(D)/COF-OMe | 639.9 | 159.9 | 480.0 | 3.0 | 0.613 | 0.153 |
| Sample | C 1s/ %(at.) | N 1s/ %(at.) | O 1s/ %(at.) | Fe 2p/ %(at.) |
|---|---|---|---|---|
| NM88 | 59.17 | 6.92 | 29.00 | 4.90 |
| COF-OMe | 84.32 | 6.92 | 8.76 | 0.00 |
| NM88(D) | 59.30 | 6.67 | 29.21 | 4.82 |
| NM88/COF-OMe | 67.73 | 6.40 | 22.64 | 3.23 |
| NM88(D)/COF-OMe | 70.10 | 7.33 | 19.53 | 3.05 |
表2 XPS测试的样品的表面元素(C、N、O和Fe)含量
Table 2 Surface element (C, N, O and Fe) distribution of samples determined from XPS
| Sample | C 1s/ %(at.) | N 1s/ %(at.) | O 1s/ %(at.) | Fe 2p/ %(at.) |
|---|---|---|---|---|
| NM88 | 59.17 | 6.92 | 29.00 | 4.90 |
| COF-OMe | 84.32 | 6.92 | 8.76 | 0.00 |
| NM88(D) | 59.30 | 6.67 | 29.21 | 4.82 |
| NM88/COF-OMe | 67.73 | 6.40 | 22.64 | 3.23 |
| NM88(D)/COF-OMe | 70.10 | 7.33 | 19.53 | 3.05 |
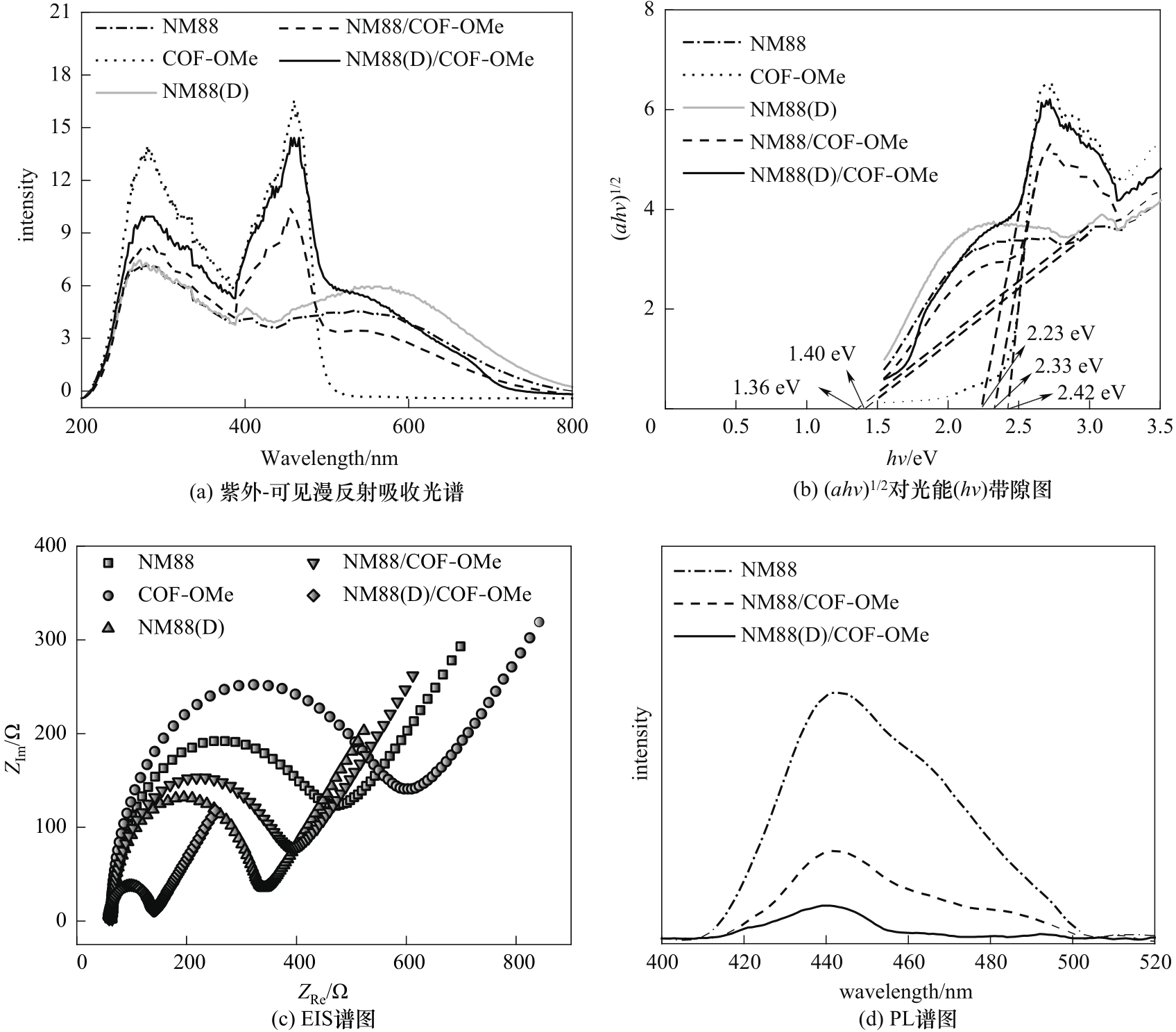
图3 不同材料的紫外-可见漫反射吸收光谱(a)、(ahv)1/2对光能(hv)带隙图(b)、电化学阻抗谱(c)和光致发光光谱(d)
Fig.3 UV-Vis DRS spectra(a), plots of (ahv)1/2versus photon energy (hv)(b), electrochemical impedance spectroscopy(c) and PL spectra(d) for different materials
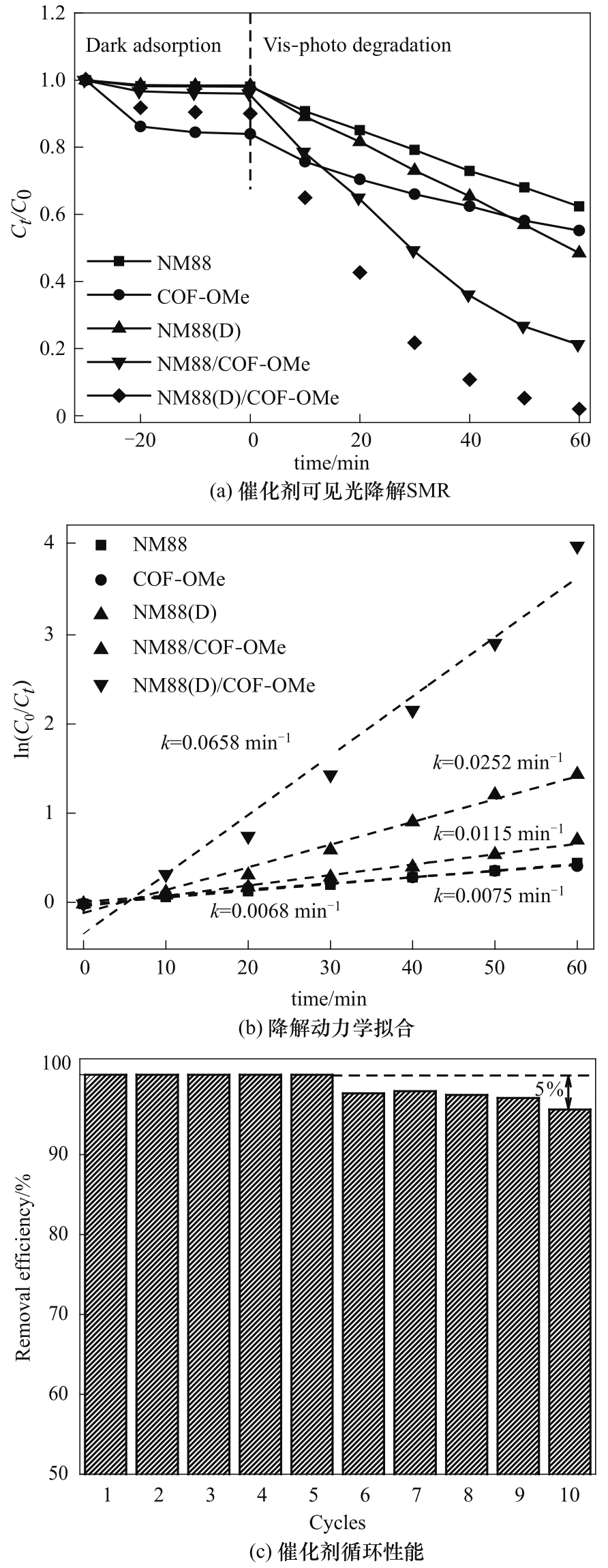
图4 在可见光下不同材料的光芬顿SMR降解(a)、降解动力学拟合(b)和NM88(D)/COF-OMe的循环性能(c)
Fig.4 Photo-Fenton degradation of SMR(a), calculated degradation kinetics constants under visible light for different materials(b) and recycling performance of NM88(D)/COF-OMe(c)
| Catalyst | Pollutant | Concentration/(mg/L) | V①/ml | Catalyst/mg | H2O2/ (mmol/L) | pH | k②×104/ (L/(mmol·min)) | Ref. |
|---|---|---|---|---|---|---|---|---|
| SBC | SMT③ | 10 | 78 | 40 | 10 | 7.4 | 1.1 | [ |
| Fe2O3-CeO2 | SMR | 20 | 250 | 125 | 8 | 3.0 | 1.3 | [ |
| CuFeO | SMT | 50 | 100 | 50 | 60 | 6.0 | 1.7 | [ |
| CUS-MIL-100(Fe) | SMT | 20 | 80 | 40 | 6 | 3.0 | 3.1 | [ |
| FeCu@C | SMT | 20 | 80 | 20 | 1.5 | 3.0 | 18.6 | [ |
| NM88(D)/COF-OMe | SMR | 10 | 100 | 10 | 2 | 6.2 | 32.9 | this work |
表3 对比不同材料降解相似磺胺类抗生素的降解速率
Table 3 Comparison of degradation rate of similar sulfonamide antibiotics by different materials
| Catalyst | Pollutant | Concentration/(mg/L) | V①/ml | Catalyst/mg | H2O2/ (mmol/L) | pH | k②×104/ (L/(mmol·min)) | Ref. |
|---|---|---|---|---|---|---|---|---|
| SBC | SMT③ | 10 | 78 | 40 | 10 | 7.4 | 1.1 | [ |
| Fe2O3-CeO2 | SMR | 20 | 250 | 125 | 8 | 3.0 | 1.3 | [ |
| CuFeO | SMT | 50 | 100 | 50 | 60 | 6.0 | 1.7 | [ |
| CUS-MIL-100(Fe) | SMT | 20 | 80 | 40 | 6 | 3.0 | 3.1 | [ |
| FeCu@C | SMT | 20 | 80 | 20 | 1.5 | 3.0 | 18.6 | [ |
| NM88(D)/COF-OMe | SMR | 10 | 100 | 10 | 2 | 6.2 | 32.9 | this work |
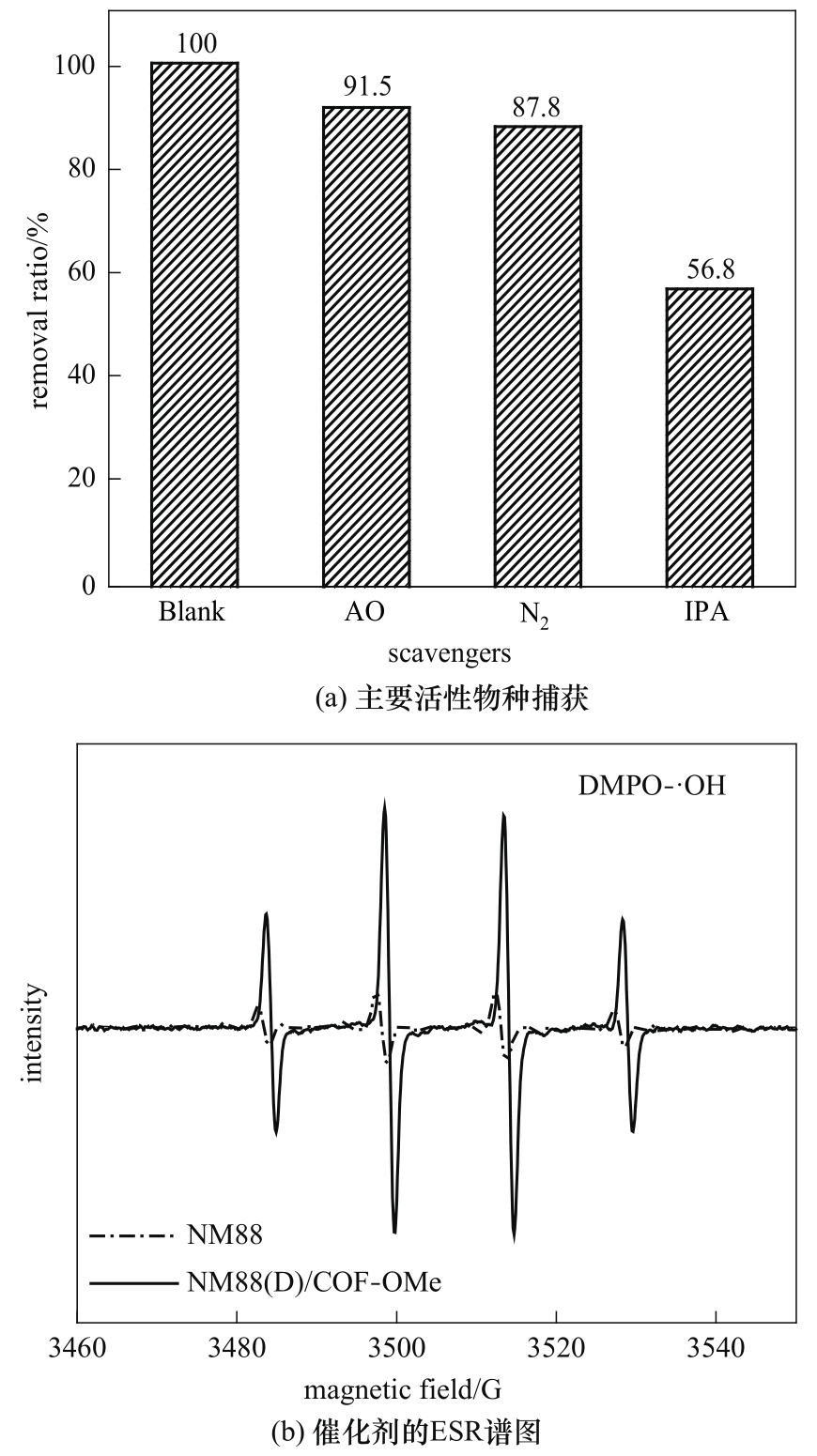
图5 NM88(D)/COF-OMe捕获主要活性物种实验(a)和可见光照下DMPO-·OH的ESR谱图(b)
Fig.5 Trapping experiments for major active species(a) and ESR spectra of DMPO-·OH under visible light irradiation(b)
| 1 | Batista A P S, Teixeira A C S C, Cooper W J, et al. Correlating the chemical and spectroscopic characteristics of natural organic matter with the photodegradation of sulfamerazine[J]. Water Research, 2016, 93: 20-29. |
| 2 | Wang J L, Zhuan R, Chu L B. The occurrence, distribution and degradation of antibiotics by ionizing radiation: an overview[J]. Science of the Total Environment, 2019, 646: 1385-1397. |
| 3 | Wang J L, Wang S Z. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants[J]. Chemical Engineering Journal, 2018, 334: 1502-1517. |
| 4 | Zhuang S T, Liu Y, Wang J L. Covalent organic frameworks as efficient adsorbent for sulfamerazine removal from aqueous solution[J]. Journal of Hazardous Materials, 2020, 383: 121126. |
| 5 | Wang J L, Wang S Z. Microbial degradation of sulfamethoxazole in the environment[J]. Applied Microbiology and Biotechnology, 2018, 102(8): 3573-3582. |
| 6 | Gao P, Chen X J, Hao M J, et al. Oxygen vacancy enhancing the Fe2O3-CeO2 catalysts in Fenton-like reaction for the sulfamerazine degradation under O2 atmosphere[J]. Chemosphere, 2019, 228: 521-527. |
| 7 | Liu Y, Tan N, Guo J R, et al. Catalytic activation of O2 by Al0-CNTs-Cu2O composite for Fenton-like degradation of sulfamerazine antibiotic at wide pH range[J]. Journal of Hazardous Materials, 2020, 396: 122751. |
| 8 | Chen Y, Yang Z, Liu Y B, et al. Fenton-like degradation of sulfamerazine at nearly neutral pH using Fe-Cu-CNTs and Al0-CNTs for in situ generation of H2O2/·OH/O2-·[J]. Chemical Engineering Journal, 2020, 396: 125329. |
| 9 | Chen J, Fu X Y, Chen H, et al. Simultaneous Gd2O3 clusters decoration and O-doping of g-C3N4 by solvothermal-polycondensation method for reinforced photocatalytic activity towards sulfamerazine[J]. Journal of Hazardous Materials, 2021, 402: 123780. |
| 10 | Fabiańska A, Białk-Bielińska A, Stepnowski P, et al. Electrochemical degradation of sulfonamides at BDD electrode: kinetics, reaction pathway and eco-toxicity evaluation[J]. Journal of Hazardous Materials, 2014, 280: 579-587. |
| 11 | Batista A P S, Pires F C C, Teixeira A C S C. Photochemical degradation of sulfadiazine, sulfamerazine and sulfamethazine: relevance of concentration and heterocyclic aromatic groups to degradation kinetics[J]. Journal of Photochemistry and Photobiology A: Chemistry, 2014, 286: 40-46. |
| 12 | Costa E P, Roccamante M, Amorim C C, et al. New trend on open solar photoreactors to treat micropollutants by photo-Fenton at circumneutral pH: increasing optical pathway[J]. Chemical Engineering Journal, 2020, 385: 123982. |
| 13 | Lu S, Liu L B, Demissie H, et al. Design and application of metal-organic frameworks and derivatives as heterogeneous Fenton-like catalysts for organic wastewater treatment: a review[J]. Environment International, 2021, 146: 106273. |
| 14 | Yuan R R, Yue C L, Qiu J L, et al. Highly efficient sunlight-driven reduction of Cr(Ⅵ) by TiO2@NH2-MIL-88B(Fe) heterostructures under neutral conditions[J]. Applied Catalysis B: Environmental, 2019, 251: 229-239. |
| 15 | Wang H, Wang H, Wang Z W, et al. Covalent organic framework photocatalysts: structures and applications[J]. Chemical Society Reviews, 2020, 49(12): 4135-4165. |
| 16 | Cai M K, Li Y L, Liu Q L, et al. One-step construction of hydrophobic MOFs@COFs core-shell composites for heterogeneous selective catalysis[J]. Advanced Science, 2019, 6(8): 1802365. |
| 17 | Lv S W, Liu J M, Li C Y, et al. Two novel MOFs@COFs hybrid-based photocatalytic platforms coupling with sulfate radical-involved advanced oxidation processes for enhanced degradation of bisphenol A[J]. Chemosphere, 2020, 243: 125378. |
| 18 | Horcajada P, Salles F, Wuttke S, et al. How linker’s modification controls swelling properties of highly flexible iron(Ⅲ) dicarboxylates MIL-88[J]. Journal of the American Chemical Society, 2011, 133(44): 17839-17847. |
| 19 | Shi X F, Yao Y J, Xu Y L, et al. Imparting catalytic activity to a covalent organic framework material by nanoparticle encapsulation[J]. ACS Applied Materials & Interfaces, 2017, 9(8): 7481-7488. |
| 20 | Shi L, Wang T, Zhang H B, et al. An amine-functionalized iron(III) metal-organic framework as efficient visible-light photocatalyst for Cr(Ⅵ) reduction[J]. Advanced Science, 2015, 2(3): 1500006. |
| 21 | Cho W, Park S, Oh M. Coordination polymer nanorods of Fe-MIL-88B and their utilization for selective preparation of hematite and magnetite nanorods[J]. Chemical Communications, 2011, 47(14): 4138. |
| 22 | Wang Y X, Zhong Z, Muhammad Y, et al. Defect engineering of NH2-MIL-88B(Fe) using different monodentate ligands for enhancement of photo-Fenton catalytic performance of acetamiprid degradation[J]. Chemical Engineering Journal, 2020, 398: 125684. |
| 23 | Zhang S, Yang Q, Liu X Y, et al. High-energy metal-organic frameworks (HE-MOFs): synthesis, structure and energetic performance[J]. Coordination Chemistry Reviews, 2016, 307: 292-312. |
| 24 | Tran T V, Nguyen V H, Nong L X, et al. Hexagonal Fe-based MIL-88B nanocrystals with NH2 functional groups accelerating oxytetracycline capture via hydrogen bonding[J]. Surfaces and Interfaces, 2020, 20: 100605. |
| 25 | Li F, Wang D K, Xing Q J, et al. Design and syntheses of MOF/COF hybrid materials via postsynthetic covalent modification: an efficient strategy to boost the visible-light-driven photocatalytic performance[J]. Applied Catalysis B: Environmental, 2019, 243: 621-628. |
| 26 | Du Y R, Xu B H, Pan J S, et al. Confinement of Brønsted acidic ionic liquids into covalent organic frameworks as a catalyst for dehydrative formation of isosorbide from sorbitol[J]. Green Chemistry, 2019, 21(17): 4792-4799. |
| 27 | Li Y Y, Jiang J, Fang Y, et al. TiO2 nanoparticles anchored onto the metal-organic framework NH2-MIL-88B(Fe) as an adsorptive photocatalyst with enhanced Fenton-like degradation of organic pollutants under visible light irradiation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(12): 16186-16197. |
| 28 | Liu N, Huang W Y, Zhang X D, et al. Ultrathin graphene oxide encapsulated in uniform MIL-88A(Fe) for enhanced visible light-driven photodegradation of RhB[J]. Applied Catalysis B: Environmental, 2018, 221: 119-128. |
| 29 | Zhang F M, Sheng J L, Yang Z D, et al. Rational design of MOF/COF hybrid materials for photocatalytic H2 evolution in the presence of sacrificial electron donors[J]. Angewandte Chemie International Edition, 2018, 57(37): 12106-12110. |
| 30 | Shi L, Yang L Q, Zhou W, et al. Photoassisted construction of holey defective g-C3N4 photocatalysts for efficient visible-light-driven H2O2 production[J]. Small, 2018, 14(9): 1703142. |
| 31 | Shao L Y, Yu Z X, Li X H, et al. Carbon nanodots anchored onto the metal-organic framework NH2-MIL-88B(Fe) as a novel visible light-driven photocatalyst: photocatalytic performance and mechanism investigation[J]. Applied Surface Science, 2020, 505: 144616. |
| 32 | Lu G L, Huang X B, Li Y, et al. Covalently integrated core-shell MOF@COF hybrids as efficient visible-light-driven photocatalysts for selective oxidation of alcohols[J]. Journal of Energy Chemistry, 2020, 43: 8-15. |
| 33 | Deng R, Luo H, Huang D L, et al. Biochar-mediated Fenton-like reaction for the degradation of sulfamethazine: role of environmentally persistent free radicals[J]. Chemosphere, 2020, 255: 126975. |
| 34 | Cheng M, Liu Y, Huang D L, et al. Prussian blue analogue derived magnetic Cu-Fe oxide as a recyclable photo-Fenton catalyst for the efficient removal of sulfamethazine at near neutral pH values[J]. Chemical Engineering Journal, 2019, 362: 865-876. |
| 35 | Tang J T, Wang J L. Metal organic framework with coordinatively unsaturated sites as efficient Fenton-like catalyst for enhanced degradation of sulfamethazine[J]. Environmental Science & Technology, 2018, 52(9): 5367-5377. |
| 36 | Tang J T, Wang J L. MOF-derived three-dimensional flower-like FeCu@C composite as an efficient Fenton-like catalyst for sulfamethazine degradation[J]. Chemical Engineering Journal, 2019, 375: 122007. |
| [1] | 周晓庆, 李春煜, 杨光, 蔡爱峰, 吴静怡. 液滴撞击不同曲率过冷波纹面结冰动力学行为及机理研究[J]. 化工学报, 2023, 74(S1): 141-153. |
| [2] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [3] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [4] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [5] | 林典, 江国梅, 徐秀彬, 赵波, 刘冬梅, 吴旭. 硅基类液防原油黏附涂层的研制及其减阻性能研究[J]. 化工学报, 2023, 74(8): 3438-3445. |
| [6] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| [7] | 李正涛, 袁志杰, 贺高红, 姜晓滨. 疏水界面上的NaCl液滴蒸发过程内环流调控机制研究[J]. 化工学报, 2023, 74(5): 1904-1913. |
| [8] | 尹驰, 张正国, 凌子夜, 方晓明. 含石蜡@二氧化硅纳米胶囊和碳纤维的相变热界面材料及其散热性能[J]. 化工学报, 2023, 74(4): 1795-1804. |
| [9] | 程伟江, 汪何琦, 高翔, 李娜, 马赛男. 锂离子电池硅基负极电解液成膜添加剂的研究进展[J]. 化工学报, 2023, 74(2): 571-584. |
| [10] | 廖艺, 牛亚宾, 潘艳秋, 俞路. 复配表面活性剂对油水界面行为和性质影响的模拟研究[J]. 化工学报, 2022, 73(9): 4003-4014. |
| [11] | 葛旺鑫, 朱以华, 江宏亮, 李春忠. 二氧化碳电还原的电解质研究进展[J]. 化工学报, 2022, 73(8): 3433-3447. |
| [12] | 蔡楚玥, 方晓明, 张正国, 凌子夜. CNTs阵列增强石蜡/硅橡胶复合相变垫片的散热性能研究[J]. 化工学报, 2022, 73(7): 2874-2884. |
| [13] | 王姝焱, 张瑞阳, 刘润, 刘凯, 周莹. Mn(BO2)2/BNO界面结构调控增强催化臭氧分解性能研究[J]. 化工学报, 2022, 73(7): 3193-3201. |
| [14] | 李雯, 兰忠, 强伟丽, 任文芝, 杜宾港, 马学虎. 蒸汽冷凝近壁过渡区团簇演化特性[J]. 化工学报, 2022, 73(7): 2865-2873. |
| [15] | 于喆淼, 王志, 生梦龙, 邢广宇, 王纪孝. 界面聚合法制备用于脱氮提纯CH4的N2优先渗透ZIF-90/聚酰胺混合基质膜[J]. 化工学报, 2022, 73(7): 3273-3286. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号