化工学报 ›› 2020, Vol. 71 ›› Issue (5): 2333-2343.DOI: 10.11949/0438-1157.20191178
收稿日期:2019-10-11
修回日期:2020-01-24
出版日期:2020-05-05
发布日期:2020-05-05
通讯作者:
郭庆杰
作者简介:王燕霞(1987—),女,博士研究生,基金资助:
Yanxia WANG( ),Xiude HU,Jian HAO,Qingjie GUO(
),Xiude HU,Jian HAO,Qingjie GUO( )
)
Received:2019-10-11
Revised:2020-01-24
Online:2020-05-05
Published:2020-05-05
Contact:
Qingjie GUO
摘要:
以商业煤基活性炭为原料,经低浓度氧气焙烧、H2O2氧化改性,并以四乙烯五胺(TEPA)浸渍,得到胺负载复合氧化活性炭,用于模拟烟道气[(15%(体积)CO2+85%(体积)N2)+10%(体积)H2O]中CO2吸附。低浓度氧气焙烧后,活性炭的最大比表面积和孔体积分别为1421.82 m2/g、0.83 cm3/g。经复合氧化改性后,活性炭的介孔体积增大,表面含氧官能团增加,使得TEPA负载复合氧化活性炭的CO2吸附性能提高。焙烧时间为4 h,H2O2氧化、负载40%TEPA的样品COAC-4-40TEPA,在60℃时CO2饱和吸附量最高为2.45 mmol/g,是TEPA负载未改性活性炭AC-40TEPA的2.02倍。经过十次吸附循环后,COAC-4-40TEPA的 CO2饱和吸附量可维持在92.24%,而TEPA的浸出量仅有0.67%。失活模型研究表明,COAC-4-40TEPA的初始吸附速率常数是AC-40TEPA的1.64倍,且失活速率常数低于AC-40TEPA。
中图分类号:
王燕霞, 胡修德, 郝健, 郭庆杰. TEPA负载复合氧化活性炭吸附烟气中的CO2性能[J]. 化工学报, 2020, 71(5): 2333-2343.
Yanxia WANG, Xiude HU, Jian HAO, Qingjie GUO. The CO2 adsorption performance under flue gas for TEPA-impregnated composited oxidized activated carbon[J]. CIESC Journal, 2020, 71(5): 2333-2343.
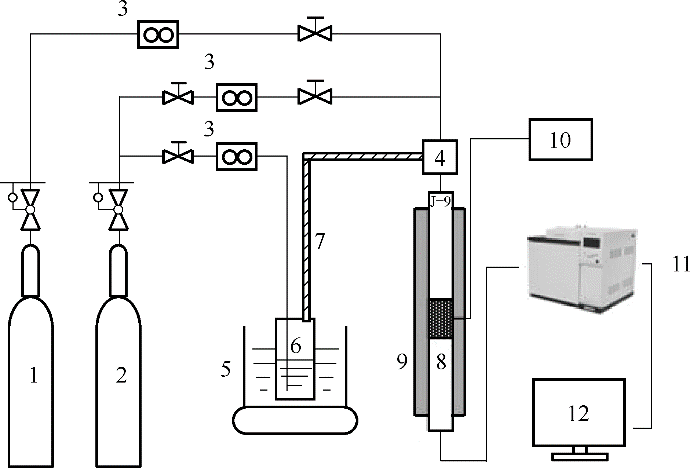
图1 CO2穿透吸附装置1—氮气;2—混合气(15%(体积)CO2/85%(体积)N2);3—质量流量计;4—混合器; 5—水浴锅; 6—鼓泡发生器; 7—加热带;8—吸附反应器;9—固定床;10—温度控制器;11—气相色谱仪;12—数据记录
Fig.1 Schematic diagram of CO2 adsorption setup
| 样品名称 | 比表面积/ (m2/g) | 孔体积/ (cm3/g) | 微孔体积/ (cm3/g) | 氧含量/ %(质量) |
|---|---|---|---|---|
| AC | 1256.69 | 0.70 | 0.38 | 6.39 |
| OAC-4 | 1360.80 | 0.81 | 0.28 | 10.54 |
| COAC-4 | 1228.59 | 0.76 | 0.24 | 16.91 |
| COAC-4-20TEPA | 543.39 | 0.35 | 0.12 | — |
| COAC-4-30TEPA | 69.44 | 0.09 | 0 | — |
| COAC-4-40TEPA | 23.15 | 0.04 | 0 | — |
| COAC-4-50TEPA | 16.21 | 0.02 | 0 | — |
表1 不同吸附剂的孔结构参数与氧含量
Table 1 Textural properties and oxygen content of adsorbents
| 样品名称 | 比表面积/ (m2/g) | 孔体积/ (cm3/g) | 微孔体积/ (cm3/g) | 氧含量/ %(质量) |
|---|---|---|---|---|
| AC | 1256.69 | 0.70 | 0.38 | 6.39 |
| OAC-4 | 1360.80 | 0.81 | 0.28 | 10.54 |
| COAC-4 | 1228.59 | 0.76 | 0.24 | 16.91 |
| COAC-4-20TEPA | 543.39 | 0.35 | 0.12 | — |
| COAC-4-30TEPA | 69.44 | 0.09 | 0 | — |
| COAC-4-40TEPA | 23.15 | 0.04 | 0 | — |
| COAC-4-50TEPA | 16.21 | 0.02 | 0 | — |
| 样品名称 | 比表面积/(m2/g) | 孔体积/ (cm3/g) | 微孔体积/(cm3/g) | 介孔体积/(cm3/g) |
|---|---|---|---|---|
| AC | 1256.70 | 0.70 | 0.38 | 0.32 |
| OAC-2 | 1421.82 | 0.83 | 0.39 | 0.44 |
| OAC-4 | 1360.80 | 0.81 | 0.28 | 0.51 |
| OAC-8 | 1071.78 | 0.69 | 0.21 | 0.48 |
表2 不同焙烧时间得到活性炭的孔结构参数
Table 2 Textural properties of activated carbons
| 样品名称 | 比表面积/(m2/g) | 孔体积/ (cm3/g) | 微孔体积/(cm3/g) | 介孔体积/(cm3/g) |
|---|---|---|---|---|
| AC | 1256.70 | 0.70 | 0.38 | 0.32 |
| OAC-2 | 1421.82 | 0.83 | 0.39 | 0.44 |
| OAC-4 | 1360.80 | 0.81 | 0.28 | 0.51 |
| OAC-8 | 1071.78 | 0.69 | 0.21 | 0.48 |
| 样品名称 | 穿透 时间/ min | 穿透 吸附量/ (mmol/g) | 饱和 吸附量/ (mmol/g) | 胺效率/ (mmol CO2/(g TEPA)) |
|---|---|---|---|---|
| COAC-4-20TEPA | 4 | 0.62 | 1.40 | 8.31 |
| COAC-4-30TEPA | 6 | 0.93 | 1.83 | 7.12 |
| COAC-4-40TEPA | 10 | 1.55 | 2.45 | 6.66 |
| COAC-4-50TEPA | 6 | 0.93 | 2.20 | 4.49 |
表3 不同样品的CO2吸附量和胺效率
Table 3 CO2 adsorption capacity and amine efficiency of samples
| 样品名称 | 穿透 时间/ min | 穿透 吸附量/ (mmol/g) | 饱和 吸附量/ (mmol/g) | 胺效率/ (mmol CO2/(g TEPA)) |
|---|---|---|---|---|
| COAC-4-20TEPA | 4 | 0.62 | 1.40 | 8.31 |
| COAC-4-30TEPA | 6 | 0.93 | 1.83 | 7.12 |
| COAC-4-40TEPA | 10 | 1.55 | 2.45 | 6.66 |
| COAC-4-50TEPA | 6 | 0.93 | 2.20 | 4.49 |
| 样品名称 | k0/(ml/(min·g)) | kd/(1/min) | R2 |
|---|---|---|---|
| AC | 66.0498 | 2.0001 | 0.9996 |
| AC-40TEPA | 43.6547 | 0.4577 | 0.9793 |
| COAC-4-40TEPA | 71.8006 | 0.3943 | 0.9775 |
表4 对比样品AC、AC-40TEPA、COAC-4-40TEPA的失活模型参数
Table 4 Parameters of deactivation model for fitting controlled samples
| 样品名称 | k0/(ml/(min·g)) | kd/(1/min) | R2 |
|---|---|---|---|
| AC | 66.0498 | 2.0001 | 0.9996 |
| AC-40TEPA | 43.6547 | 0.4577 | 0.9793 |
| COAC-4-40TEPA | 71.8006 | 0.3943 | 0.9775 |
| 1 | Greer K, Zeller D, Woroniak J, et al. Global trends in carbon dioxide (CO2) emissions from fuel combustion in marine fisheries from 1950 to 2016[J]. Marine Policy, 2019, 107: 103382. |
| 2 | Cox P, Betts R, Jones C, et al. Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model[J]. Nature, 2000, 408(6809): 184-187. |
| 3 | Goeppert A, Czaun M, Surya Prakash G K, et al. Air as the renewable carbon source of the future: an overview of CO2 capture from the atmosphere[J]. Energy & Environmental Science, 2012, 5(7): 7833. |
| 4 | Peng H L, Zhang J B, Zhang J Y, et al. Chitosan-derived mesoporous carbon with ultrahigh pore volume for amine impregnation and highly efficient CO2 capture[J]. Chemical Engineering Journal, 2019, 359: 1159-1165. |
| 5 | Mondal M K, Balsora H K, Varshney P. Progress and trends in CO2 capture/separation technologies: a review[J]. Energy, 2012, 46(1): 431-441. |
| 6 | Mardani A, Streimikiene D, Cavallaro F, et al. Carbon dioxide (CO2) emissions and economic growth: a systematic review of two decades of research from 1995 to 2017[J]. Science of The Total Environment, 2019, 649: 31-49. |
| 7 | 阎海宇, 付强, 周言, 等. 真空变压吸附捕集烟道气中二氧化碳的模拟、实验及分析[J]. 化工学报, 2016, 67(6): 2371-2379. |
| Yan H Y, Fu Q, Zhou Y, et al. Simulation, experimentation and analyzation of vacuum pressure swing adsorption process for CO2 capture from dry flue gas[J]. CIESC Journal, 2016, 67(6): 2371-2379. | |
| 8 | 刘亚敏, 彭蕾, 苏凤英, 等. 多孔胺基化氧化石墨烯基材料对CO2的吸附性能研究[J]. 化工学报, 2019, 70(5): 2016-2024. |
| Liu Y M, Peng L, Su F Y, et al. Study of CO2 adsorption on amine functionalized graphene oxide porous materials[J]. CIESC Journal, 2019, 70(5): 2016-2024. | |
| 9 | 何凯武, 唐思扬, 刘长军, 等. 有机胺功能化介孔固体吸附剂吸附分离CO2性能研究[J]. 化工学报, 2018, 69(9): 3887-3895. |
| He K W, Tang S Y, Liu C J, et al. Performance of amine functionalized mesoprous adsorbents for CO2 adsorption[J]. CIESC Journal, 2018, 69(9): 3887-3895. | |
| 10 | 彭召静, 赵彦杰, 黄成德, 等. 用于燃烧后CO2捕集系统的胺基固态吸附材料研究进展[J]. 化工进展, 2018, 37(2): 610-620. |
| Peng Z J, Zhao Y J, Huang C D, et al. Recent advances in amine-based solid sorbents for post-combustion CO2 capture[J]. Chemical Industry and Engineering Process, 2018, 37(2): 610-620. | |
| 11 | Kishor R, Ghoshal A K. APTES grafted ordered mesoporous silica KIT-6 for CO2 adsorption[J]. Chemical Engineering Journal, 2015, 262: 882-890. |
| 12 | Ren Y P, Ding R Y, Yue H R, et al. Amine-grafted mesoporous copper silicates as recyclable solid amine sorbents for post-combustion CO2 capture[J]. Applied Energy, 2017, 198: 250-260. |
| 13 | Harlick P J E, Sayari A. Applications of pore-expanded mesoporous silicas (3): Triamine silane grafting for enhanced CO2 adsorption[J]. Industrial & Engineering Chemistry Research, 2006, 45(9): 3248-3255. |
| 14 | Jung H, Jeon S, Jo D H, et al. Effect of crosslinking on the CO2 adsorption of polyethyleneimine-impregnated sorbents[J]. Chemical Engineering Journal, 2017, 307: 836-844. |
| 15 | Zhang G J, Zhao P Y, Hao L X, et al. Amine-modified SBA-15(P): a promising adsorbent for CO2 capture[J]. Journal of CO2 Utilization, 2018, 24: 22-33. |
| 16 | Kishor R, Ghoshal A K. Amine-modified mesoporous milica for CO2 adsorption: the role of structural parameters[J]. Industrial & Engineering Chemistry Research, 2017, 56(20): 6078-6087. |
| 17 | Rao N, Wang M, Shang Z M, et al. CO2 adsorption by amine-functionalized MCM-41: a comparison between impregnation and grafting modification methods[J]. Energy & Fuels, 2018, 32(1): 670-677. |
| 18 | Wang Y S, Du T, Qiu Z Y, et al. CO2 adsorption on polyethylenimine-modified ZSM-5 zeolite synthesized from rice husk ash[J]. Materials Chemistry and Physics, 2018, 207: 105-113. |
| 19 | Wang X, Chen L L, Guo Q J. Development of hybrid amine-functionalized MCM-41 sorbents for CO2 capture[J]. Chemical Engineering Journal, 2015, 260: 573-581. |
| 20 | Zhang Z Z, Wang H, Chen X Q, et al. CO2 sorption in wet ordered mesoporous silica kit-6: effects of water content and mechanism on enhanced sorption capacity[J]. Adsorption, 2014, 20(7): 883-888. |
| 21 | Jiao J, Cao J, Xia Y, et al. Improvement of adsorbent materials for CO2 capture by amine functionalized mesoporous silica with worm-hole framework structure[J]. Chemical Engineering Journal, 2016, 306: 9-16. |
| 22 | Wang X, Guo Q J. CO2 adsorption behavior of activated coal char modified with tetraethylenepentamine[J]. Energy & Fuels, 2016, 30(4): 3281-3288. |
| 23 | Gibson J A A, Gromov A V, Brandani S, et al. The effect of pore structure on the CO2 adsorption efficiency of polyamine impregnated porous carbons[J]. Microporous and Mesoporous Materials, 2015, 208: 129-139. |
| 24 | Siegelman R L, Mcdonald T M, Gonzalez M I, et al. Controlling cooperative CO2 adsorption in diamine-appended Mg2(dobpdc) metal-organic frameworks[J]. Journal of the American Chemical Society, 2017, 139(30): 10526-10538. |
| 25 | Darunte L A, Terada Y, Murdock C R, et al. Monolith-supported amine-functionalized Mg2(dobpdc) adsorbents for CO2 capture[J]. ACS Appl. Mater. Interfaces, 2017, 9(20): 17042-17050. |
| 26 | Gholidoust A, Atkinson J D, Hashisho Z. Enhancing CO2 adsorption via amine-impregnated activated carbon from oil sands coke[J]. Energy & Fuels, 2017, 31(2): 1756-1763. |
| 27 | Guo Y F, Zhao C W, Li C H, et al. Application of PEI-K2CO3/AC for capturing CO2 from flue gas after combustion[J]. Applied Energy, 2014, 129: 17-24. |
| 28 | Daud W M A W, Houshamnd A H. Textural characteristics, surface chemistry and oxidation of activated carbon[J]. Journal of Natural Gas Chemistry, 2010, 19(3): 267-279. |
| 29 | El-Hendawy A A. Influence of HNO3 oxidation on the structure and adsorptive properties of corncob-based activated carbon[J]. Carbon, 2003, 41(4): 713-722. |
| 30 | Macías-García A, Díaz-Díez M A, Cuerda-Correa E M, et al. Study of the pore size distribution and fractal dimension of HNO3-treated activated carbons[J]. Applied Surface Science, 2006, 252(17): 5972-5975. |
| 31 | Wang X, Guo Q, Zhao J, et al. Mixed amine-modified MCM-41 sorbents for CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 37: 90-98. |
| 32 | Wang X, Guo Q, Kong T T. Tetraethylenepentamine-modified MCM-41/silica gel with hierarchical mesoporous structure for CO2 capture[J]. Chemical Engineering Journal, 2015, 273: 472-480. |
| 33 | Wang Y X, Hu X D, Hao J, et al. Nitrogen and oxygen codoped porous carbon with superior CO2 adsorption performance: a combined experimental and DFT calculation study[J]. Industrial & Engineering Chemistry Research, 2019, 58(29): 13390-13400. |
| 34 | Zhang G J, Zhao P Y, Hao L X, et al. A novel amine double functionalized adsorbent for carbon dioxide capture using original mesoporous silica molecular sieves as support[J]. Separation and Purification Technology, 2019, 209: 516-527. |
| 35 | Shao L S, Wang S Q, Liu M Q, et al. Triazine-based hyper-cross-linked polymers derived porous carbons for CO2 capture[J]. Chemical Engineering Journal, 2018, 339: 509-518. |
| 36 | Liu F Q, Wang L L, Li G H, et al. Hierarchically structured graphene coupled microporous organic polymers for superior CO2 capture[J]. ACS Applied Materials & Interfaces, 2017, 9(39): 33997-34004. |
| 37 | Lai Q H, Diao Z J, Kong L L, et al. Amine-impregnated silicic acid composite as an efficient adsorbent for CO2 capture[J]. Applied Energy, 2018, 223: 293-301. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [3] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [4] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [5] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [6] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [7] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [8] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [9] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [10] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [11] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [12] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [13] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [14] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [15] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号