化工学报 ›› 2023, Vol. 74 ›› Issue (1): 276-289.DOI: 10.11949/0438-1157.20221574
张浩1( ), 王子悦1, 程钰洁1, 何晓辉1,3(
), 王子悦1, 程钰洁1, 何晓辉1,3( ), 纪红兵1,2,3,4(
), 纪红兵1,2,3,4( )
)
收稿日期:2022-12-07
修回日期:2023-01-11
出版日期:2023-01-05
发布日期:2023-03-20
通讯作者:
何晓辉,纪红兵
作者简介:张浩(1995—),男,博士研究生,zhangh577@mail2.sysu.edu.cn
基金资助:
Hao ZHANG1( ), Ziyue WANG1, Yujie CHENG1, Xiaohui HE1,3(
), Ziyue WANG1, Yujie CHENG1, Xiaohui HE1,3( ), Hongbing JI1,2,3,4(
), Hongbing JI1,2,3,4( )
)
Received:2022-12-07
Revised:2023-01-11
Online:2023-01-05
Published:2023-03-20
Contact:
Xiaohui HE, Hongbing JI
摘要:
单原子催化剂兼备均相催化剂活性中心明确和多相催化剂易于分离等优点,被视为传统均相催化和多相催化之间的桥梁。单原子催化剂所具备的高原子利用率和独特的电子-几何结构等特性,使其在一系列重要反应中表现出相当优异的催化性能,具有较好的工业化应用前景。但目前报道的单原子催化剂的制备量级仍大多局限在克级甚至毫克级,远不能满足未来的工业应用需求。本文介绍了目前间歇式(热解法、物理混合法和气体迁移法)和连续式(前体微胶囊法、前体雾化法、光化学合成法、两段式微反应器法和电场辅助合成法)这两种大规模制备单原子催化剂的典型合成策略,可为单原子催化剂的工业化生产和应用提供借鉴。
中图分类号:
张浩, 王子悦, 程钰洁, 何晓辉, 纪红兵. 单原子催化剂规模化制备的研究进展[J]. 化工学报, 2023, 74(1): 276-289.
Hao ZHANG, Ziyue WANG, Yujie CHENG, Xiaohui HE, Hongbing JI. Progress in the mass production of single-atom catalysts[J]. CIESC Journal, 2023, 74(1): 276-289.
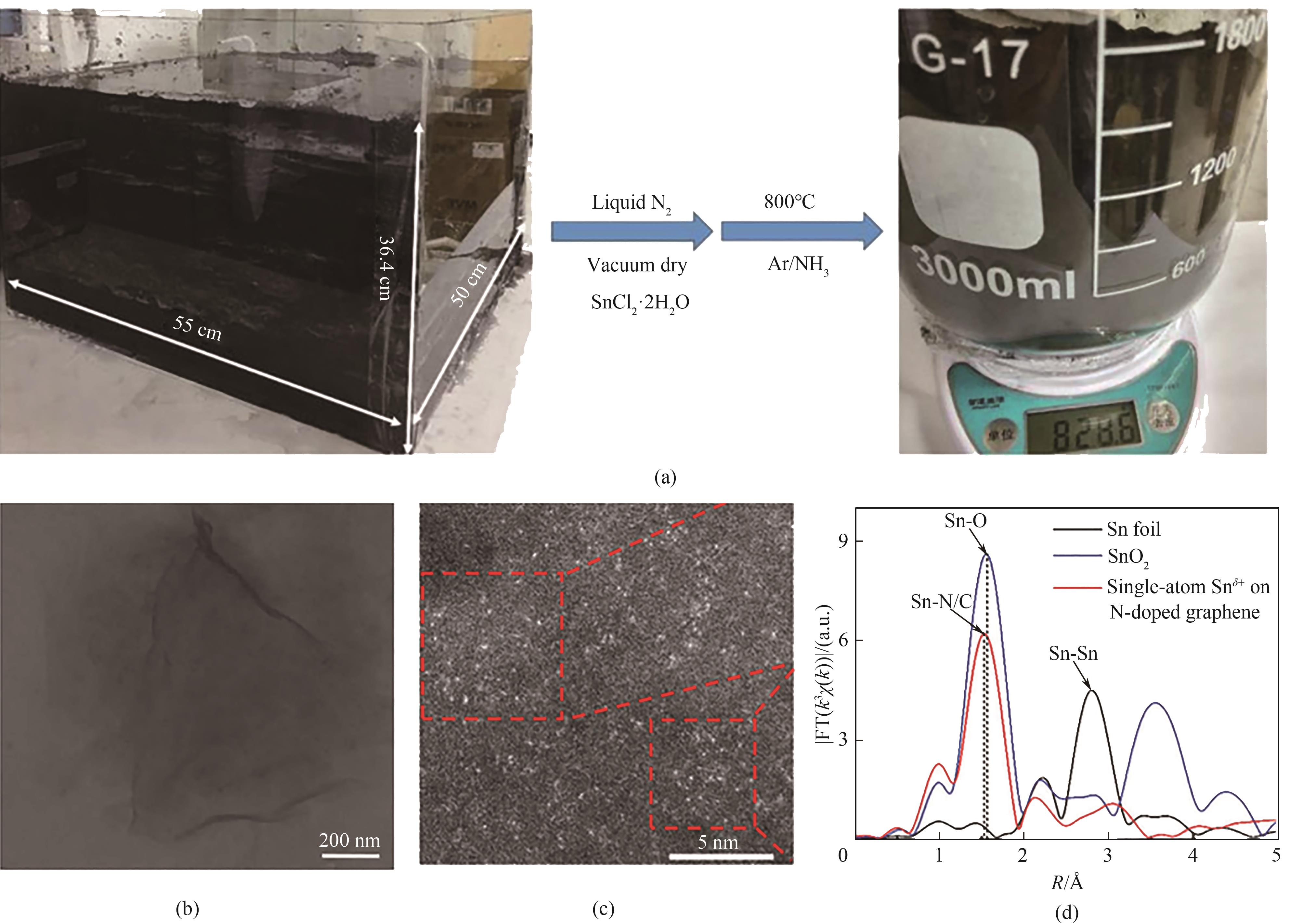
图1 Sn δ+单原子催化剂的合成示意图(a);Sn δ+单原子催化剂的TEM图(b);Sn δ+单原子催化剂的AC HAADF-STEM图(c);Sn δ+单原子催化剂的FT-EXAFS光谱图(d)[44] (1 Å=0.1 nm)
Fig.1 Synthesis diagram of the synthesis of Sn δ+ single-atom catalyst (a); TEM image of Sn δ+ single-atom catalyst (b); AC HAADF-STEM image of Sn δ+ single-atom catalyst (c); FT-EXAFS spectra of Sn δ+ single-atom catalyst (d) [44]

图2 配体介导策略合成单原子催化剂的示意图(a);2.5% (b)、3.4% (c)、4.5% (d)和5.3% (e)金属Ni负载量的Ni单原催化剂的AC HAADF-STEM图;大规模2.5%金属载量Ni单原子催化剂的照片(f) [47];UHD-SACs的制备策略(g) [47-48]
Fig.2 Schematic diagram of the ligand-mediated strategy for the synthesis of single-atom catalysts (a); AC HAADF-STEM images of different Ni single-atom catalysts with 2.5% (b), 3.4% (c), 4.5% (d), and 5.3% (e) Ni loding; Photograph of a large-scale 2.5% metal-loaded Ni single-atom catalyst (f) [47]; Strategy for the preparation of UHD-SACs (g)[47-48]

图3 Pd1/ZnO的合成示意图(a);不同规模Pd1/ZnO的AC HAADF-STEM图(b); Pd1/ZnO-10和Pd1/ZnO-1000的FT-EXAFS图(c);不同规模单原子催化剂的图片(d) [53]
Fig.3 Schematic diagram of the synthesis of Pd1/ZnO (a); AC HAADF-STEM images of different scales of Pd1/ZnO (b); FT-EXAFS spectra of Pd1/ZnO-10 and Pd1/ZnO-1000 (c); Pictures of different scales of single-atom catalysts (d) [53]
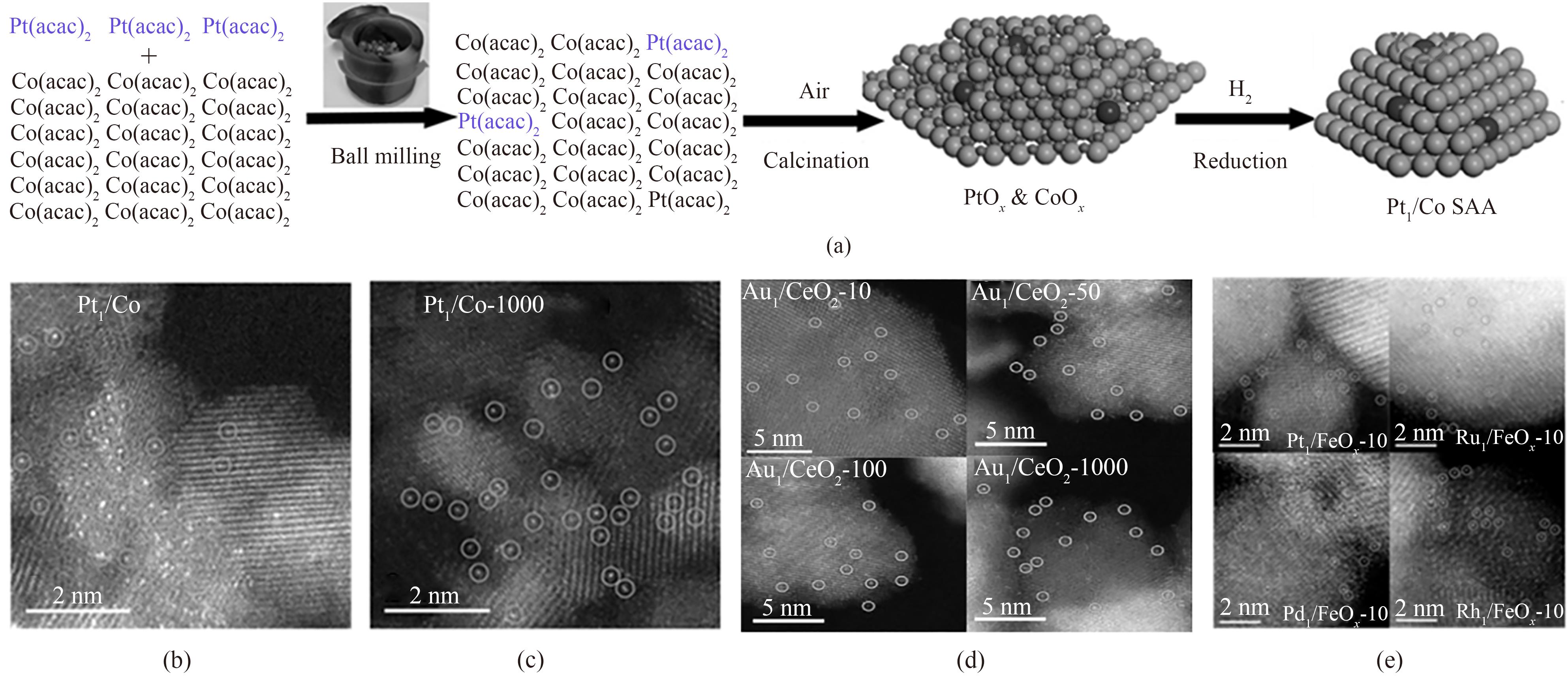
图4 Pt1/Co的合成示意图(a);Pt1/Co和Pt1/Co-1000的AC HAADF-STEM图[(b)、(c)];不同规模单原子催化剂的AC HAADF-STEM图(d);不同种类单原子催化剂的AC HAADF-STEM图(e) [55-57]
Fig.4 Schematic diagram of the synthesis of Pt1/Co (a); AC HAADF-STEM images of Pt1/Co and Pt1/Co-1000 [(b),(c)]; AC HAADF-STEM images of different scales of single-atom catalysts (d); AC HAADF-STEM images of different types of single-atom catalysts (e) [55-57]

图5 千克级Ru单原子催化剂的合成示意图(a);Ru1/MAFO的STEM图(b);Ru1/MAFO的AC HAADF-STEM图[(c)、(d)] [59]
Fig.5 Schematic diagram of the synthesis of kilogram-scale Ru single-atom catalysts (a); STEM image of Ru1/MAFO (b); AC HAADF-STEM images of Ru1/MAFO[ (c), (d)] [59]

图7 气体迁移策略的实验装置和形成机理示意图(a);Cu1/N-C的AC HAADF-STEM图[(b)、(c)];Cu1/N-C的FT-EXAFS图(d) [67]
Fig.7 Schematic diagram of the experimental setup and formation mechanism of the gas migration strategy (a); AC HAADF-STEM image of Cu1/N-C [(b), (c)]; FT-EXAFS spectra of Cu1/N-C (d) [67]

图8 Fe/SNC的准连续合成示意图(a);微胶囊的扫描电镜(SEM)图(b);Fe/SNC (c)、Co/SNC (d)和Ni/SNC (e)的AC HAADF-STEM图[68]
Fig.8 Schematic diagram for the quasi-continuous synthesis of Fe/SNC (a); Scanning electron microscopy image of microcapsules (b); AC AC HAADF-STEM image of Fe/SNC (c), Co/SNC (d), and Ni/SNC (e) [68]

图9 Pd1/FeO x 的制备实验示意图(a);单原子催化剂合成生产线(b);不同合成批次Pd1/FeO x 的AC HAADF-STEM图[(c)~(f)];Pd1/FeO x -1和Pd1/FeO x -4的FT-EXAFS图(g)[70]
Fig.9 Experimental plot of the preparation of Pd1/FeO x (a); Single-atom catalyst synthesis production line (b); AC HAADF-STEM image of Pd1/FeO x from different synthesis batches [(c)-(f)]; FT-EXAFS spectra of Pd1/FeO x -1 and Pd1/FeO x -4 (g)[70]
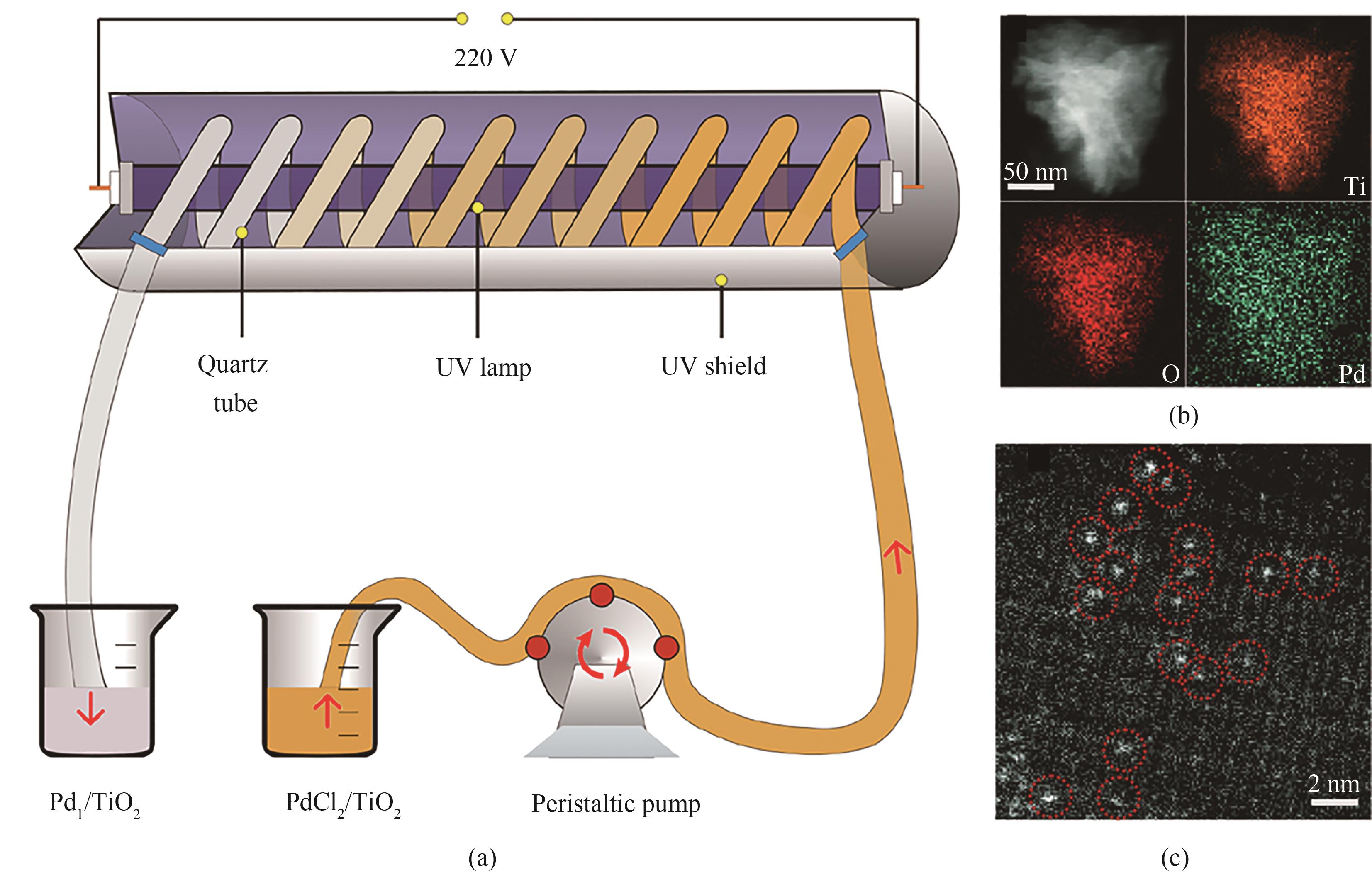
图10 Pd1/TiO2的连续合成方案(a);单个Pd1/TiO2纳米片的STEM-EDS元素谱图(b);Pd1/TiO2的AC HAADF-STEM图(c) [2]
Fig.10 Scheme for the continuous synthesis of Pd1/TiO2 (a); STEM-EDS elemental mapping of a single Pd1/TiO2 nanosheet (b); AC HAADF-STEM image of Pd1/TiO2 (c) [2]
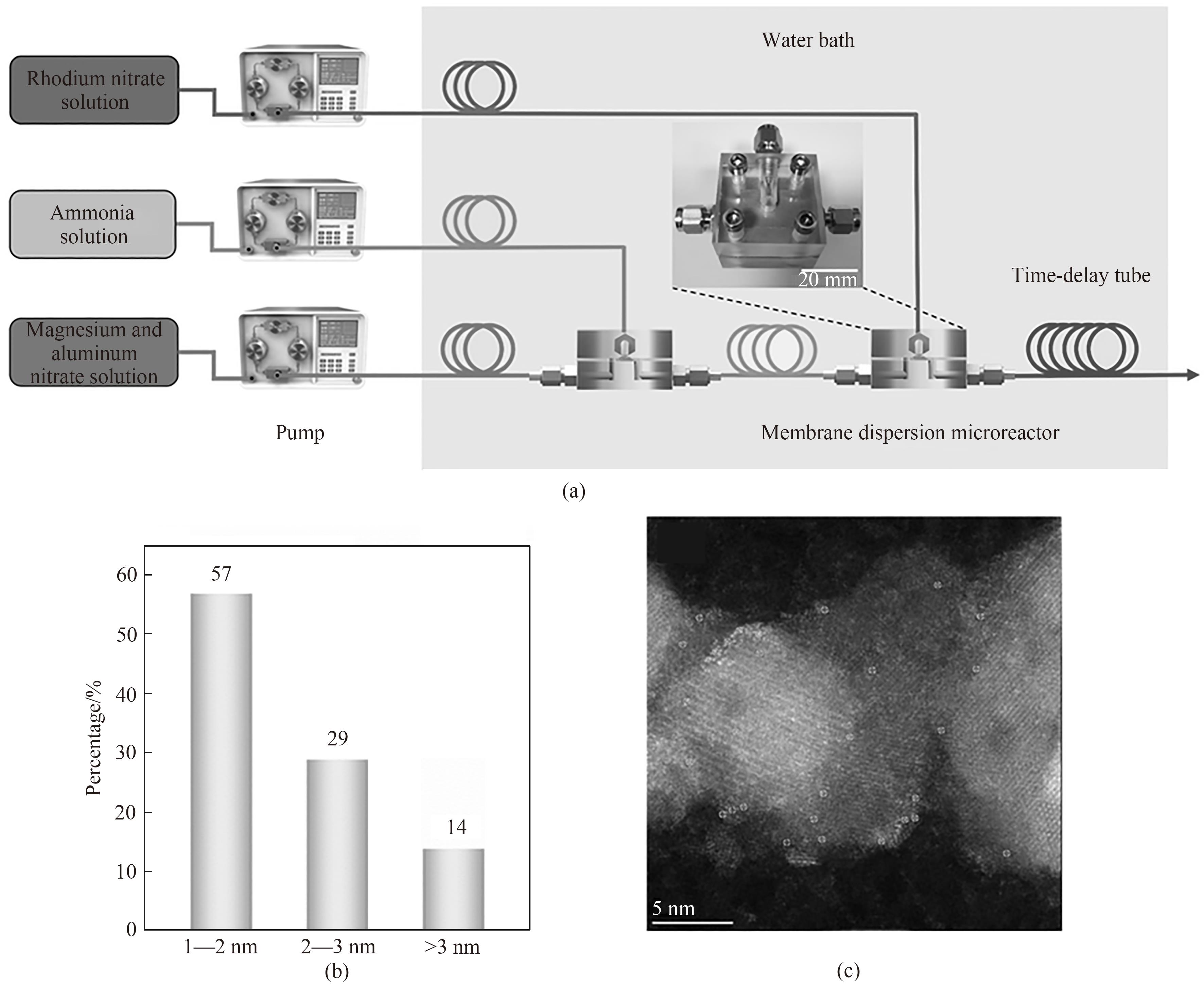
图11 Rh单原子催化剂的连续合成示意图(a);催化剂的粒径分布直方图(b);Rh单原子催化剂的AC HAADF-STEM图(c)[71]
Fig.11 Schematic of the continuous synthesis of catalysts (a); Histogram of particle size distribution of catalysts (b); AC HAADF-STEM image of Rh single-atom catalyst (c)[71]
| 1 | Kyriakou G, Boucher M B, Jewell A D, et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations[J]. Science, 2012, 335(6073): 1209-1212. |
| 2 | Liu P X, Zhao Y, Qin R X, et al. Photochemical route for synthesizing atomically dispersed palladium catalysts[J]. Science, 2016, 352(6287): 797-801. |
| 3 | Pei G X, Liu X Y, Chai M Q, et al. Isolation of Pd atoms by Cu for semi-hydrogenation of acetylene: effects of Cu loading[J]. Chinese Journal of Catalysis, 2017, 38(9): 1540-1548. |
| 4 | Lin L L, Yao S Y, Gao R, et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation[J]. Nature Nanotechnology, 2019, 14(4): 354-361. |
| 5 | Huang F, Deng Y C, Chen Y L, et al. Atomically dispersed Pd on nanodiamond/graphene hybrid for selective hydrogenation of acetylene[J]. Journal of the American Chemical Society, 2018, 140(41): 13142-13146. |
| 6 | Zhang Z L, Zhu Y H, Asakura H, et al. Thermally stable single atom Pt/m-Al2O3 for selective hydrogenation and CO oxidation[J]. Nature Communications, 2017, 8: 16100. |
| 7 | Wei H S, Liu X Y, Wang A Q, et al. FeO x -supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes[J]. Nature Communications, 2014, 5: 5634. |
| 8 | Nie L, Mei D H, Xiong H F, et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation[J]. Science, 2017, 358(6369): 1419-1423. |
| 9 | Liu W G, Zhang L L, Liu X, et al. Discriminating catalytically active FeN x species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C—H bond[J]. Journal of the American Chemical Society, 2017, 139(31): 10790-10798. |
| 10 | Cao L N, Liu W, Luo Q Q, et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2 [J]. Nature, 2019, 565(7741): 631-635. |
| 11 | Sun Q D, Wang X Y, Wang H, et al. Crystal facet effects of platinum single-atom catalysts in hydrolytic dehydrogenation of ammonia borane[J]. Journal of Materials Chemistry A, 2022, 10(20): 10837-10843. |
| 12 | Lin L L, Zhou W, Gao R, et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544(7648): 80-83. |
| 13 | Chen L N, Hou K P, Liu Y S, et al. Efficient hydrogen production from methanol using a single-site Pt1/CeO2 catalyst[J]. Journal of the American Chemical Society, 2019, 141(45): 17995-17999. |
| 14 | Guo X G, Fang G Z, Li G, et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen[J]. Science, 2014, 344(6184): 616-619. |
| 15 | Chen Z P, Vorobyeva E, Mitchell S, et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling[J]. Nature Nanotechnology, 2018, 13(8): 702-707. |
| 16 | Cao S F, Yang M, Elnabawy A O, et al. Single-atom gold oxo-clusters prepared in alkaline solutions catalyse the heterogeneous methanol self-coupling reactions[J]. Nature Chemistry, 2019, 11(12): 1098-1105. |
| 17 | Gu J, Hsu C S, Bai L C, et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO[J]. Science, 2019, 364(6445): 1091-1094. |
| 18 | Zhao C M, Dai X Y, Yao T, et al. Ionic exchange of metal-organic frameworks to access single nickel sites for efficient electroreduction of CO2 [J]. Journal of the American Chemical Society, 2017, 139(24): 8078-8081. |
| 19 | Yang H B, Hung S F, Liu S, et al. Atomically dispersed Ni(i) as the active site for electrochemical CO2 reduction[J]. Nature Energy, 2018, 3(2): 140-147. |
| 20 | Jung E, Shin H, Lee B H, et al. Atomic-level tuning of Co-N-C catalyst for high-performance electrochemical H2O2 production[J]. Nature Materials, 2020, 19(4): 436-442. |
| 21 | Tang J L, Xu S H, Sun K, et al. Recycling synthesis of single-atom Zn-nitrogen-carbon catalyst for electrocatalytic reduction of O2 to H2O2 [J]. Science China Materials, 2022, 65(12): 3490-3496. |
| 22 | Wang X N, Xu H X, Luo Y B, et al. High selective direct synthesis of H2O2 over Pd1 @γ-Al2O3 single-atom catalyst[J]. ChemCatChem, 2022, 14(21): e202200853. |
| 23 | Wu Y H, Ma H, Feng Y R, et al. Harnessing optimized surface reconstruction of single-atom Ni-doped Ni-NiO/NC precatalysts toward robust H2O2 production[J]. ACS Applied Materials & Interfaces, 2022, 14(23): 26803-26813. |
| 24 | Xiong Y, Dong J C, Huang Z Q, et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation[J]. Nature Nanotechnology, 2020, 15(5): 390-397. |
| 25 | Zhou H, Wu Y E. Formic acid oxidation by iridium single-atom catalysts on nitrogen-doped carbon[J]. Science China Chemistry, 2020, 63(9): 1171-1172. |
| 26 | Han A L, Zhang Z D, Yang J R, et al. Carbon-supported single-atom catalysts for formic acid oxidation and oxygen reduction reactions[J]. Small, 2021, 17(16): e2004500. |
| 27 | Li M F, Duanmu K N, Wan C Z, et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis[J]. Nature Catalysis, 2019, 2(6): 495-503. |
| 28 | Lu Z Y, Wang B, Hu Y F, et al. An isolated zinc-cobalt atomic pair for highly active and durable oxygen reduction[J]. Angewandte Chemie International Edition, 2019, 58(9): 2622-2626. |
| 29 | Liu D B, Li X Y, Chen S M, et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution[J]. Nature Energy, 2019, 4(6): 512-518. |
| 30 | Ye S H, Luo F Y, Zhang Q L, et al. Highly stable single Pt atomic sites anchored on aniline-stacked graphene for hydrogen evolution reaction[J]. Energy & Environmental Science, 2019, 12(3): 1000-1007. |
| 31 | Cui X J, Li W, Ryabchuk P, et al. Bridging homogeneous and heterogeneous catalysis by heterogeneous single-metal-site catalysts[J]. Nature Catalysis, 2018, 1(6): 385-397. |
| 32 | Chen Y J, Ji S F, Chen C, et al. Single-atom catalysts: synthetic strategies and electrochemical applications[J]. Joule, 2018, 2(7): 1242-1264. |
| 33 | Cao S F, Zhao Y Y, Lee S, et al. High-loading single Pt atom sites[Pt-O(OH) x ] catalyze the CO PROX reaction with high activity and selectivity at mild conditions[J]. Science Advances, 2020, 6(25): eaba3809. |
| 34 | Tao H C, Choi C, Ding L X, et al. Nitrogen fixation by Ru single-atom electrocatalytic reduction[J]. Chem, 2019, 5(1): 204-214. |
| 35 | Qiao B T, Wang A Q, Yang X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeO x [J]. Nature Chemistry, 2011, 3(8): 634-641. |
| 36 | Vajda S, White M G. Catalysis applications of size-selected cluster deposition[J]. ACS Catalysis, 2015, 5(12): 7152-7176. |
| 37 | Zhao L, Zhang Y, Huang L B, et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts[J]. Nature Communications, 2019, 10: 1278. |
| 38 | Fan L L, Liu P F, Yan X C, et al. Atomically isolated nickel species anchored on graphitized carbon for efficient hydrogen evolution electrocatalysis[J]. Nature Communications, 2016, 7: 10667. |
| 39 | Yao S Y, Zhang X, Zhou W, et al. Atomic-layered Au clusters on α-MoC as catalysts for the low-temperature water-gas shift reaction[J]. Science, 2017, 357(6349): 389-393. |
| 40 | Wu M F, Wang H, Zhong L S, et al. Effects of acid pretreatment on Fe-ZSM-5 and Fe-beta catalysts for N2O decomposition[J]. Chinese Journal of Catalysis, 2016, 37(6): 898-907. |
| 41 | Yan H, Lin Y, Wu H, et al. Bottom-up precise synthesis of stable platinum dimers on graphene[J]. Nature Communications, 2017, 8: 1070. |
| 42 | Zhang L, Banis M N, Sun X L. Single-atom catalysts by the atomic layer deposition technique[J]. National Science Review, 2018, 5(5): 628-630. |
| 43 | Sun S H, Zhang G X, Gauquelin N, et al. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Scientific Reports, 2013, 3: 1775. |
| 44 | Zu X L, Li X D, Liu W, et al. Efficient and robust carbon dioxide electroreduction enabled by atomically dispersed Sn δ + sites[J]. Advanced Materials, 2019, 31(15): e1808135. |
| 45 | Wen N, Xia Y G, Wang H H, et al. Large-scale synthesis of spinel Ni x Mn3- x O4 solid solution immobilized with iridium single atoms for efficient alkaline seawater electrolysis[J]. Advanced Science, 2022, 9(16): 2200529. |
| 46 | Lyu F C, Zeng S S, Jia Z, et al. Two-dimensional mineral hydrogel-derived single atoms-anchored heterostructures for ultrastable hydrogen evolution[J]. Nature Communications, 2022, 13: 6249. |
| 47 | Yang H Z, Shang L, Zhang Q H, et al. A universal ligand mediated method for large scale synthesis of transition metal single atom catalysts[J]. Nature Communications, 2019, 10: 4585. |
| 48 | Hai X, Xi S B, Mitchell S, et al. Scalable two-step annealing method for preparing ultra-high-density single-atom catalyst libraries[J]. Nature Nanotechnology, 2022, 17(2): 174-181. |
| 49 | James S L, Adams C J, Bolm C, et al. Mechanochemistry: opportunities for new and cleaner synthesis[J]. Chemical Society Reviews, 2012, 41(1): 413-447. |
| 50 | Baláž P, Achimovičová M, Baláž M, et al. Hallmarks of mechanochemistry: from nanoparticles to technology[J]. Chemical Society Reviews, 2013, 42(18): 7571-7637. |
| 51 | Deng D H, Chen X Q, Yu L, et al. A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature[J]. Science Advances, 2015, 1(11): e1500462. |
| 52 | Cui X J, Li H B, Wang Y, et al. Room-temperature methane conversion by graphene-confined single iron atoms[J]. Chem, 2018, 4(8): 1902-1910. |
| 53 | He X H, Deng Y C, Zhang Y, et al. Mechanochemical kilogram-scale synthesis of noble metal single-atom catalysts[J]. Cell Reports Physical Science, 2020, 1(1): 100004. |
| 54 | Guo Y L, Huang Y K, Zeng B, et al. Photo-thermo semi-hydrogenation of acetylene on Pd1/TiO2 single-atom catalyst[J]. Nature Communications, 2022, 13: 2648. |
| 55 | Gan T, Liu Y F, He Q, et al. Facile synthesis of kilogram-scale Co-alloyed Pt single-atom catalysts via ball milling for hydrodeoxygenation of 5-hydroxymethylfurfural[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(23): 8692-8699. |
| 56 | Gan T, He Q, Zhang H, et al. Unveiling the kilogram-scale gold single-atom catalysts via ball milling for preferential oxidation of CO in excess hydrogen[J]. Chemical Engineering Journal, 2020, 389: 124490. |
| 57 | Zhang H, Zhang X C, Shi S L, et al. Highly efficient fabrication of kilogram-scale palladium single-atom catalysts for the suzuki-miyaura cross-coupling reaction[J]. ACS Applied Materials & Interfaces, 2022, 14(48): 53755-53760. |
| 58 | Han G F, Li F, Rykov A I, et al. Abrading bulk metal into single atoms[J]. Nature Nanotechnology, 2022, 17(4): 403-407. |
| 59 | Liu K P, Zhao X T, Ren G Q, et al. Strong metal-support interaction promoted scalable production of thermally stable single-atom catalysts[J]. Nature Communications, 2020, 11: 1263. |
| 60 | Jones J, Xiong H F, DeLaRiva A T, et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping[J]. Science, 2016, 353(6295): 150-154. |
| 61 | Wei S J, Li A, Liu J C, et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms[J]. Nature Nanotechnology, 2018, 13(9): 856-861. |
| 62 | Qu Y T, Li Z J, Chen W X, et al. Direct transformation of bulk copper into copper single sites via emitting and trapping of atoms[J]. Nature Catalysis, 2018, 1(10): 781-786. |
| 63 | Qu Y T, Chen B X, Li Z J, et al. Thermal emitting strategy to synthesize atomically dispersed Pt metal sites from bulk Pt metal[J]. Journal of the American Chemical Society, 2019, 141(11): 4505-4509. |
| 64 | Zhou P, Li N, Chao Y G, et al. Thermolysis of noble metal nanoparticles into electron-rich phosphorus-coordinated noble metal single atoms at low temperature[J]. Angewandte Chemie International Edition, 2019, 58(40): 14184-14188. |
| 65 | Qiao B T, Liang J X, Wang A Q, et al. Ultrastable single-atom gold catalysts with strong covalent metal-support interaction (CMSI)[J]. Nano Research, 2015, 8(9): 2913-2924. |
| 66 | Yang J, Qiu Z Y, Zhao C M, et al. In situ thermal atomization to convert supported nickel nanoparticles into surface-bound nickel single-atom catalysts[J]. Angewandte Chemie International Edition, 2018, 57(43): 14095-14100. |
| 67 | Yang Z K, Chen B X, Chen W X, et al. Directly transforming copper (Ⅰ) oxide bulk into isolated single-atom copper sites catalyst through gas-transport approach[J]. Nature Communications, 2019, 10: 3734. |
| 68 | Huang L Y, Wu K, He Q, et al. Quasi-continuous synthesis of iron single atom catalysts via a microcapsule pyrolysis strategy[J]. AIChE Journal, 2021, 67(6): e17197. |
| 69 | Huang L Y, Zhang H, Cheng Y J, et al. Quasi-continuous synthesis of cobalt single atom catalysts for transfer hydrogenation of quinoline[J]. Chinese Chemical Letters, 2022, 33(5): 2569-2572. |
| 70 | He X H, Zhang H, Zhang X C, et al. Building up libraries and production line for single atom catalysts with precursor-atomization strategy[J]. Nature Communications, 2022, 13: 5721. |
| 71 | Xue Q Q, Yan B H, Wang Y J, et al. Continuous synthesis of atomically dispersed Rh supported on MgAl2O4 using two-stage microreactor[J]. AIChE Journal, 2022, 68(11): e17841. |
| 72 | Yan B, Song H L, Yang G W. A facile and green large-scale fabrication of single atom catalysts for high photocatalytic H2 evolution activity[J]. Chemical Engineering Journal, 2022, 427: 131795. |
| [1] | 张双星, 刘舫辰, 张义飞, 杜文静. R-134a脉动热管相变蓄放热实验研究[J]. 化工学报, 2023, 74(S1): 165-171. |
| [2] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [3] | 陈爱强, 代艳奇, 刘悦, 刘斌, 吴翰铭. 基板温度对HFE7100液滴蒸发过程的影响研究[J]. 化工学报, 2023, 74(S1): 191-197. |
| [4] | 刘明栖, 吴延鹏. 导光管直径和长度对传热影响的模拟分析[J]. 化工学报, 2023, 74(S1): 206-212. |
| [5] | 王志国, 薛孟, 董芋双, 张田震, 秦晓凯, 韩强. 基于裂隙粗糙性表征方法的地热岩体热流耦合数值模拟与分析[J]. 化工学报, 2023, 74(S1): 223-234. |
| [6] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [7] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [8] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [9] | 王玉兵, 李杰, 詹宏波, 朱光亚, 张大林. R134a在菱形离散肋微小通道内的流动沸腾换热实验研究[J]. 化工学报, 2023, 74(9): 3797-3806. |
| [10] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [11] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [12] | 李科, 文键, 忻碧平. 耦合蒸气冷却屏的真空多层绝热结构对液氢储罐自增压过程的影响机制研究[J]. 化工学报, 2023, 74(9): 3786-3796. |
| [13] | 陈天华, 刘兆轩, 韩群, 张程宾, 李文明. 喷雾冷却换热强化研究进展及影响因素[J]. 化工学报, 2023, 74(8): 3149-3170. |
| [14] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [15] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号