化工学报 ›› 2021, Vol. 72 ›› Issue (5): 2697-2705.DOI: 10.11949/0438-1157.20201319
黄艳1( ),陈功2,王睿猛2,邵珊2,张正生2,杨东晓2,卢真保1,黄佳1,赵祯霞2,赵钟兴2(
),陈功2,王睿猛2,邵珊2,张正生2,杨东晓2,卢真保1,黄佳1,赵祯霞2,赵钟兴2( )
)
收稿日期:2020-09-17
修回日期:2021-02-10
出版日期:2021-05-05
发布日期:2021-05-05
通讯作者:
赵钟兴
作者简介:黄艳(1974—),女,博士,高级工程师,基金资助:
HUANG Yan1( ),CHEN Gong2,WANG Ruimeng2,SHAO Shan2,ZHANG Zhengsheng2,YANG Dongxiao2,LU Zhenbao1,HUANG Jia1,ZHAO Zhenxia2,ZHAO Zhongxing2(
),CHEN Gong2,WANG Ruimeng2,SHAO Shan2,ZHANG Zhengsheng2,YANG Dongxiao2,LU Zhenbao1,HUANG Jia1,ZHAO Zhenxia2,ZHAO Zhongxing2( )
)
Received:2020-09-17
Revised:2021-02-10
Online:2021-05-05
Published:2021-05-05
Contact:
ZHAO Zhongxing
摘要:
提出“高挥发性分子协同脱附”策略,即利用高挥发性乙醇分子与低挥发性香料香兰素分子间的氢键作用,提升香兰素/乙醇MIL-100(Fe)共吸附体系中香兰素分子在MIL-100(Fe)上的脱附效率,并通过分子模拟计算香兰素与乙醇分子间的氢键作用,以及MIL-100(Fe)中香兰素和乙醇之间结合能的影响关系。结果发现:MIL-100(Fe)对香兰素乙醇溶液中的香兰素具有较高的吸附量 (780 mg/g),并且将吸附香兰素后MIL-100(Fe)在60℃干燥预处理后,由于乙醇的协同脱附作用使香兰素在MIL-100(Fe)上的脱附效率显著上升,其脱附峰温为190℃。同时,考察不同香兰素吸附量对MIL-100(Fe)上香兰素脱附率的影响,发现香兰素的脱附率随香兰素吸附量的增加呈现先增加后下降的趋势,在吸附量约606 mg/g条件时达到最大脱附率(59.1%)。最后,采用分子模拟计算方法发现香兰素和乙醇之间存在强氢键作用,导致在乙醇存在的条件下香兰素与MIL-100(Fe)之间的结合能从-103.47 kJ/mol下降到-66.58 kJ/mol,使得香兰素分子更容易从MIL-100(Fe)上脱附。
中图分类号:
黄艳, 陈功, 王睿猛, 邵珊, 张正生, 杨东晓, 卢真保, 黄佳, 赵祯霞, 赵钟兴. MIL-100(Fe)中乙醇对低挥发性香兰素的协同脱附研究[J]. 化工学报, 2021, 72(5): 2697-2705.
HUANG Yan, CHEN Gong, WANG Ruimeng, SHAO Shan, ZHANG Zhengsheng, YANG Dongxiao, LU Zhenbao, HUANG Jia, ZHAO Zhenxia, ZHAO Zhongxing. Synergistic desorption of low volatile vanillin with ethanol on MIL-100(Fe)[J]. CIESC Journal, 2021, 72(5): 2697-2705.

图1 MIL-100(Fe)的SEM与PXRD谱图(a); N2吸附脱附等温线及DFT孔径分布(b); 傅里叶红外光谱图(c); 热重曲线(d)
Fig.1 SEM and PXRD images (a); N2 adsorption desorption isotherm and DFT pore diameter distribution (b); Fourier infrared spectrum (c) and thermogravimetric curve (d) of MIL-100(Fe)
| Sample | SLang/ (m2/g) | SBET/ (m2/g) | SMic/(m2/g) | SMeso/SMic | Vt/ (cm3/g) | VMic/(cm3/g) |
|---|---|---|---|---|---|---|
| MIL-100(Fe) | 2594.1 | 1895.6 | 1473.3 | 0.29 | 0.90 | 0.58 |
表1 MIL-100(Fe)的孔隙结构参数
Table 1 Physical properties of MIL-100(Fe)
| Sample | SLang/ (m2/g) | SBET/ (m2/g) | SMic/(m2/g) | SMeso/SMic | Vt/ (cm3/g) | VMic/(cm3/g) |
|---|---|---|---|---|---|---|
| MIL-100(Fe) | 2594.1 | 1895.6 | 1473.3 | 0.29 | 0.90 | 0.58 |
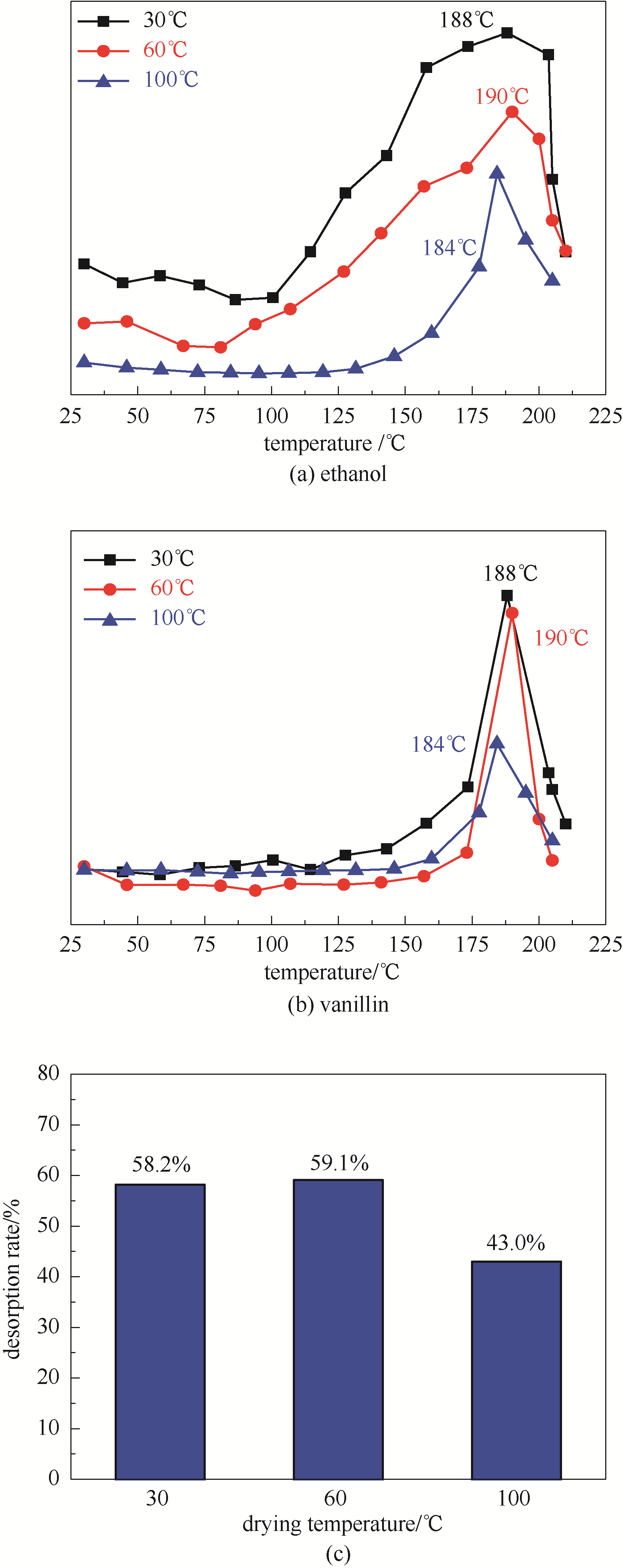
图3 不同干燥温度下共吸附于MIL-100(Fe)中的乙醇(a)和香兰素(b)的脱附曲线以及香兰素脱附率(c)
Fig.3 Desorption curves of ethanol (a) and vanillin (b) adsorbed in MIL-100(Fe) under different drying methods and vanillin desorption rate(c)
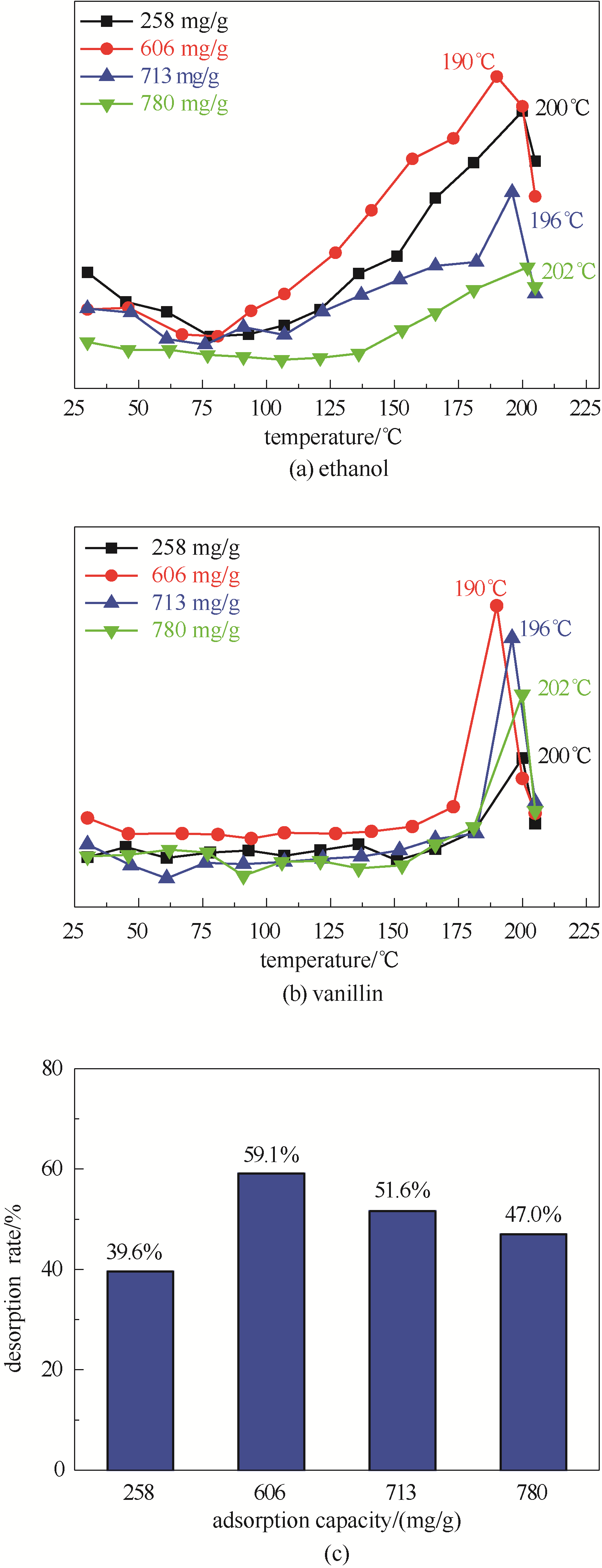
图4 不同吸附量下共吸附于MIL-100(Fe)中的乙醇(a)和香兰素(b)的脱附曲线以及香兰素脱附率(c)
Fig.4 Desorption curves of ethanol (a) and vanillin (b) adsorbed in MIL-100(Fe) under different adsorption capacity and vanillin desorption rate(c)

图5 MIL-100(Fe)的循环实验(a)及5次吸脱附前后MIL-100(Fe)的N2吸附脱附等温线和DFT孔径分布(b)
Fig.5 Cycle experiments of MIL-100(Fe)(a) and N2 adsorption and desorption isotherms and DFT pore size distribution of MIL-100(Fe) before and after used 5 times(b)
| Sample | SLang/(m2/g) | SBET/(m2/g) | SMic/(m2/g) | SMeso/SMic | Vt/(cm3/g) | VMic/(cm3/g) |
|---|---|---|---|---|---|---|
| original MIL-100(Fe) | 2594.1 | 1895.6 | 1473.3 | 0.29 | 0.90 | 0.58 |
| used 5 times MIL-100(Fe) | 1669.2 | 1323.6 | 995.2 | 0.33 | 0.53 | 0.30 |
表2 MIL-100(Fe)使用前后的孔隙结构参数
Table 2 Physical properties of MIL-100(Fe) before and after used 5times
| Sample | SLang/(m2/g) | SBET/(m2/g) | SMic/(m2/g) | SMeso/SMic | Vt/(cm3/g) | VMic/(cm3/g) |
|---|---|---|---|---|---|---|
| original MIL-100(Fe) | 2594.1 | 1895.6 | 1473.3 | 0.29 | 0.90 | 0.58 |
| used 5 times MIL-100(Fe) | 1669.2 | 1323.6 | 995.2 | 0.33 | 0.53 | 0.30 |
| 体系 | 吸附能/(kJ/mol) |
|---|---|
| 乙醇 | -64.35 |
| 香兰素 | -103.47 |
| 乙醇/香兰素 | -66.58 |
表3 香兰素、乙醇和香兰素/乙醇在MIL-100(Fe)上的吸附能数据
Table 3 Adsorption energy data of vanillin, ethanol and vanillin/ethanol on MIL-100(Fe)
| 体系 | 吸附能/(kJ/mol) |
|---|---|
| 乙醇 | -64.35 |
| 香兰素 | -103.47 |
| 乙醇/香兰素 | -66.58 |
| 1 | 周珠贤, 胡静, 江黎明, 等. 纳米香料技术的现状与发展[J]. 中国科学: 化学, 2019, 49(4): 575-580. |
| Zhou Z X, Hu J, Jiang L M, et al. Nanotechnology in fragrances: current status and future prospects[J]. Scientia Sinica (Chimica), 2019, 49(4): 575-580. | |
| 2 | Liu Y H, Wang Y X, Huang J X, et al. Encapsulation and controlled release of fragrances from functionalized porous metal-organic frameworks[J]. AIChE Journal, 2019, 65(2): 491-499. |
| 3 | 高海有, 刘秀明, 高莉, 等. 烟用香精香料研究现状与发展趋势[J]. 香料香精化妆品, 2019, (2): 70-73, 69. |
| Gao H Y, Liu X M, Gao L, et al. The current situation and trends of tobacco flavor research[J]. Flavour Fragrance Cosmetics, 2019, (2): 70-73, 69. | |
| 4 | 王璐, 毛海涛, 张磊, 等. 基于反向机器学习的调香设计方法[J]. 化工学报, 2019, 70(12): 4722-4729. |
| Wang L, Mao H T, Zhang L, et al. Inverse machine learning-based fragrance tuned design method[J]. CIESC Journal, 2019, 70(12): 4722-4729. | |
| 5 | 陈功, 武煜翔, 卢真保, 等. 苯乙醇和茴香醚在含Fe蚕沙基生物碳材料的储香与释放性能研究[J]. 化工学报, 2020, 71(12): 5628-5635. |
| Chen G, Wu Y X, Lu Z B, et al. Storage and release properties of pheneylethanol and anisole flavor on Fe-based silkworm excrement biocarbon[J]. CIESC Journal, 2020, 71(12): 5628-5635. | |
| 6 | 周枫, 李智宇, 李根, 等. 茴香醚在葡萄糖基多孔碳材料上缓释机理[J]. 化工学报, 2017, 68(12): 4625-4632. |
| Zhou F, Li Z Y, Li G, et al. Release-slowing mechanism of anisole on glucose-based porous carbon materials[J]. CIESC Journal, 2017, 68(12): 4625-4632. | |
| 7 | Liu Y X, Liu K Y, Zhao M N, et al. A pH-responsive fragrance release system based on pseudopeptide polymeric micelles[J]. Reactive and Functional Polymers, 2018, 132: 138-144. |
| 8 | Saffarionpour S, Tam S Y S, van der Wielen L A M, et al. Influence of ethanol and temperature on adsorption of flavor-active esters on hydrophobic resins[J]. Separation and Purification Technology, 2019, 210: 219-230. |
| 9 | 刘宇航, 周珠贤, 江黎明, 等. 氨基修饰的锆基金属有机框架材料对香料的吸附和缓控释[J]. 中国科学: 化学, 2019, 49(4): 607-612. |
| Liu Y H, Zhou Z X, Jiang L M, et al. A zirconium-based and amine-functionalized metal-organic framework material for adsorption and controlled release of fragrance[J]. Scientia Sinica (Chimica), 2019, 49(4): 607-612. | |
| 10 | 王颖, 张亮, 吕乔, 等. 活性炭对茉莉香精的吸附及缓释性能研究[J]. 轻工科技, 2015, 31(11): 41-42. |
| Wang Y, Zhang L, Lyu Q, et al. Study on the adsorption and slow-release properties of jasmine flavor by activated carbon [J]. Light Industry Science and Technology, 2015, 31(11): 41-42. | |
| 11 | 徐川辉, 邹长军. 环糊精改性羧甲基纤维素水凝胶的制备、表征和封装/释放性能研究[J]. 化学通报, 2018, 81(7): 630-635. |
| Xu C H, Zou C J. Study on preparation, characterization, and encapsulation/release propeties of β-CD modified carboxymethyl cellulose hydrogel[J]. Chemistry, 2018, 81(7): 630-635. | |
| 12 | Yuan S, Feng L, Wang K C, et al. Stable metal-organic frameworks: stable metal-organic frameworks: design, synthesis, and applications [J]. Advanced Materials, 2018, 30(37): 1870277. |
| 13 | Duan J G, Pan Y C, Liu G P, et al. Metal-organic framework adsorbents and membranes for separation applications[J]. Current Opinion in Chemical Engineering, 2018, 20: 122-131. |
| 14 | Kaneti Y V, Tang J, Salunkhe R R, et al. Nanoarchitectured design of porous materials and nanocomposites from metal-organic frameworks[J]. Advanced Materials, 2017, 29(12): 1604898. |
| 15 | Jiang D N, Chen M, Wang H, et al. The application of different typological and structural MOFs-based materials for the dyes adsorption[J]. Coordination Chemistry Reviews, 2019, 380: 471-483. |
| 16 | Shi Z N, Li L, Xiao Y X, et al. Synthesis of mixed-ligand Cu-MOFs and their adsorption of malachite green[J]. RSC Advances, 2017, 7(49): 30904-30910. |
| 17 | Li W, Li S. CO2 adsorption performance of functionalized metal-organic frameworks of varying topologies by molecular simulations[J]. Chemical Engineering Science, 2018, 189: 65-74. |
| 18 | Mao D S, Xie C J, Li Z Y, et al. Adsorption and controlled release of three kinds of flavors on UiO-66[J]. Food Science & Nutrition, 2020, 8(4): 1914-1922. |
| 19 | Wang H, Lashkari E, Lim H, et al. The moisture-triggered controlled release of a natural food preservative from a microporous metal-organic framework[J]. Chemical Communications, 2016, 52(10): 2129-2132. |
| 20 | Shen H, Huang M Q, Zhao M M, et al. Interactions of selected ketone flavours with porcine myofibrillar proteins: the role of molecular structure of flavour compounds[J]. Food Chemistry, 2019, 298: 125060. |
| 21 | 刘俊. UiO-66、TiO2/MIL-53(Al)光催化还原水体中Cr(Ⅵ)及UiO-66吸附缓释乙基香兰素的研究[D]. 昆明: 云南大学, 2018. |
| Liu J. Photocatalytic reduction of Cr(Ⅵ) by UiO-66 and TiO2/MIL-53(Al) and adsorption and control-release of ethyl vanillin by UiO-66[D]. Kunming: Yunnan University, 2018. | |
| 22 | Zhai C P, Sun F, Zhang P, et al. Interactions of dopamine and dopamine hydrochloride with ethanol[J]. Journal of Molecular Liquids, 2016, 223: 420-426. |
| 23 | Zhong Z, Li M, Fu J H, et al. Construction of Cu-bridged Cu2O/MIL(Fe/Cu) catalyst with enhanced interfacial contact for the synergistic photo-Fenton degradation of thiacloprid[J]. Chemical Engineering Journal, 2020, 395: 125184. |
| 24 | Fang Y, Yang Z G, Li H P, et al. MIL-100(Fe) and its derivatives: from synthesis to application for wastewater decontamination[J]. Environmental Science and Pollution Research, 2020, 27(5): 4703-4724. |
| 25 | Wu Y X, Huang Y, Huang H, et al. Porous Fe@C composites derived from silkworm excrement for effective separation of anisole compounds[J]. ACS Omega, 2019, 4(25): 21204-21213. |
| 26 | Wei Y N, Wang B F, Cui X F, et al. Highly advanced degradation of thiamethoxam by synergistic chemisorption-catalysis strategy using MIL(Fe)/Fe-SPC composites with ultrasonic irradiation[J]. ACS Applied Materials & Interfaces, 2018, 10(41): 35260-35272. |
| 27 | Zhang H B, Wen J, Fang Y, et al. Influence of fulvic acid on Pb(Ⅱ) removal from water using a post-synthetically modified MIL-100(Fe)[J]. Journal of Colloid and Interface Science, 2019, 551: 155-163. |
| 28 | Guo X Z, Han S S, Yang J M, et al. Effect of synergistic interplay between surface charge, crystalline defects, and pore volume of MIL-100(Fe) on adsorption of aqueous organic dyes[J]. Industrial & Engineering Chemistry Research, 2020, 59(5): 2113-2122. |
| 29 | Mahmoodi N M, Abdi J, Oveisi M, et al. Metal-organic framework (MIL-100 (Fe)): synthesis, detailed photocatalytic dye degradation ability in colored textile wastewater and recycling[J]. Materials Research Bulletin, 2018, 100: 357-366. |
| 30 | Yuan B Q, Wang X, Zhou X, et al. Novel room-temperature synthesis of MIL-100(Fe) and its excellent adsorption performances for separation of light hydrocarbons[J]. Chemical Engineering Journal, 2019, 355: 679-686. |
| 31 | Simon M A, Anggraeni E, Soetaredjo F E, et al. Hydrothermal synthesize of HF-free MIL-100(Fe) for isoniazid-drug delivery[J]. Scientific Reports, 2019, 9(1): 16907. |
| 32 | Ghoufi A, Artzner F, Malfreyt P. Physical properties and hydrogen-bonding network of water-ethanol mixtures from molecular dynamics simulations[J]. The Journal of Physical Chemistry B, 2016, 120(4): 793-802. |
| 33 | Sun H, Ren D N, Kong R Q, et al. Tuning 1-hexene/n-hexane adsorption on MOF-74 via constructing Co-Mg bimetallic frameworks[J]. Microporous and Mesoporous Materials, 2019, 284: 151-160. |
| 34 | Zhang H Y, Yang C, Geng Q, et al. Adsorption of hydrogen sulfide by amine-functionalized metal organic framework (MOF-199): an experimental and simulation study[J]. Applied Surface Science, 2019, 497: 143815. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [5] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [6] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [7] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [8] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [9] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [10] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [11] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [12] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [13] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [14] | 刘远超, 蒋旭浩, 邵钶, 徐一帆, 钟建斌, 李耑. 几何尺寸及缺陷对石墨炔纳米带热输运特性的影响[J]. 化工学报, 2023, 74(6): 2708-2716. |
| [15] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号