化工学报 ›› 2023, Vol. 74 ›› Issue (6): 2624-2638.DOI: 10.11949/0438-1157.20230121
收稿日期:2023-02-17
修回日期:2023-03-27
出版日期:2023-06-05
发布日期:2023-07-27
通讯作者:
钱勇
作者简介:卫雪岩(1998—),男,硕士研究生,moiswxy@foxmail.com
基金资助:Received:2023-02-17
Revised:2023-03-27
Online:2023-06-05
Published:2023-07-27
Contact:
Yong QIAN
摘要:
微米级铁粉是极具潜力的新型无碳固体燃料,为实现金属燃料规模化高效清洁燃烧利用,需要掌握其基础燃烧特性及其化学反应动力学机理。这需要大量关于动力学参数的研究,特别是活化能。热重分析(TGA)是实验获得动力学数据最常用的工具,而等转换动力学分析是处理TGA数据计算的最有效方法。本文利用热重分析仪对6、25、30、40、55及120 μm 6种微米级铁粉进行实验,采用Friedman等转化率对TGA数据进行处理与分析,主要包括TGA数据原始数据分析,获得转化率数据,定转化率数据插值选点,根据Friedman等转化率拟合,计算六种铁粉活化能与转换函数,不同粒径铁粉数据联合对比分析。结果表明大部分情况下,微米级铁粉粒径越小,同温度下反应越充分;在反应速率达到峰值前,铁粉粒径越小,反应速率越快;在反应速率达到峰值后,铁粉粒径越小,反应速率越慢;在转化率大于0.300时,对于30、40、55及120 μm的铁粉,粒径越小,活化能越小。研究成果基于氧浓度、粒径大小、加热速率多变量取得,研究了微米级铁粉的中低温全过程氧化特性,计算了多种微米级铁粉的活化能数据,为金属燃料应用提供理论基础,为建立铁粉燃烧反应动力学机理提供实验参数,为金属燃烧仿真实验提供理论参考,为金属燃料应用提供基础。
中图分类号:
卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638.
Xueyan WEI, Yong QIAN. Experimental study on the low to medium temperature oxidation characteristics and kinetics of micro-size iron powder[J]. CIESC Journal, 2023, 74(6): 2624-2638.
| α | R2 | α | R2 | α | R2 | α | R2 |
|---|---|---|---|---|---|---|---|
| 0.050 | 0.7910 | 0.275 | 0.9207 | 0.500 | 0.9785 | 0.725 | 0.9562 |
| 0.075 | 0.9248 | 0.300 | 0.9529 | 0.525 | 0.9766 | 0.750 | 0.8709 |
| 0.100 | 0.7338 | 0.325 | 0.9671 | 0.550 | 0.9733 | 0.775 | 0.9317 |
| 0.125 | 0.8453 | 0.350 | 0.9679 | 0.575 | 0.9705 | 0.800 | 0.9008 |
| 0.150 | 0.9798 | 0.375 | 0.9677 | 0.600 | 0.9652 | 0.825 | 0.8853 |
| 0.175 | 0.9697 | 0.400 | 0.9698 | 0.625 | 0.9583 | 0.850 | 0.8516 |
| 0.200 | 0.9433 | 0.425 | 0.9737 | 0.650 | 0.9481 | 0.875 | 0.8249 |
| 0.225 | 0.9159 | 0.450 | 0.9768 | 0.675 | 0.9287 | 0.900 | 0.6846 |
| 0.250 | 0.9033 | 0.475 | 0.9783 | 0.700 | 0.8838 |
表1 6、25、30、40、55及120 μm铁粉平均拟合效果
Table 1 Average correlation coefficients of linear regressions of 6,25,30,40,55 and 120 μm samples
| α | R2 | α | R2 | α | R2 | α | R2 |
|---|---|---|---|---|---|---|---|
| 0.050 | 0.7910 | 0.275 | 0.9207 | 0.500 | 0.9785 | 0.725 | 0.9562 |
| 0.075 | 0.9248 | 0.300 | 0.9529 | 0.525 | 0.9766 | 0.750 | 0.8709 |
| 0.100 | 0.7338 | 0.325 | 0.9671 | 0.550 | 0.9733 | 0.775 | 0.9317 |
| 0.125 | 0.8453 | 0.350 | 0.9679 | 0.575 | 0.9705 | 0.800 | 0.9008 |
| 0.150 | 0.9798 | 0.375 | 0.9677 | 0.600 | 0.9652 | 0.825 | 0.8853 |
| 0.175 | 0.9697 | 0.400 | 0.9698 | 0.625 | 0.9583 | 0.850 | 0.8516 |
| 0.200 | 0.9433 | 0.425 | 0.9737 | 0.650 | 0.9481 | 0.875 | 0.8249 |
| 0.225 | 0.9159 | 0.450 | 0.9768 | 0.675 | 0.9287 | 0.900 | 0.6846 |
| 0.250 | 0.9033 | 0.475 | 0.9783 | 0.700 | 0.8838 |
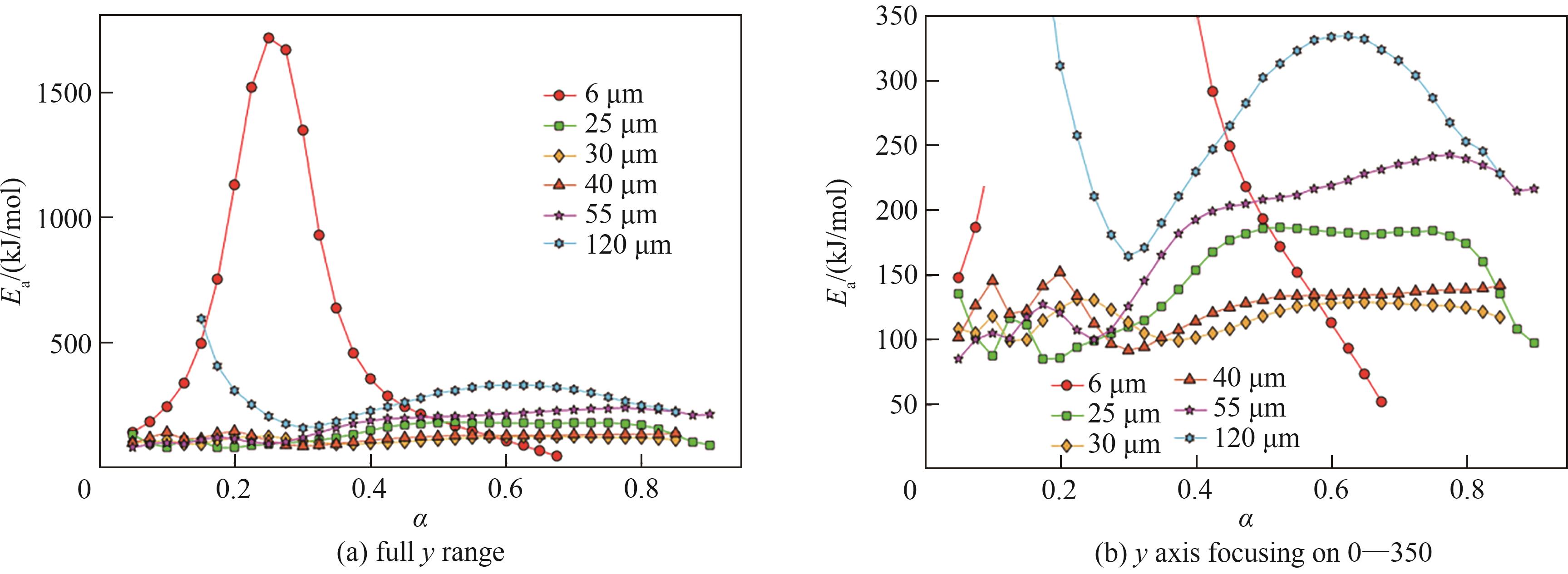
图11 6、25、30、40、55和120 μm铁粉全y轴及选定y轴范围0~350各转化率下Ea
Fig.11 Ea as a function of conversion for 6, 25, 30, 40, 55, and 120 μm samples in full y range and y axis range focusing on 0—350
| 1 | Wen D S. Nanofuel as a potential secondary energy carrier[J]. Energy & Environmental Science, 2010, 3(5): 591-600. |
| 2 | Glassman I. Metal Combustion Processes[M]. New York: American Rocket Society Preprint, 1959. |
| 3 | Mikhail N L. Reaction of aluminum powders with liquid water and steam[M]//Metal Nanopowders. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA, 2014: 163-198. |
| 4 | Shkolnikov E I, Zhuk A Z, Vlaskin M S. Aluminum as energy carrier: feasibility analysis and current technologies overview[J]. Renewable and Sustainable Energy Reviews, 2011, 15(9): 4611-4623. |
| 5 | Gromov A A, Il'in A P, Foerter-Barth U, et al. Effect of the passivating coating type, particle size, and storage time on oxidation and nitridation of aluminum powders[J]. Combustion, Explosion and Shock Waves, 2006, 42(2): 177-184. |
| 6 | Beach D B, Rondinone A J, Sumpter B G, et al. Solid-state combustion of metallic nanoparticles: new possibilities for an alternative energy carrier[J]. Journal of Energy Resources Technology, 2007, 129(1): 29-32. |
| 7 | Bergthorson J M, Goroshin S, Soo M J, et al. Direct combustion of recyclable metal fuels for zero-carbon heat and power[J]. Applied Energy, 2015, 160: 368-382. |
| 8 | Ning D G, Shoshin Y, van Stiphout M, et al. Temperature and phase transitions of laser-ignited single iron particle[J]. Combustion and Flame, 2022, 236: 111801. |
| 9 | Gan Y N, Lim Y S, Qiao L. Combustion of nanofluid fuels with the addition of boron and iron particles at dilute and dense concentrations[J]. Combustion and Flame, 2012, 159(4): 1732-1740. |
| 10 | 王金云, 王孟军, 杨在林, 等. 金属燃料技术研究进展[C]//第五届空天动力联合会议暨中国航天第三专业信息网第41届技术交流会论文集(第三册). 南京, 2020: 206-219. |
| Wang J Y, Wang M J, Yang Z L, et al. Research progress on metal fuel technology[C]// Proceedings of the 5th Aerospace Power Joint Conference and the 41st Technical Exchange Meeting of the Third Professional Information Network of China Aerospace (Volume 3). Nanjing, 2020: 206-219. | |
| 11 | Fang C, Li S F. Experimental research of the effects of superfine aluminum powders on the combustion characteristics of NEPE propellants[J]. Propellants, Explosives, Pyrotechnics, 2002, 27(1): 34-38. |
| 12 | Julien P, Whiteley S, Goroshin S, et al. Flame structure and particle-combustion regimes in premixed methane-iron-air suspensions[J]. Proceedings of the Combustion Institute, 2015, 35(2): 2431-2438. |
| 13 | Schiemann M, Fischer P, Bergthorson J. Iron particles as carbon-neutral fuel in spray roasting reactors[C]// Proceedings of the Digital Proceedings of the 8th European Combustion Meeting. 2017. |
| 14 | Wen D S, Song P X, Zhang K, et al. Thermal oxidation of iron nanoparticles and its implication for chemical-looping combustion[J]. Journal of Chemical Technology & Biotechnology, 2011, 86(3): 375-380. |
| 15 | Mandilas C, Karagiannakis G, Konstandopoulos A G, et al. Study of oxidation and combustion characteristics of iron nanoparticles under idealized and enginelike conditions[J]. Energy & Fuels, 2016, 30(5): 4318-4330. |
| 16 | 高文静, 金晶, 曾武勇. 纳米铁粉的燃烧动力学模型研究[J]. 科学技术与工程, 2013, 13(33): 9808-9812. |
| Gao W J, Jin J, Zeng W Y. Kinetic model study on combustion of nano iron powders[J]. Science Technology and Engineering, 2013, 13(33): 9808-9812. | |
| 17 | Jiang X, Wang L, Shen F M. Shaft furnace direct reduction technology-midrex and energiron[J]. Advanced Materials Research, 2013, 805/806: 654-659. |
| 18 | Beckstead M W. Correlating aluminum burning times[J]. Combustion, Explosion and Shock Waves, 2005, 41(5): 533-546. |
| 19 | Wilson R P, Williams F A. Experimental study of the combustion of single aluminum particles in O2/Ar[J]. Symposium (International) on Combustion, 1971, 13(1): 833-845. |
| 20 | Cassel H M, Liebman I. Combustion of magnesium particles (Ⅰ)[J]. Combustion and Flame, 1962, 6: 153-156. |
| 21 | Cassel H M, Liebman I. Combustion of magnesium particles (Ⅱ):Ignition temperatures and thermal conductivities of ambient atmospheres[J]. Combustion and Flame, 1963, 7: 79-81. |
| 22 | Law C, Williams F. Experiments on combustion of magnesium particles in oxygen-inert atmospheres[C]//9th Propulsion Conference. Reston, Virginia: AIAA, 1973: 1195. |
| 23 | Marion M, Chauveau C, GöKALP I. Studies on the ignition and burning of levitated aluminum particles[J]. Combustion Science and Technology, 1996, 115(4/5/6): 369-390. |
| 24 | Legrand B, Shafirovich E, Marion M, et al. Ignition and combustion of levitated magnesium particles in carbon dioxide[J]. Symposium (International) on Combustion, 1998, 27(2): 2413-2419. |
| 25 | Legrand B, Marion M, Chauveau C, et al. Ignition and combustion of levitated magnesium and aluminum particles in carbon dioxide[J]. Combustion Science and Technology, 2001, 165(1): 151-174. |
| 26 | Liebman I, Corry J, Perlee H E. Ignition and incendivity of laser irradiated single micron-size magnesium particles[J]. Combustion Science and Technology, 1972, 5(1): 21-30. |
| 27 | Wright A, Goroshin S, Higgins A. Combustion time and ignition temperature of iron particles in different oxidizing environments[C]//Proceedings of the 25th International Colloquium on the Dynamics of Explosions and Reactive Systems. 2015. |
| 28 | 谢启源, 陈丹丹, 丁延伟. 热重分析技术及其在高分子表征中的应用[J]. 高分子学报, 2022, 53(2): 193-210. |
| Xie Q Y, Chen D D, Ding Y W. Thermogravimetric analysis and its applications in polymer characterization[J]. Acta Polymerica Sinica, 2022, 53(2): 193-210. | |
| 29 | Cai J M, Xu D, Dong Z J, et al. Processing thermogravimetric analysis data for isoconversional kinetic analysis of lignocellulosic biomass pyrolysis: case study of corn stalk[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 2705-2715. |
| 30 | Sbirrazzuoli N, Vincent L, Mija A, et al. Integral, differential and advanced isoconversional methods[J]. Chemometrics and Intelligent Laboratory Systems, 2009, 96(2): 219-226. |
| 31 | Vyazovkin S, Wight C A. Isothermal and nonisothermal reaction kinetics in solids: in search of ways toward consensus[J]. The Journal of Physical Chemistry A, 1997, 101(44): 8279-8284. |
| 32 | Lysenko E N, Surzhikov A P, Zhuravkov S P, et al. The oxidation kinetics study of ultrafine iron powders by thermogravimetric analysis[J]. Journal of Thermal Analysis and Calorimetry, 2014, 115(2): 1447-1452. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 张化福, 童莉葛, 张振涛, 杨俊玲, 王立, 张俊浩. 机械蒸汽压缩蒸发技术研究现状与发展趋势[J]. 化工学报, 2023, 74(S1): 8-24. |
| [4] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [5] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [6] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [7] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [8] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [9] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [10] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [11] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [12] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [13] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [14] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [15] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
