化工学报 ›› 2021, Vol. 72 ›› Issue (5): 2688-2696.DOI: 10.11949/0438-1157.20201277
王莹1( ),郑柏树1(
),郑柏树1( ),王刘盛1,汪冠宇1,曾文江1,汪朝旭1,阳庆元2(
),王刘盛1,汪冠宇1,曾文江1,汪朝旭1,阳庆元2( )
)
收稿日期:2020-09-07
修回日期:2020-12-29
出版日期:2021-05-05
发布日期:2021-05-05
通讯作者:
郑柏树,阳庆元
作者简介:王莹(1998—),女,硕士研究生,基金资助:
WANG Ying1( ),ZHENG Baishu1(
),ZHENG Baishu1( ),WANG Liusheng1,WANG Guanyu1,ZENG Wenjiang1,WANG Zhaoxu1,YANG Qingyuan2(
),WANG Liusheng1,WANG Guanyu1,ZENG Wenjiang1,WANG Zhaoxu1,YANG Qingyuan2( )
)
Received:2020-09-07
Revised:2020-12-29
Online:2021-05-05
Published:2021-05-05
Contact:
ZHENG Baishu,YANG Qingyuan
摘要:
存在于建筑材料中的氡(Rn)已成为污染室内空气的一种重要放射性气体,对人体的健康可造成严重的危害,因此开发对其具有高效分离性能的新型多孔材料具有重要的意义。基于巨正则系综的Monte Carlo模拟方法,系统地研究了163种锆基金属-有机骨架材料(Zr-MOF)在常温常压下对Rn/N2和Rn/O2混合气体的吸附分离性能。研究结果表明材料的孔径在5.6~8 ?(1 ? =0.1 nm)、可接触比表面积在140 ~870 m2/g范围内,材料对Rn的分离效果最佳,并发现在材料骨架上引入强极性功能基团如羧基(—COOH)和硫磺基(—SO3H)等,有利于强化材料对Rn的分离性能。研究结果可为今后理性设计与可控合成相关高性能MOF分离材料提供理论参考。
中图分类号:
王莹, 郑柏树, 王刘盛, 汪冠宇, 曾文江, 汪朝旭, 阳庆元. 锆基金属-有机骨架材料分离放射性气体Rn的计算筛选研究[J]. 化工学报, 2021, 72(5): 2688-2696.
WANG Ying, ZHENG Baishu, WANG Liusheng, WANG Guanyu, ZENG Wenjiang, WANG Zhaoxu, YANG Qingyuan. Computational screening study of radioactive gas Rn from zirconium-based metal organic frameworks materials[J]. CIESC Journal, 2021, 72(5): 2688-2696.
| 原子类型 | (ε/kB)/ K | σ/ ? | q/ e |
|---|---|---|---|
| O_O2 | 49.0 | 3.02 | -0.113 |
| COM_O2 | — | — | 0.226 |
| N_N2 | 38.298 | 3.306 | -0.405 |
| COM_N2 | — | — | 0.810 |
| Rn | 300.0 | 4.17 | — |
| H | 7.649 | 2.846 | — |
| C | 47.859 | 3.473 | — |
| N | 38.948 | 3.263 | — |
| O | 48.156 | 3.033 | — |
| P | 161.024 | 3.697 | — |
| S | 173.101 | 3.590 | — |
| F | 36.482 | 3.093 | — |
| Cl | 142.557 | 3.519 | — |
| Br | 186.184 | 3.519 | — |
| I | 256.632 | 3.697 | — |
| Co | 7.045 | 2.559 | — |
| Ni | 7.549 | 2.525 | — |
| Cu | 2.516 | 3.114 | — |
| Zn | 62.403 | 2.462 | — |
| Zr | 34.724 | 2.783 | — |
| Fe | 6.542 | 2.594 | — |
表1 不同流体分子与Zr-MOF材料的LJ势能模型参数
Table 1 Potential energy parameters of different fluid molecules and Zr-MOF
| 原子类型 | (ε/kB)/ K | σ/ ? | q/ e |
|---|---|---|---|
| O_O2 | 49.0 | 3.02 | -0.113 |
| COM_O2 | — | — | 0.226 |
| N_N2 | 38.298 | 3.306 | -0.405 |
| COM_N2 | — | — | 0.810 |
| Rn | 300.0 | 4.17 | — |
| H | 7.649 | 2.846 | — |
| C | 47.859 | 3.473 | — |
| N | 38.948 | 3.263 | — |
| O | 48.156 | 3.033 | — |
| P | 161.024 | 3.697 | — |
| S | 173.101 | 3.590 | — |
| F | 36.482 | 3.093 | — |
| Cl | 142.557 | 3.519 | — |
| Br | 186.184 | 3.519 | — |
| I | 256.632 | 3.697 | — |
| Co | 7.045 | 2.559 | — |
| Ni | 7.549 | 2.525 | — |
| Cu | 2.516 | 3.114 | — |
| Zn | 62.403 | 2.462 | — |
| Zr | 34.724 | 2.783 | — |
| Fe | 6.542 | 2.594 | — |
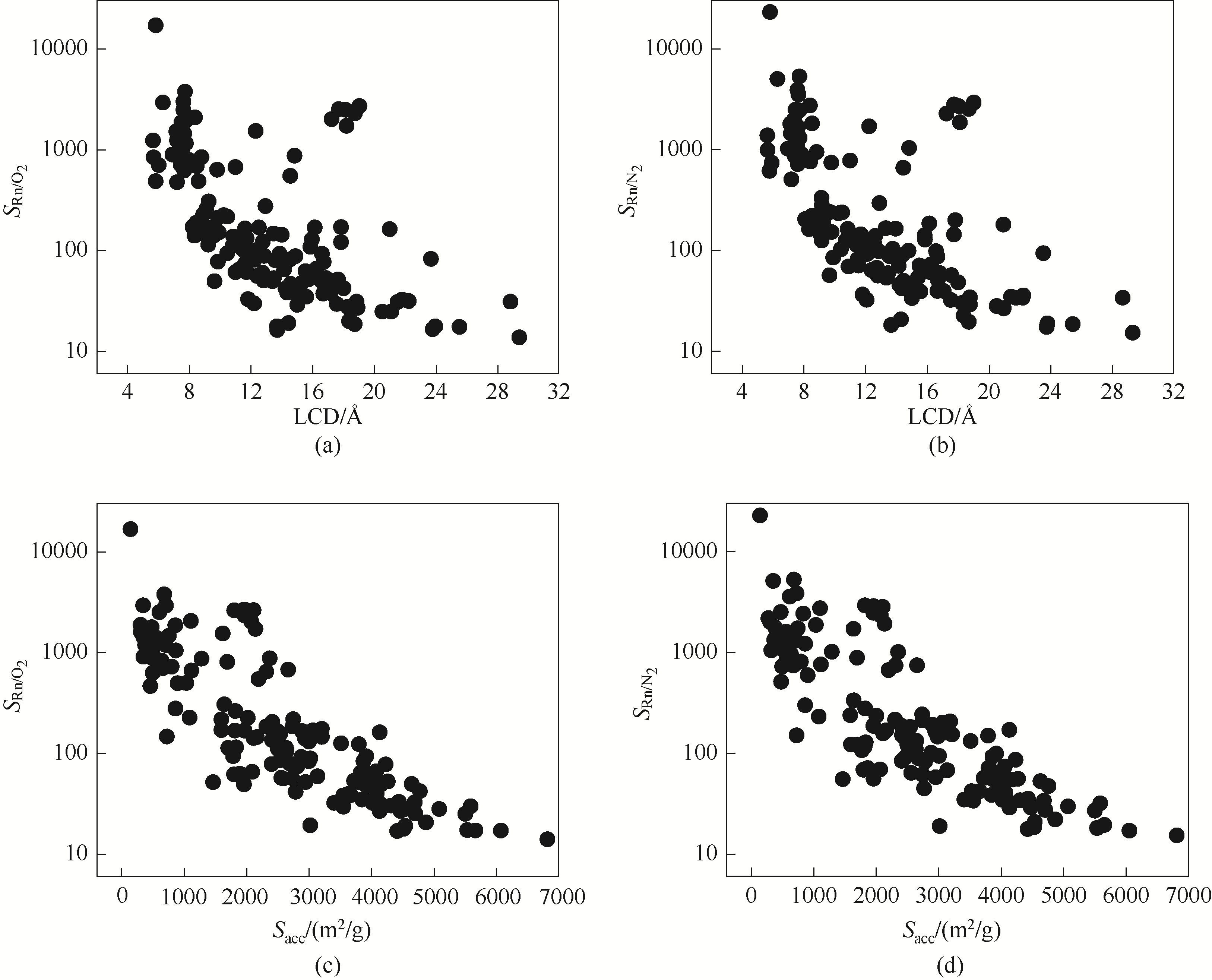
图1 Rn/O2和Rn/N2混合气体的吸附选择性与163种Zr-MOF材料结构的孔径、比表面积之间的关系
Fig.1 The relationship between selectivity of binary gas mixture Rn/O2 (Rn/N2) and the pore size, specific surface area of 163 Zr-MOF
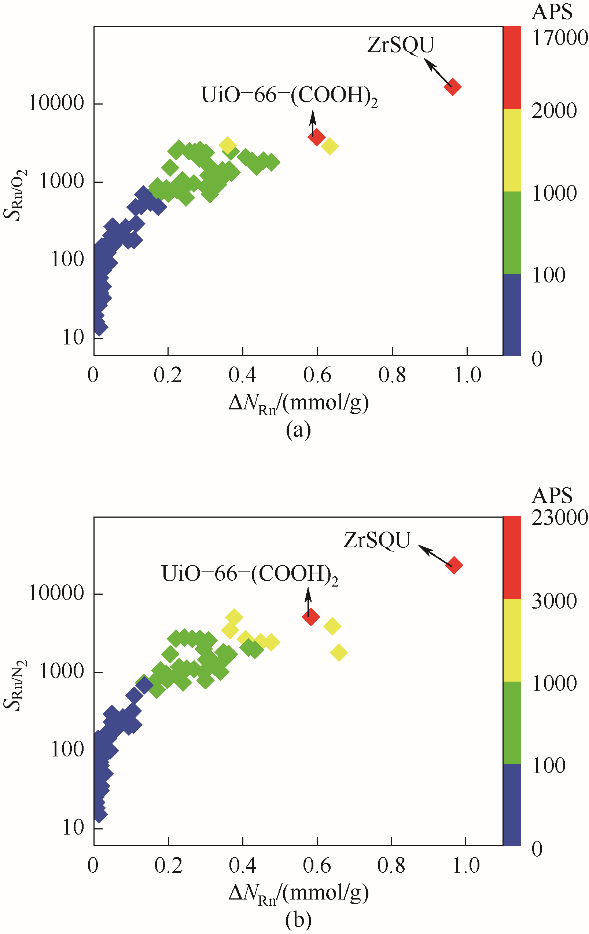
图2 Rn/O2(a)和Rn/N2(b)的吸附选择性与Rn的工作容量之间的关系(以APS作为颜色映射)
Fig.2 The relationship between Rn/O2 (a), Rn/N2(b) selectivity and the working capacity of Rn(coloured by APS)
| 材料 | Rn/O2 | Rn/N2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NRn/ (mmol/g) | ΔNRn/ (mmol/g) | NRn /(mmol/g) | ΔNRn /(mmol/g) | |||||||
| ZrSQU | 1.951 | 0.1153 | 0.962 | 16903.6 | 16261.26 | 1.979 | 0.0838 | 0.952 | 23603.6 | 22470.63 |
| ZrSQU (DDEC) | 1.95 | 0.1152 | 0.94 | 16906.8 | 15886.66 | 1.966 | 0.0834 | 0.965 | 23552.2 | 22729.67 |
| UiO-66-(COOH)2 | 1.099 | 0.289 | 0.597 | 3797.3 | 2266.99 | 1.081 | 0.2042 | 0.594 | 5287.8 | 3140.95 |
表2 候选材料的气体吸附情况
Table 2 Gas adsorption of candidate materials
| 材料 | Rn/O2 | Rn/N2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| NRn/ (mmol/g) | ΔNRn/ (mmol/g) | NRn /(mmol/g) | ΔNRn /(mmol/g) | |||||||
| ZrSQU | 1.951 | 0.1153 | 0.962 | 16903.6 | 16261.26 | 1.979 | 0.0838 | 0.952 | 23603.6 | 22470.63 |
| ZrSQU (DDEC) | 1.95 | 0.1152 | 0.94 | 16906.8 | 15886.66 | 1.966 | 0.0834 | 0.965 | 23552.2 | 22729.67 |
| UiO-66-(COOH)2 | 1.099 | 0.289 | 0.597 | 3797.3 | 2266.99 | 1.081 | 0.2042 | 0.594 | 5287.8 | 3140.95 |
| 结构名称 | LCD/ ? | Sacc/ (m2/g) | φ |
|---|---|---|---|
| ZrSQU | 5.82 | 143.44 | 0.33 |
| UiO-66-(COOH)2 | 7.18 | 690.59 | 0.39 |
表3 候选材料的结构性能参数
Table 3 The structural performance parameters of the candidate materials
| 结构名称 | LCD/ ? | Sacc/ (m2/g) | φ |
|---|---|---|---|
| ZrSQU | 5.82 | 143.44 | 0.33 |
| UiO-66-(COOH)2 | 7.18 | 690.59 | 0.39 |
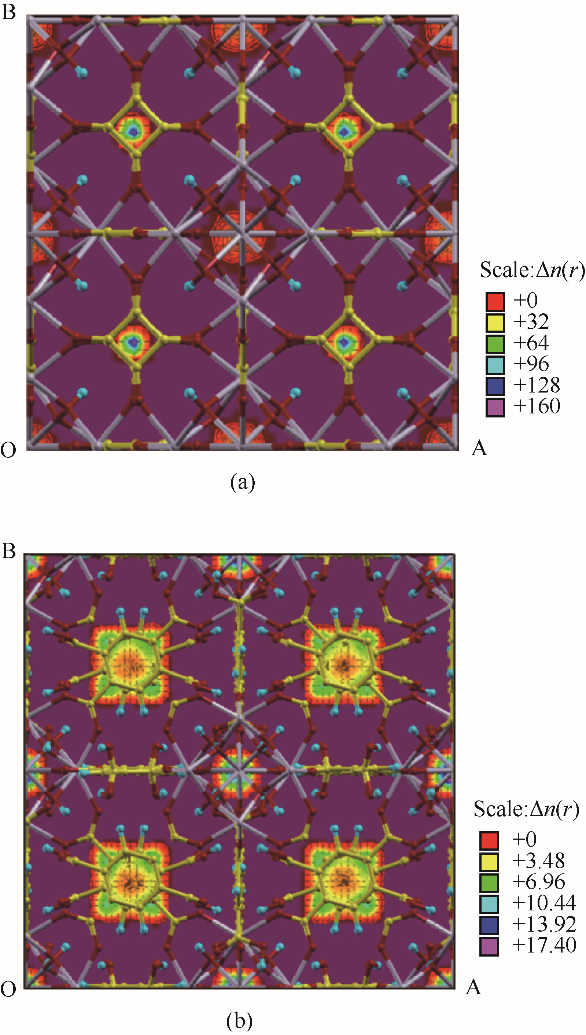
图3 在298 K、1 bar条件下, ZrSQU(a)和UiO-66-(COOH)2(b) 对Rn/N2混合气体中Rn吸附的质心分布图(碳原子为黄色、氧原子为红色、锆原子为灰色、氢原子为蓝色)
Fig.3 The centroid distribution diagram (COM) of ZrSQU(a) and UiO-66-(COOH)2 (b) for the adsorption of Rn in Rn/N2 under the condition of 298 K and 1 bar(carbon atoms are showed in yellow, oxygen atoms in red, zirconium atoms in gray, and hydrogen atoms in blue, respectively)
| 结构名称 | Rn/N2 | Rn/O2 | ||
|---|---|---|---|---|
| NRn/ (mmol/g) | NRn/ (mmol/g) | |||
| UiO-66 | 0.676 | 1850.88 | 0.148 | 499.19 |
| UiO-66-F2 | 0.249 | 829.17 | 0.206 | 694.50 |
| UiO-66-(NH2)2 | 0.296 | 1339.68 | 0.403 | 1168.33 |
| UiO-66-Cl2 | 0.555 | 1715.94 | 0.477 | 1357.43 |
| UiO-66-Br2 | 0.518 | 1601.72 | 0.425 | 1400.73 |
| UiO-66-(CH3)2 | 0.406 | 2014.44 | 0.581 | 1597.75 |
| UiO-66-(OCH3)2 | 0.762 | 2477.02 | 0.665 | 1878.11 |
| UiO-66-(CF3)2 | 0.809 | 3583.05 | 0.702 | 2499.25 |
| UiO66-(SO3H)2 | 1.719 | 5135.59 | 1.181 | 2962.31 |
| UiO-66-(COOH)2 | 1.143 | 5287.77 | 1.099 | 3797.28 |
| UiO-66-(NO2)2 | 0.646 | 2163.26 | 0.661 | 1861.25 |
表4 UiO-66系列材料对气体的吸附分离情况
Table 4 The adsorption and separation of UiO-66 series materials to gases
| 结构名称 | Rn/N2 | Rn/O2 | ||
|---|---|---|---|---|
| NRn/ (mmol/g) | NRn/ (mmol/g) | |||
| UiO-66 | 0.676 | 1850.88 | 0.148 | 499.19 |
| UiO-66-F2 | 0.249 | 829.17 | 0.206 | 694.50 |
| UiO-66-(NH2)2 | 0.296 | 1339.68 | 0.403 | 1168.33 |
| UiO-66-Cl2 | 0.555 | 1715.94 | 0.477 | 1357.43 |
| UiO-66-Br2 | 0.518 | 1601.72 | 0.425 | 1400.73 |
| UiO-66-(CH3)2 | 0.406 | 2014.44 | 0.581 | 1597.75 |
| UiO-66-(OCH3)2 | 0.762 | 2477.02 | 0.665 | 1878.11 |
| UiO-66-(CF3)2 | 0.809 | 3583.05 | 0.702 | 2499.25 |
| UiO66-(SO3H)2 | 1.719 | 5135.59 | 1.181 | 2962.31 |
| UiO-66-(COOH)2 | 1.143 | 5287.77 | 1.099 | 3797.28 |
| UiO-66-(NO2)2 | 0.646 | 2163.26 | 0.661 | 1861.25 |

图5 取代基材料对Rn的吸附热 (a)和吸附量(b)的增量百分比
Fig.5 Contribution of functional groups to the increased percentages of isosteric heat (a) and adsorption amount(b) of Rn at initial dilution
| 1 | Lubin J H, Boice J D, Edling C, et al. Lung cancer in radon-exposed miners and estimation of risk from indoor exposure[J]. JNCI: Journal of the National Cancer Institute, 1995, 87(11): 817-827. |
| 2 | Garzillo C, Pugliese M, Loffredo F, et al. Indoor radon exposure and lung cancer risk: a meta-analysis of case-control studies[J]. Translational Cancer Research, 2017, 6(S5): S934-S943. |
| 3 | Martín Sánchez A, de la Torre Pérez J, Ruano Sánchez A B, et al. Radon in workplaces in Extremadura (Spain)[J]. Journal of Environmental Radioactivity, 2012, 107: 86-91. |
| 4 | Cavka J H, Jakobsen S, Olsbye U, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. |
| 5 | Li H L, Eddaoudi M, O'Keeffe M, et al. Design and synthesis of an exceptionally stable and highly porous metal-organic framework[J]. Nature, 1999, 402(6759): 276-279. |
| 6 | Perry J J, Teich-Mcgoldrick S L, Meek S T, et al. Noble gas adsorption in metal-organic frameworks containing open metal sites[J]. The Journal of Physical Chemistry C, 2014, 118(22): 11685-11698. |
| 7 | Tang Z L, Chen H J, Zhang Y, et al. Functional two-dimensional coordination polymer exhibiting luminescence detection of nitroaromatics[J]. Crystal Growth & Design, 2019, 19(2): 1172-1182. |
| 8 | Wang Z X, Zheng B S, Liu H T, et al. A highly porous 4, 4-paddlewheel-connected NbO-type metal-organic framework with a large gas-uptake capacity[J]. Dalton Transactions, 2013, 42(31): 11304-11311. |
| 9 | Yang Q, Wiersum A D, Llewellyn P L, et al. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: a computational exploration[J]. Chemical Communications, 2011, 47(34): 9603-9605. |
| 10 | Zhang S W, Chen H J, Tian H J, et al. A 3D supramolecular network constructed from {Ni9} cluster and benzotriazole[J]. Inorganic Chemistry Communications, 2017, 86: 87-89. |
| 11 | Zheng B S, Lin X, Wang Z X, et al. Enhanced water stability of a microporous acylamide-functionalized metal-organic framework via interpenetration and methyl decoration[J]. CrystEngComm, 2014, 16(41): 9586-9589. |
| 12 | Zheng B S, Wang H, Wang Z X, et al. A highly porous rht-type acylamide-functionalized metal-organic framework exhibiting large CO2 uptake capabilities[J]. Chemical Communications, 2016, 52(88): 12988-12991. |
| 13 | Zheng B S, Yun R R, Bai J F, et al. Expanded porous MOF-505 analogue exhibiting large hydrogen storage capacity and selective carbon dioxide adsorption[J]. Inorganic Chemistry, 2013, 52(6): 2823-2829. |
| 14 | Dincă M, Long J R. Hydrogen storage in microporous metal-organic frameworks with exposed metal sites[J]. Angewandte Chemie (International Edition in English), 2008, 47(36): 6766-6779. |
| 15 | Liao J X, Zeng W J, Zheng B S, et al. Highly efficient CO2 capture and conversion of a microporous acylamide functionalized rht-type metal-organic framework[J]. Inorganic Chemistry Frontiers, 2020, 7(9): 1939-1948. |
| 16 | Rosi N L, Eckert J, Eddaoudi M, et al. Hydrogen storage in microporous metal-organic frameworks[J]. Science, 2003, 300(5622): 1127-1129. |
| 17 | Wang Z X, Luo X, Zheng B S, et al. Highly selective carbon dioxide capture and cooperative catalysis of a water-stable acylamide-functionalized metal-organic framework[J]. European Journal of Inorganic Chemistry, 2018, 2018(11): 1309-1314. |
| 18 | Wang Z X, Zheng B S, Liu H T, et al. High-capacity gas storage by a microporous oxalamide-functionalized NbO-type metal-organic framework[J]. Crystal Growth & Design, 2013, 13(11): 5001-5006. |
| 19 | Zhou W. Methane storage in porous metal-organic frameworks: current records and future perspectives[J]. The Chemical Record, 2010, 10(3): 200-204. |
| 20 | Yun R R, Cui R R, Qian F J, et al. Formation of a metal-organic framework with high gas uptakes based upon amino-decorated polyhedral cages[J]. RSC Advances, 2015, 5(4): 2374-2377. |
| 21 | 原野, 王明, 周云琪, 等. 金属有机框架孔径调控进展[J]. 化工学报, 2020, 71(2): 429-450. |
| Yuan Y, Wang M, Zhou Y Q, et al. Progress in pore size regulation of metal-organic frameworks[J]. CIESC Journal, 2020, 71(2): 429-450. | |
| 22 | 阳庆元, 刘大欢, 仲崇立. 金属-有机骨架材料的计算化学研究[J]. 化工学报, 2009, 60(4): 805-819. |
| Yang Q Y, Liu D H, Zhong C L. Computational study of metal-organic frameworks[J]. CIESC Journal, 2009, 60(4): 805-819. | |
| 23 | Zheng B S, Luo X, Wang Z X, et al. An unprecedented water stable acylamide-functionalized metal-organic framework for highly efficient CH4/CO2 gas storage/separation and acid-base cooperative catalytic activity[J]. Inorganic Chemistry Frontiers, 2018, 5(9): 2355-2363. |
| 24 | 刘宇, 赵双良, 胡军, 等. 气体在金属-有机骨架材料中的吸附分离: 经典密度泛函理论的应用[J]. 化工学报, 2016, 67(1): 89-96. |
| Liu Y, Zhao S L, Hu J, et al. Gas adsorption and separation in metal-organic framework: application of classical density functional theory[J]. CIESC Journal, 2016, 67(1): 89-96. | |
| 25 | Zheng B S, Liu H T, Wang Z X, et al. Porous NbO-type metal-organic framework with inserted acylamide groups exhibiting highly selective CO2 capture[J]. CrystEngComm, 2013, 15(18): 3517. |
| 26 | 王磊, 方桂英, 阳庆元. 金属-有机骨架材料CO2捕获性能的大规模计算筛选[J]. 化工学报, 2019, 70(3): 1135-1143. |
| Wang L, Fang G Y, Yang Q Y. Performance of metal-organic frameworks for CO2 capture from large-scale computational screening[J]. CIESC Journal, 2019, 70(3): 1135-1143. | |
| 27 | Zheng B S, Huang L, Cao X Y, et al. A highly porous acylamide decorated MOF-505 analogue exhibiting high and selective CO2 gas uptake capability[J]. CrystEngComm, 2018, 20(13): 1874-1881. |
| 28 | 张所瀛, 刘红, 刘朋飞, 等. 金属有机骨架材料在CO2/CH4吸附分离中的研究进展[J]. 化工学报, 2014, 65(5): 1563-1570. |
| Zhang S Y, Liu H, Liu P F, et al. Progress of adsorption-based CO2/CH4 separation by metal organic frameworks[J]. CIESC Journal, 2014, 65(5): 1563-1570. | |
| 29 | Yang Q Y, Zhong C L. Molecular simulation of carbon dioxide/methane/hydrogen mixture adsorption in metal-organic frameworks[J]. The Journal of Physical Chemistry B, 2006, 110(36): 17776-17783. |
| 30 | Wang M, Guo L, Cao D P. Amino-functionalized luminescent metal-organic framework test paper for rapid and selective sensing of SO2 gas and its derivatives by luminescence turn-on effect[J]. Analytical Chemistry, 2018, 90(5): 3608-3614. |
| 31 | Qiao Z W, Peng C W, Zhou J, et al. High-throughput computational screening of 137953 metal-organic frameworks for membrane separation of a CO2/N2/CH4 mixture[J]. Journal of Materials Chemistry A, 2016, 4(41): 15904-15912. |
| 32 | Peng X, Cao D P. Computational screening of porous carbons, zeolites, and metal organic frameworks for desulfurization and decarburization of biogas, natural gas, and flue gas[J]. AIChE Journal, 2013, 59(8): 2928-2942. |
| 33 | Zhao X X, Zhang S W, Yan J Q, et al. Polyoxometalate-based metal-organic frameworks as visible-light-induced photocatalysts[J]. Inorganic Chemistry, 2018, 57(9): 5030-5037. |
| 34 | Xie Y, Ning S G, Zhang Y, et al. A 3D supramolecular network as highly selective and sensitive luminescent sensor for PO43- and Cu2+ ions in aqueous media[J]. Dyes and Pigments, 2018, 150: 36-43. |
| 35 | Dhakshinamoorthy A, Alvaro M, Garcia H. Commercial metal-organic frameworks as heterogeneous catalysts[J]. Chemical Communications, 2012, 48(92): 11275-11288. |
| 36 | Zeng X F, Chen F, Cao D P. Screening metal-organic frameworks for capturing radioactive gas Rn in indoor air[J]. Journal of Hazardous Materials, 2019, 366: 624-629. |
| 37 | Sumer Z, Keskin S. Molecular simulations of MOF adsorbents and membranes for noble gas separations[J]. Chemical Engineering Science, 2017, 164: 108-121. |
| 38 | Zhou F X, Zheng B S, Liu D H, et al. Large-scale structural refinement and screening of zirconium metal-organic frameworks for H2S/CH4 separation[J]. ACS Applied Materials & Interfaces, 2019, 11(50): 46984-46992. |
| 39 | Willems T F, Rycroft C H, Kazi M, et al. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials[J]. Microporous and Mesoporous Materials, 2012, 149(1): 134-141. |
| 40 | Mellot C, Lignieres J. Monte Carlo simulations of N2 and O2 adsorption in silicalite and CaLSX zeolites[J]. Molecular Simulation, 1997, 18(6): 349-365. |
| 41 | Makrodimitris K, Papadopoulos G K, Theodorou D N. Prediction of permeation properties of CO2 and N2 through silicalite via molecular simulations[J]. The Journal of Physical Chemistry B, 2001, 105(4): 777-788. |
| 42 | Pellenq R J M, Nicholson D. Intermolecular potential function for the physical adsorption of rare gases in silicalite[J]. The Journal of Physical Chemistry, 1994, 98(50): 13339-13349. |
| 43 | Rappé A K, Casewit C J, Colwell K S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. Journal of the American Chemical Society, 1992, 114(25): 10024-10035. |
| 44 | Mayo S L, Olafson B D, Goddard W A. DREIDING: a generic force field for molecular simulations[J]. The Journal of Physical Chemistry, 1990, 94(26): 8897-8909. |
| 45 | Yang Q Y, Liu D H, Zhong C L, et al. Development of computational methodologies for metal-organic frameworks and their application in gas separations[J]. Chemical Reviews, 2013, 113(10): 8261-8323. |
| 46 | Lan Y S, Han X Y, Tong M M, et al. Materials genomics methods for high-throughput construction of COFs and targeted synthesis [J]. Nature Communications, 2018, 9:5274-5283. |
| 47 | Breneman C M, Wiberg K B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis[J]. Journal of Computational Chemistry, 1990, 11(3): 361-373. |
| 48 | Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, Condensed Matter, 1996, 54(16): 11169-11186. |
| 49 | Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758. |
| 50 | Manz T A, Sholl D S. Chemically meaningful atomic charges that reproduce the electrostatic potential in periodic and nonperiodic materials[J]. Journal of Chemical Theory and Computation, 2010, 6(8): 2455-2468. |
| 51 | 许红, 童敏曼, 吴栋, 等. 金属-有机骨架材料用于去除天然气中H2S的计算研究[J]. 物理化学学报, 2015, 31(1): 41-50. |
| Xu H, Tong M M, Wu D, et al. Computational study of metal-organic frameworks for removing H2S from natural gas[J]. Acta Physico-Chimica Sinica, 2015, 31(1): 41-50. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [6] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [7] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [8] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [9] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [10] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [11] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [12] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [13] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [14] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [15] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号