化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4786-4795.DOI: 10.11949/0438-1157.20201921
收稿日期:2020-12-28
修回日期:2021-06-14
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
张海涛
作者简介:王伟(1996—),男,硕士研究生,基金资助:
Wei WANG( ),Weixing QIAN,Hongfang MA,Weiyong YING,Haitao ZHANG(
),Weixing QIAN,Hongfang MA,Weiyong YING,Haitao ZHANG( )
)
Received:2020-12-28
Revised:2021-06-14
Online:2021-09-05
Published:2021-09-05
Contact:
Haitao ZHANG
摘要:
氢型丝光沸石(H-MOR)分子筛是二甲醚(DME)羰基化制乙酸甲酯(MA)的一种高效催化剂,经研究吡啶的修饰可以有效提高其稳定性及催化寿命。为了从原子尺度上研究吡啶对其改性的本质机理,基于Monte Carlo及分子动力学模拟,分别对H-AlMOR及Py-H-AlMOR周期性模型内羰基化主反应物CO、DME及产物MA的吸附-扩散行为进行了对比研究。结果表明,吡啶的引入会使H-MOR分子筛模型内主反应物CO、DME的吸附量产生一定下降(24%~33%),但有助于改善二者分子筛内的吸附平衡,并提升活性孔道8-MR内的反应物浓度。同时,吡啶引入后将对各分子扩散产生较大影响(21%~58%),尤其产物MA扩散性能下降约58%。此外,吡啶的引入也会使达到高反应活性所需的高进料比PCO/PDME有所降低。
中图分类号:
王伟, 钱伟鑫, 马宏方, 应卫勇, 张海涛. 吡啶修饰H-MOR上二甲醚羰基化吸附-扩散理论研究[J]. 化工学报, 2021, 72(9): 4786-4795.
Wei WANG, Weixing QIAN, Hongfang MA, Weiyong YING, Haitao ZHANG. A theoretical study on adsorption-diffusion of dimethyl ether carbonylation on pyridine-modified H-MOR[J]. CIESC Journal, 2021, 72(9): 4786-4795.
| 分子/模型 | Nads/(mol/mol) | Eads/(kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | |||||
| Py-H-AlMOR (2×1×1) | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | ||||
| Py-H-AlMOR (2×1×2) | 7.039 | 17.434 | 6.154 | -5.179 | -11.421 | -13.177 | ||||
表1 于493 K-2.5 MPa z轴延伸方向不同周期性模型中主要反应物及产物分子的平均吸附量(Nads)和吸附能(Eads) (以Al-T1O7为例)
Table 1 The average adsorption capacity (Nads) and adsorption energy (Eads) of the main reactants and product molecules in different periodic models in the 493 K-2.5 MPa z-axis extension direction (Al-T1O7 as an example)
| 分子/模型 | Nads/(mol/mol) | Eads/(kcal/mol) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | |||||
| Py-H-AlMOR (2×1×1) | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | ||||
| Py-H-AlMOR (2×1×2) | 7.039 | 17.434 | 6.154 | -5.179 | -11.421 | -13.177 | ||||
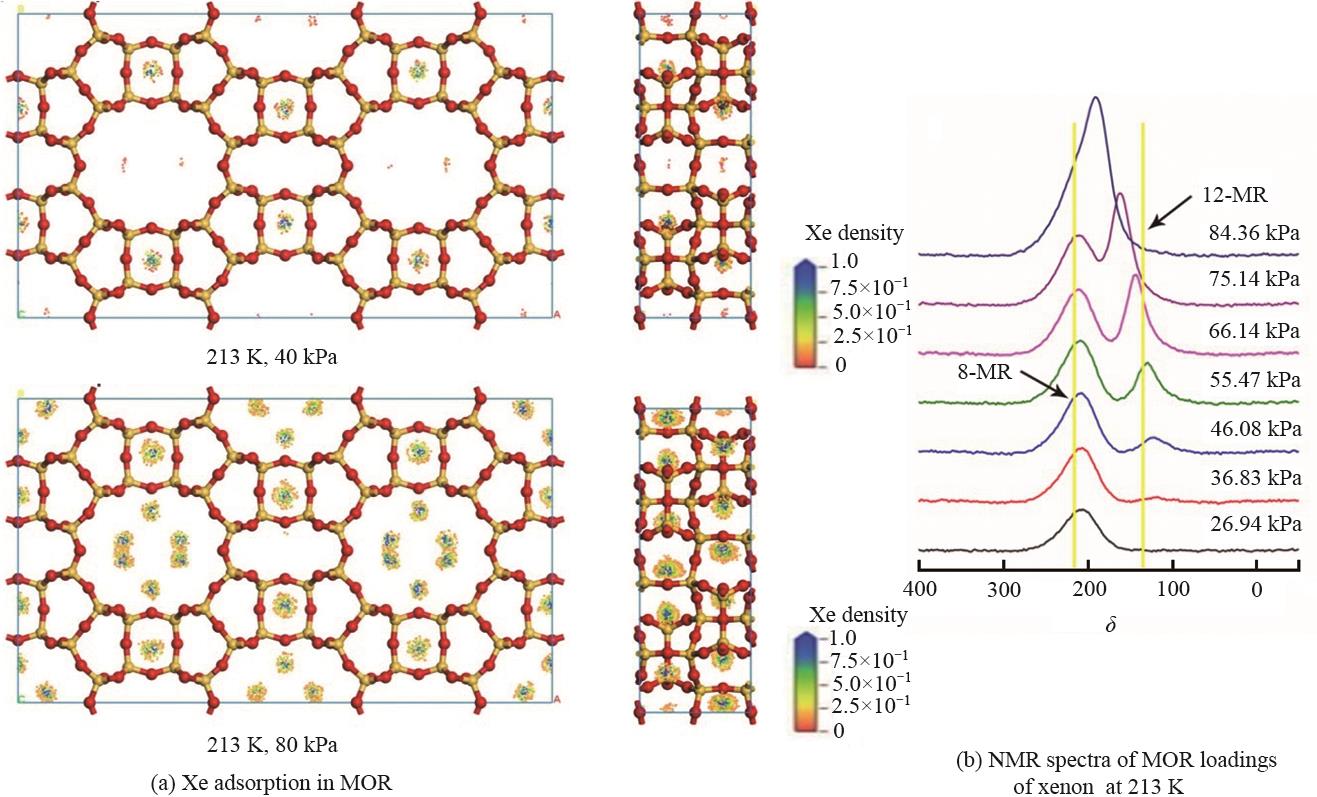
图3 129Xe于213 K在MOR(2×1×1)周期模型上的吸附密度分布 (a) 参照实验213 K下不同压力下129Xe的NMR光谱(b)
Fig.3 The adsorption density distribution of 129Xe at 213 K on the MOR (2×1×1) periodic model (a) NMR spectra of 129Xe under different pressures in reference experiment (b)
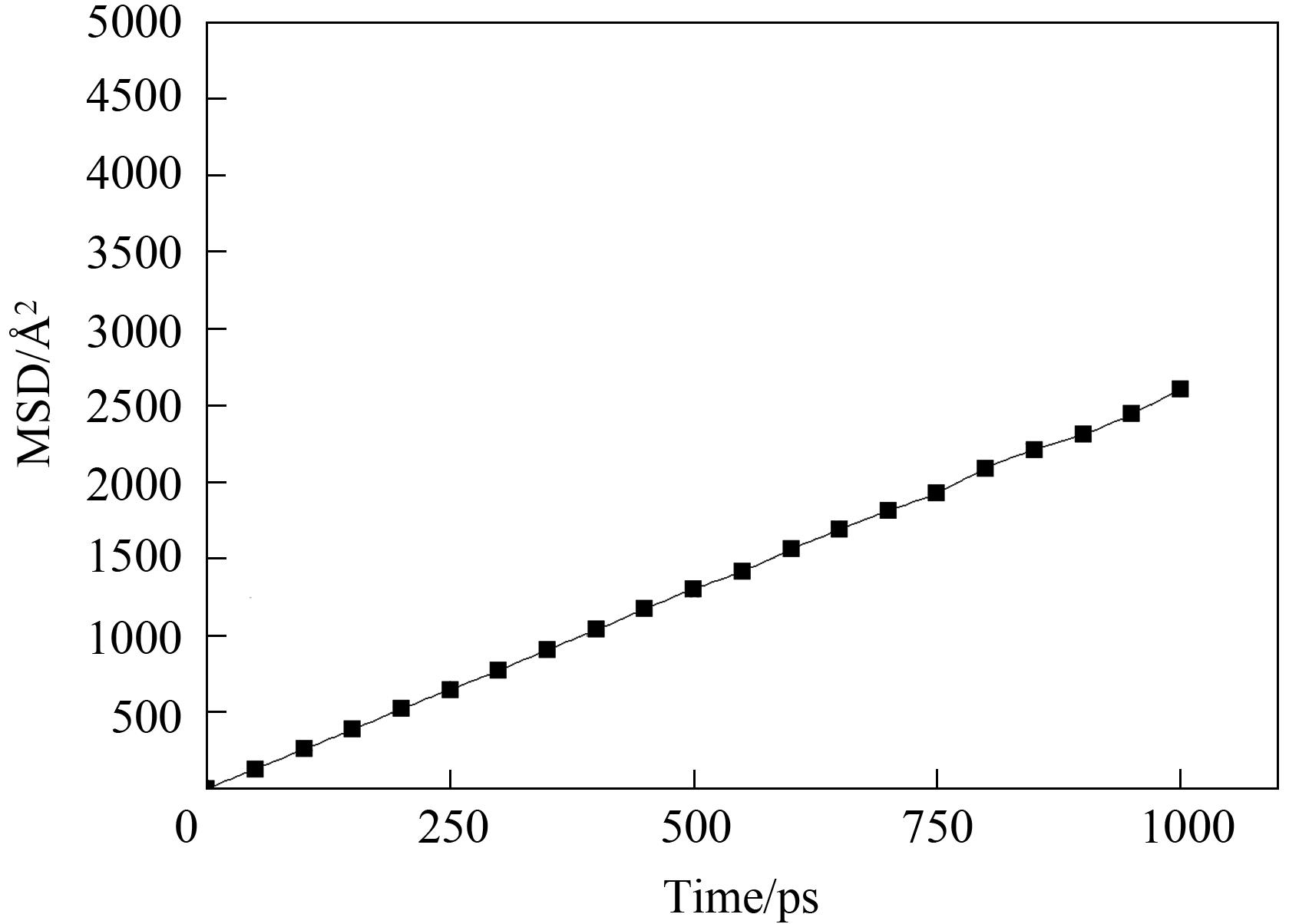
图4 MOR(2×1×1)周期模型内CH4分子于493 K下均方位移(MSD)与模拟时间关系
Fig.4 The relationship between the mean square displacement (MSD) of the CH4 molecule at 493 K and the simulation time in the MOR (2×1×1) period model
| 模型/分子 | Nads/(mol/mol) | Eads/(kcal/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | ||||||
| Al-T1O7 | 4.531 | 12.815 | 7.550 | -4.902 | -12.241 | -14.856 | |||||
| Py-Al-T1O7 | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | |||||
| Al-T2O2 | 4.459 | 13.519 | 8.150 | -4.904 | -12.298 | -15.772 | |||||
| Py-Al-T2O2 | 3.462 | 8.988 | 2.785 | -5.198 | -11.313 | -13.085 | |||||
| Al-T3O1 | 4.632 | 12.338 | 7.254 | -4.917 | -12.328 | -15.201 | |||||
| Py-Al-T3O1 | 3.432 | 8.695 | 3.107 | -5.196 | -11.263 | -13.142 | |||||
| Al-T4O2 | 4.489 | 13.444 | 7.949 | -4.893 | -12.143 | -14.516 | |||||
| Py-Al-T4O2 | 3.359 | 8.224 | 2.777 | -5.195 | -11.251 | -13.062 | |||||
表2 于493 K-2.5 MPa各模型中主要反应物及产物分子的平均吸附量(Nads)和吸附能(Eads)
Table 2 Average adsorption capacity (Nads) and adsorption energy (Eads) of main reactants and product molecules in different acid models at 493 K-2.5 MPa
| 模型/分子 | Nads/(mol/mol) | Eads/(kcal/mol) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CO | DME | MA | CO | DME | MA | ||||||
| Al-T1O7 | 4.531 | 12.815 | 7.550 | -4.902 | -12.241 | -14.856 | |||||
| Py-Al-T1O7 | 3.504 | 8.666 | 2.957 | -5.167 | -11.270 | -13.085 | |||||
| Al-T2O2 | 4.459 | 13.519 | 8.150 | -4.904 | -12.298 | -15.772 | |||||
| Py-Al-T2O2 | 3.462 | 8.988 | 2.785 | -5.198 | -11.313 | -13.085 | |||||
| Al-T3O1 | 4.632 | 12.338 | 7.254 | -4.917 | -12.328 | -15.201 | |||||
| Py-Al-T3O1 | 3.432 | 8.695 | 3.107 | -5.196 | -11.263 | -13.142 | |||||
| Al-T4O2 | 4.489 | 13.444 | 7.949 | -4.893 | -12.143 | -14.516 | |||||
| Py-Al-T4O2 | 3.359 | 8.224 | 2.777 | -5.195 | -11.251 | -13.062 | |||||
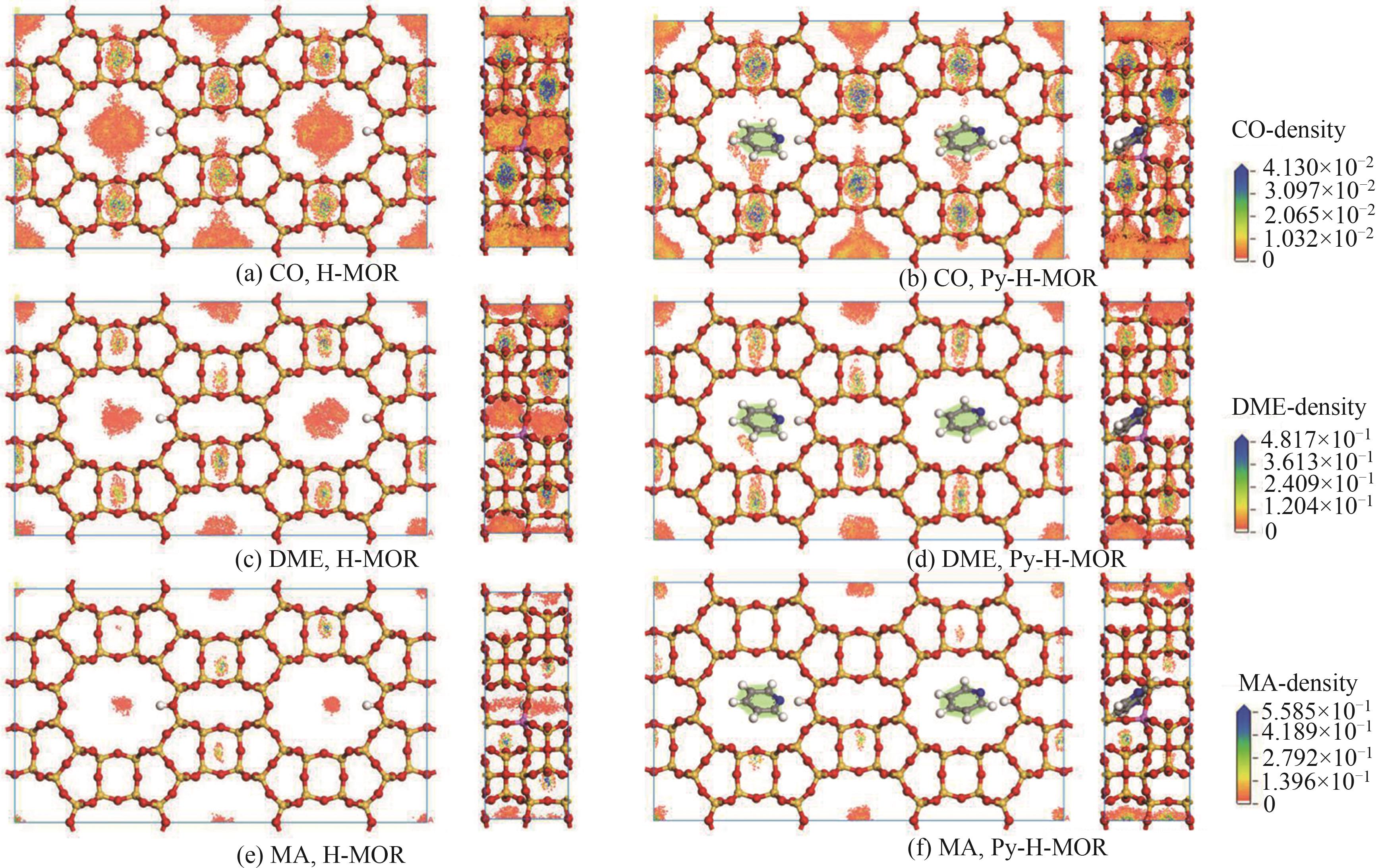
图5 493 K-2.5 MPa各模型中主要反应物及产物分子的吸附密度(以Al-T1O7为例)
Fig. 5 Adsorption density maps of different reactants and products molecule in different acid models at 493 K-2.5 MPa (Al-T1O7 as example)

图6 纯净气各分子于493 K下均方位移(MSD)与模拟时间关系图(5分子/模型)
Fig.6 The relationship between the mean square displacement (MSD) and simulation time of pure gases at 493 K (5 molecules/model)

图7 无吡啶修饰不同酸性位模型中于493 K-2.5 MPa不同进料比下混合物的平均吸附量Nads
Fig.7 Average adsorption capacity Nads of mixture under different feed ratios at 493 K-2.5 MPa in each acid model without pyridine modification

图8 含吡啶修饰不同酸性位模型中于493 K-2.5 MPa不同进料比下混合物的平均吸附量Nads
Fig.8 Average adsorption capacity Nads of mixture under different feed ratios at 493 K-2.5 MPa in each acid model with pyridine modification
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Al-T1O7 | Al-T2O2 | Al-T3O1 | Al-T4O2 | |
| 1∶1 | 0.045 | 0.058 | 0.048 | 0.055 |
| 2∶1 | 0.082 | 0.098 | 0.082 | 0.092 |
| 5∶1 | 0.227 | 0.222 | 0.302 | 0.280 |
| 10∶1 | 0.417 | 0.450 | 0.422 | 0.402 |
| 20∶1 | 0.794 | 0.847 | 0.800 | 0.758 |
| 27∶1(opt) | 0.990 | 0.971 | 1.020 | 1.042 |
| 50∶1 | 1.754 | 1.724 | 2.000 | 1.754 |
表3 H-AlMOR模型于493 K-2.5 MPa不同进料比下CO和DME的平均吸附量比
Table 3 Average adsorption ratio under different feed ratios at 493 K-2.5 MPa in H-AlMOR
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Al-T1O7 | Al-T2O2 | Al-T3O1 | Al-T4O2 | |
| 1∶1 | 0.045 | 0.058 | 0.048 | 0.055 |
| 2∶1 | 0.082 | 0.098 | 0.082 | 0.092 |
| 5∶1 | 0.227 | 0.222 | 0.302 | 0.280 |
| 10∶1 | 0.417 | 0.450 | 0.422 | 0.402 |
| 20∶1 | 0.794 | 0.847 | 0.800 | 0.758 |
| 27∶1(opt) | 0.990 | 0.971 | 1.020 | 1.042 |
| 50∶1 | 1.754 | 1.724 | 2.000 | 1.754 |
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Py-Al- T1O7 | Py-Al- T2O2 | Py-Al- T3O1 | Py-Al- T4O2 | |
| 1∶1 | 0.299 | 0.267 | 0.270 | 0.269 |
| 2∶1 | 0.427 | 0.391 | 0.402 | 0.385 |
| 5∶1 | 0.934 | 0.855 | 0.926 | 0.926 |
| 8∶1(opt) | 0.980 | 1.000 | 1.042 | 0.990 |
| 10∶1 | 1.053 | 1.053 | 1.111 | 1.075 |
| 20∶1 | 1.724 | 1.754 | 1.667 | 1.754 |
| 50∶1 | 3.571 | 3.448 | 3.226 | 3.226 |
表4 Py-H-AlMOR模型于493 K-2.5 MPa不同进料比下CO和DME的平均吸附量比
Table 4 Average adsorption ratio under different feed ratios at 493 K-2.5 MP in Py-H-AlMOR
| PCO/PDME | CO/DME吸附量比值 | |||
|---|---|---|---|---|
| Py-Al- T1O7 | Py-Al- T2O2 | Py-Al- T3O1 | Py-Al- T4O2 | |
| 1∶1 | 0.299 | 0.267 | 0.270 | 0.269 |
| 2∶1 | 0.427 | 0.391 | 0.402 | 0.385 |
| 5∶1 | 0.934 | 0.855 | 0.926 | 0.926 |
| 8∶1(opt) | 0.980 | 1.000 | 1.042 | 0.990 |
| 10∶1 | 1.053 | 1.053 | 1.111 | 1.075 |
| 20∶1 | 1.724 | 1.754 | 1.667 | 1.754 |
| 50∶1 | 3.571 | 3.448 | 3.226 | 3.226 |
| 1 | Boronat M, Martínez-Sánchez C, Law D, et al. Enzyme-like specificity in zeolites: a unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO[J]. Journal of the American Chemical Society, 2008, 130(48): 16316-16323. |
| 2 | Rasmussen D B, Christensen J M, Temel B, et al. Ketene as a reaction intermediate in the carbonylation of dimethyl ether to methyl acetate over mordenite[J]. Angewandte Chemie International Edition, 2015, 54(25): 7261-7264. |
| 3 | Li X J, Liu X H, Liu S L, et al. Activity enhancement of ZSM-35 in dimethyl ether carbonylation reaction through alkaline modifications[J]. RSC Advances, 2013, 3(37): 16549. |
| 4 | Zhou H, Zhu W L, Shi L, et al. Promotion effect of Fe in mordenite zeolite on carbonylation of dimethyl ether to methyl acetate[J]. Catalysis Science & Technology, 2015, 5(3): 1961-1968. |
| 5 | 宋庆锋, 张勇, 曾清湖. 合成气直接转化制乙醇工艺路线的技术经济分析[J]. 工业催化, 2013, 21(6): 17-21. |
| Song Q F, Zhang Y, Zeng Q H. Techno-economic analysis of production process of syngas to ethanol[J]. Industrial Catalysis, 2013, 21(6): 17-21. | |
| 6 | 王辉, 吴志连, 邰志军, 等. 合成气经二甲醚羰基化及乙酸甲酯加氢制无水乙醇的研究进展[J]. 化工进展, 2019, 38(10): 4497-4503. |
| Wang H, Wu Z L, Tai Z J, et al. Advances in synthesis of anhydrous ethanol from syngas via carbonylation of dimethyl ether and hydrogenation of methyl acetate[J]. Chemical Industry and Engineering Progress, 2019, 38(10): 4497-4503. | |
| 7 | Bhan A, Iglesia E. A link between reactivity and local structure in acid catalysis on zeolites[J]. Accounts of Chemical Research, 2008, 41(4): 559-567. |
| 8 | Cheung P, Bhan A, Sunley G J, et al. Site requirements and elementary steps in dimethyl ether carbonylation catalyzed by acidic zeolites[J]. Journal of Catalysis, 2007, 245(1): 110-123. |
| 9 | Cheung P, Bhan A, Sunley G J, et al. Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites[J]. Angewandte Chemie International Edition, 2006, 45(10): 1617-1620. |
| 10 | Bhan A, Allian A D, Sunley G J, et al. Specificity of sites within eight-membered ring zeolite channels for carbonylation of methyls to acetyls[J]. Journal of the American Chemical Society, 2007, 129(16): 4919-4924. |
| 11 | Feng P, Zhang G Q, Zang K L, et al. A theoretical study on the selective adsorption behavior of dimethyl ether and carbon monoxide on H-FER zeolites[J]. Chemical Physics Letters, 2017, 684: 279-284. |
| 12 | Li B J, Xu J, Han B, et al. Insight into dimethyl ether carbonylation reaction over mordenite zeolite from in situ solid-state NMR spectroscopy[J]. The Journal of Physical Chemistry C, 2013, 117(11): 5840-5847. |
| 13 | Boronat M, Martínez C, Corma A. Mechanistic differences between methanol and dimethyl ether carbonylation in side pockets and large channels of mordenite[J]. Physical Chemistry Chemical Physics, 2011, 13(7): 2603-2612. |
| 14 | Rasmussen D B, Christensen J M, Temel B, et al. Reaction mechanism of dimethyl ether carbonylation to methyl acetate over mordenite-a combined DFT/experimental study[J]. Catalysis Science & Technology, 2017, 7(5): 1141-1152. |
| 15 | 赵娜, 牛君阳, 刘亚华, 等. 预处理条件及金属离子改性对H-MOR分子筛的DME羰基化性能影响[J]. 化工学报, 2015, 66(9): 3504-3510. |
| Zhao N, Niu J Y, Liu Y H, et al. Influence of pretreatment and metal cation modification of H-MOR zeolite on performance of DME carbonylation[J]. CIESC Journal, 2015, 66(9): 3504-3510. | |
| 16 | Wang M X, Huang S Y, Lyu J, et al. Modifying the acidity of H-MOR and its catalytic carbonylation of dimethyl ether[J]. Chinese Journal of Catalysis, 2016, 37(9): 1530-1537. |
| 17 | Liu J L, Xue H F, Huang X M, et al. Stability enhancement of H-mordenite in dimethyl ether carbonylation to methyl acetate by pre-adsorption of pyridine[J]. Chinese Journal of Catalysis, 2010, 31(7): 729-738. |
| 18 | 袁淑萍, 段云波, 王建国, 等. 吡啶在H-MOR分子筛孔道中吸附的量子化学研究[J]. 催化学报, 2006, 27(8): 664-670. |
| Yuan S P, Duan Y B, Wang J G, et al. Study on pyridine adsorption in H-MOR by quantum chemistry[J]. Chinese Journal of Catalysis, 2006, 27(8): 664-670. | |
| 19 | Sano T, Wakabayashi S, Oumi Y, et al. Synthesis of large mordenite crystals in the presence of aliphatic alcohol[J]. Microporous and Mesoporous Materials, 2001, 46(1): 67-74. |
| 20 | Millini R, Frigerio F, Bellussi G, et al. A priori selection of shape-selective zeolite catalysts for the synthesis of 2, 6-dimethylnaphthalene[J]. Journal of Catalysis, 2003, 217(2): 298-309. |
| 21 | Cox S D, Gier T E, Stucky G D, et al. Inclusion tuning of nonlinear optical materials: switching the SHG of p-nitroaniline and 2-methyl-p-nitroaniline with molecular sieve hosts[J]. Journal of the American Chemical Society, 1988, 110(9): 2986-2987. |
| 22 | Chibani S, Chebbi M, Lebègue S, et al. A DFT investigation of the adsorption of iodine compounds and water in H-, Na-, Ag-, and Cu-mordenite[J]. The Journal of Chemical Physics, 2016, 144(24): 244705. |
| 23 | Liu Z Q, Yi X F, Wang G R, et al. Roles of 8-ring and 12-ring channels in mordenite for carbonylation reaction: from the perspective of molecular adsorption and diffusion[J]. Journal of Catalysis, 2019, 369: 335-344. |
| 24 | Baerlocher C, McCusker L B, Liu Z. Database of Zeolite Structures[EB/OL]. [2019-12-10]. . |
| 25 | Meunier M. Introduction to Materials Studio[J]. EPJ Web of Conferences, 2012, 30: 04001. |
| 26 | Akten E D, Siriwardane R, Sholl D S. Monte Carlo simulation of single-and binary-component adsorption of CO2, N2, and H2 in zeolite Na-4A[J]. Energy & Fuels, 2003, 17(4): 977-983. |
| 27 | Dunne L J, Manos G, Du Z M. Exact statistical mechanical one-dimensional lattice model of alkane binary mixture adsorption in zeolites and comparision with Monte-Carlo simulations[J]. Chemical Physics Letters, 2003, 377(5/6): 551-556. |
| 28 | 殷开梁, 邹定辉, 杨波, 等. Materials Studio软件涉及力场中氢键的研究[J]. 计算机与应用化学, 2006, 23(12): 1335-1340. |
| Yin K L, Zou D H, Yang B, et al. Investigation of H-bonding for the related force fields in materials studio software[J]. Computers and Applied Chemistry, 2006, 23(12): 1335-1340. | |
| 29 | Wu C F, Xu W J. Atomistic simulation study of absorbed water influence on structure and properties of crosslinked epoxy resin[J]. Polymer, 2007, 48(18): 5440-5448. |
| 30 | Sun H. COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds[J]. The Journal of Physical Chemistry B, 1998, 102(38): 7338-7364. |
| 31 | Wang C M, Li B W, Wang Y D, et al. Insight into the topology effect on the diffusion of ethene and propene in zeolites: a molecular dynamics simulation study[J]. Journal of Energy Chemistry, 2013, 22(6): 914-918. |
| 32 | Wu J Y, Liu Q L, Xiong Y, et al. Molecular simulation of water/alcohol mixtures' adsorption and diffusion in zeolite 4A membranes[J]. The Journal of Physical Chemistry B, 2009, 113(13): 4267-4274. |
| 33 | Zhou J, Zhou T R. Material structure simulation techniques and applications[J]. Materials Science Forum, 2007, 561/562/563/564/565: 1793-1796. |
| 34 | Frenkel D, Smit B. Monte Carlo simulations in various ensembles[M]//Understanding Molecular Simulation. Amsterdam: Elsevier, 2002: 111-137. |
| 35 | He T, Liu X C, Xu S T, et al. Role of 12-ring channels of mordenite in DME carbonylation investigated by solid-state NMR[J]. The Journal of Physical Chemistry C, 2016, 120(39): 22526-22531. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [9] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [10] | 韩奎奎, 谭湘龙, 李金芝, 杨婷, 张春, 张永汾, 刘洪全, 于中伟, 顾学红. 四通道中空纤维MFI分子筛膜用于二甲苯异构体分离[J]. 化工学报, 2023, 74(6): 2468-2476. |
| [11] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [12] | 周继鹏, 何文军, 李涛. 异形催化剂上乙烯催化氧化失活动力学反应工程计算[J]. 化工学报, 2023, 74(6): 2416-2426. |
| [13] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [14] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| [15] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号