化工学报 ›› 2021, Vol. 72 ›› Issue (S1): 421-429.DOI: 10.11949/0438-1157.20201315
收稿日期:2020-09-17
修回日期:2020-10-10
出版日期:2021-06-20
发布日期:2021-06-20
通讯作者:
黄仁亮
作者简介:吴中杰(1987—),男,博士研究生,基金资助:
WU Zhongjie1( ),LIU Zeyan2,XIE Lianke1,CUI Mei2,HUANG Renliang2(
),LIU Zeyan2,XIE Lianke1,CUI Mei2,HUANG Renliang2( )
)
Received:2020-09-17
Revised:2020-10-10
Online:2021-06-20
Published:2021-06-20
Contact:
HUANG Renliang
摘要:
聚偏氟乙烯(PVDF)膜因其优异的化学和力学稳定性而被广泛应用于水处理领域,但PVDF膜本身的疏水性,容易使其在处理含油废水的过程中被油滴污染,造成膜孔堵塞。以PVDF微滤膜为基底,通过单宁酸(TA)和聚乙烯亚胺(PEI)共沉积形成了TA/PEI黏附层,经戊二醛共价交联和接枝半胱氨酸(Cys),制备了一种PVDF改性膜(PVDF@TA/PEI-Cys)。改性后的PVDF膜具有良好的亲水性和水下超疏油性,水接触角和水下油接触角分别为22.2°和150.2°。在0.09 MPa下,PVDF@TA/PEI-Cys膜的纯水通量达6328 L/(m2·h),水包油型乳液分离效率高达99.9%。此外,该改性膜还可同时吸附水中的汞离子,最大吸附量为24.7 mg/g。
中图分类号:
吴中杰, 刘则艳, 谢连科, 崔美, 黄仁亮. 聚偏氟乙烯膜亲水改性及其乳液分离与重金属吸附应用[J]. 化工学报, 2021, 72(S1): 421-429.
WU Zhongjie, LIU Zeyan, XIE Lianke, CUI Mei, HUANG Renliang. Preparation of hydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation and heavy metal ions adsorption[J]. CIESC Journal, 2021, 72(S1): 421-429.
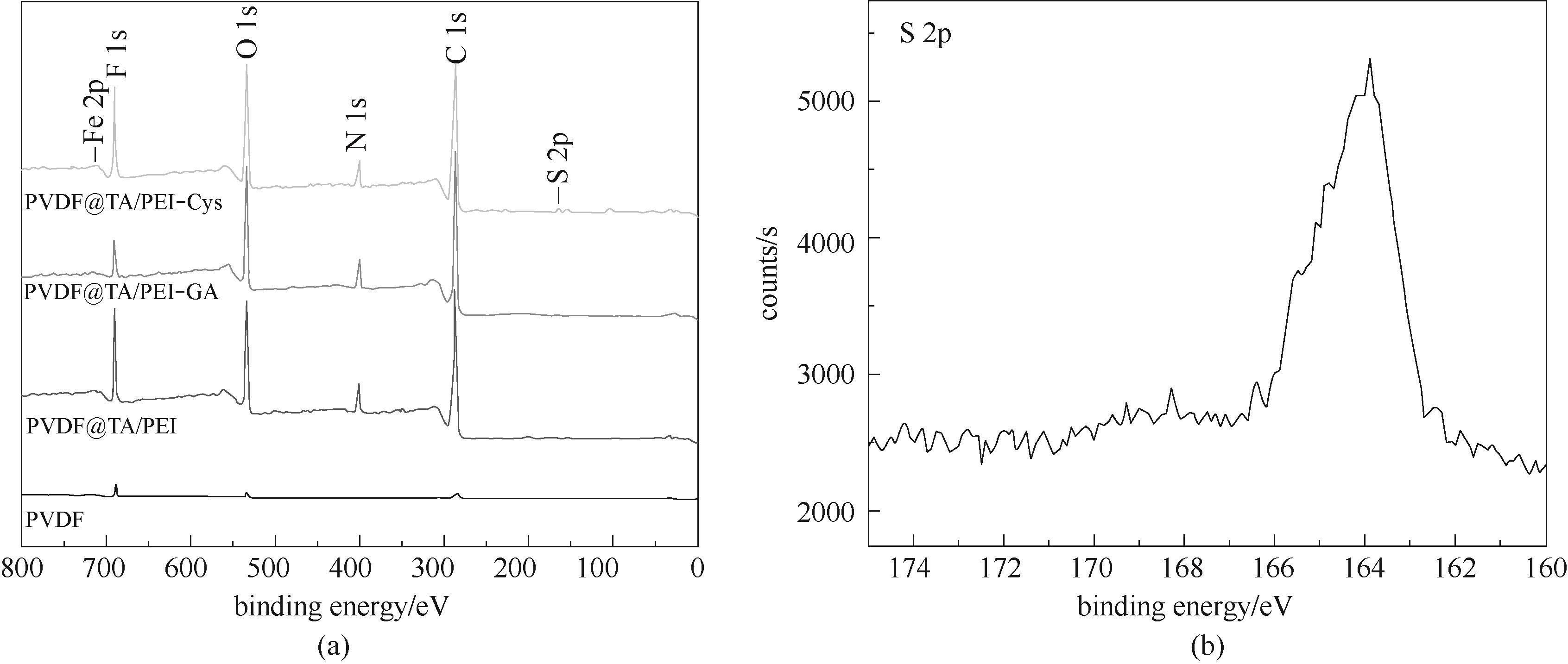
图2 不同改性PVDF膜的XPS谱图(a)和PVDF@TA/PEI-Cys膜S 2p的XPS谱图(b)
Fig.2 XPS spectra of different PVDF membranes (a) and high-resolution S 2p spectrum of PVDF@TA/PEI-Cys membrane (b)

图6 不同水包油乳液(SDS稳定)的通量和油截留率(a);不同表面活性剂稳定的水包柴油乳液的通量和油截留率(b);柴油/SDS/水乳液分离前后的照片和光学显微镜图像(c)
Fig.6 Emulsion flux and oil rejection ratio of SDS stabilized oil-in-water emulsions (a); Different surfactant stabilized diesel oil-in-water emulsions (b); Photographs and optical microscope images of SDS stabilized diesel oil-in-water emulsion and filtrate (c)
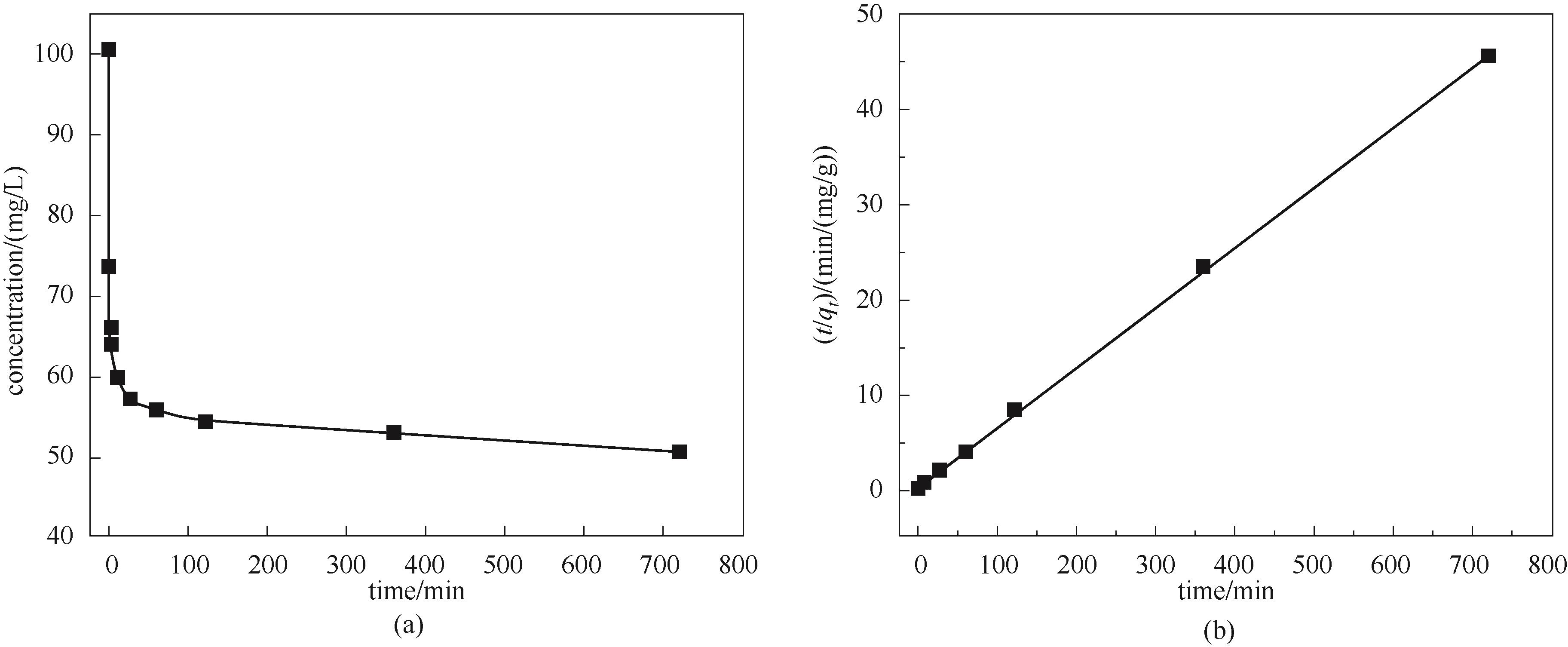
图9 Hg2+浓度随吸附时间的变化(a); Hg2+浓度随吸附时间变化的拟二级动力学拟合曲线(b)
Fig.9 Changes in Hg2+ concentrations with adsorption time (a); Pseudo-second-order kinetic fitting curve of Hg2+ concentrations with adsorption time (b)
| Ion | qe(exp)/(mg/g) | Pseudo-second-order kinetic | ||
|---|---|---|---|---|
| qe/(mg/g) | K2/(g/(mg·min)) | R2 | ||
| Hg2+ | 15.9 | 15.82 | 0.004 | 0.9994 |
表1 Hg2+吸附的拟二级动力学模型参数
Table 1 Parameters of pseudo-second-order kinetic model for Hg2+ adsorption
| Ion | qe(exp)/(mg/g) | Pseudo-second-order kinetic | ||
|---|---|---|---|---|
| qe/(mg/g) | K2/(g/(mg·min)) | R2 | ||
| Hg2+ | 15.9 | 15.82 | 0.004 | 0.9994 |

图10 不同初始Hg2+浓度下膜的吸附量(a);Langmuir等温吸附模型拟合曲线(b)
Fig.10 Adsorption capacity of PVDF@TA/PEI-Cys membrane at different initial concentrations of Hg2+(a); Fitting curve of Langmuir isotherm adsorption model (b)
| Ion | qe(exp)/(mg/g) | Langmuir model | ||
|---|---|---|---|---|
| qm/(mg/g) | KL/(L/mg) | R2 | ||
| Hg2+ | 24.7 | 25.96 | 120.9 | 0.9913 |
表2 Hg2+吸附的Langmuir模型参数
Table 2 Parameters of Langmuir model for Hg2+ adsorption
| Ion | qe(exp)/(mg/g) | Langmuir model | ||
|---|---|---|---|---|
| qm/(mg/g) | KL/(L/mg) | R2 | ||
| Hg2+ | 24.7 | 25.96 | 120.9 | 0.9913 |
| Membrane | Ion | Capacity/(mg/g) | Ref. |
|---|---|---|---|
| PVDF@TA/PEI-Cys | Hg2+ | 24.7 | this work |
| Zr(Ⅳ)-PVDF | As5+ | 21.5 | [ |
| PES/FMBO | As3+ | 73.5 | [ |
| PVDF-PAA-MEA | Hg2+ | 55.0 | [ |
| PC/HMO | Cu2+ | 29.6 | [ |
| PSf/GO | Cu2+ | 68.3 | [ |
| PSf/HFO | Pb2+ | 13.2 | [ |
| PSf/NFO | Cd2+ | 23.8 | [ |
| PVA-PVDF | Pb2+ | 121.2 | [ |
表3 不同膜材料对重金属离子的吸附量比较
Table 3 Comparison of maximum adsorption capacity of different membrane materials for heavy metal ions
| Membrane | Ion | Capacity/(mg/g) | Ref. |
|---|---|---|---|
| PVDF@TA/PEI-Cys | Hg2+ | 24.7 | this work |
| Zr(Ⅳ)-PVDF | As5+ | 21.5 | [ |
| PES/FMBO | As3+ | 73.5 | [ |
| PVDF-PAA-MEA | Hg2+ | 55.0 | [ |
| PC/HMO | Cu2+ | 29.6 | [ |
| PSf/GO | Cu2+ | 68.3 | [ |
| PSf/HFO | Pb2+ | 13.2 | [ |
| PSf/NFO | Cd2+ | 23.8 | [ |
| PVA-PVDF | Pb2+ | 121.2 | [ |
| 1 | Shi H, He Y, Pan Y, et al. A modified mussel-inspired method to fabricate TiO2 decorated superhydrophilic PVDF membrane for oil/water separation [J]. Journal of Membrane Science, 2016, 506: 60-70. |
| 2 | Zhang N, Qi Y F, Zhang Y N, et al. A review on oil/water mixture separation material [J]. Industrial & Engineering Chemistry Research, 2020, 59(33): 14546-14568. |
| 3 | Lu D W, Zhang T, Gutierrez L, et al. Influence of surface properties of filtration-layer metal oxide on ceramic membrane fouling during ultrafiltration of oil/water emulsion [J]. Environmental Science & Technology, 2016, 50(9): 4668-4674. |
| 4 | Zhu Y Z, Zhang F, Wang D, et al. A novel zwitterionic polyelectrolyte grafted PVDF membrane for thoroughly separating oil from water with ultrahigh efficiency [J]. Journal of Materials Chemistry A, 2013, 1(18): 5758. |
| 5 | Zhang G F, Gao F, Zhang Q H, et al. Enhanced oil-fouling resistance of poly(ether sulfone) membranes by incorporation of novel amphiphilic zwitterionic copolymers [J]. RSC Advances, 2016, 6(9): 7532-7543. |
| 6 | Luo C D, Liu Q X. Oxidant-induced high-efficient mussel-inspired modification on PVDF membrane with superhydrophilicity and underwater superoleophobicity characteristics for oil/water separation [J]. ACS Applied Materials & Interfaces, 2017, 9(9): 8297-8307. |
| 7 | Wu W M, Huang R L, Qi W, et al. Bioinspired peptide-coated superhydrophilic poly(vinylidene fluoride) membrane for oil/water emulsion separation [J]. Langmuir, 2018, 34(22): 6621-6627. |
| 8 | 尚茜子, 张宝泉, 李雲. 不锈钢网负载Al-β分子筛涂层的制备及其在油水分离中的应用[J]. 化工学报, 2019, 70(10): 3994-4001. |
| Shang X Z, Zhang B Q, Li Y. Fabrication of stainless steel mesh supported zeolite Al-β coatings for oil/water separation [J]. CIESC Journal, 2019, 70(10): 3994-4001. | |
| 9 | Ma W J, Li Y S, Gao S T, et al. Self-healing and superwettable nanofibrous membranes with excellent stability toward multifunctional applications in water purification [J]. ACS Applied Materials & Interfaces, 2020, 12(20): 23644-23654. |
| 10 | Wang W, Han N, Yang C, et al. Fabrication of P(AN-MA)/rGO-g-PAO superhydrophilic nanofiber membrane for removal of heavy metal ions [J]. Journal of Nanoscience and Nanotechnology, 2020, 20(3): 1685-1696. |
| 11 | Shi M B, Lin D W, Huang R L, et al. Construction of a mercapto-functionalized Zr-MOF/melamine sponge composite for the efficient removal of oils and heavy metal ions from water [J]. Industrial & Engineering Chemistry Research, 2020, 59(29): 13220-13227. |
| 12 | Chen H, Wu H, Wang Q W, et al. Separation performance of Hg2+ in desulfurization wastewater by the graphene oxide polyethersulfone membrane [J]. Energy & Fuels, 2019, 33(9): 9241-9248. |
| 13 | Song Y, Li Z L, Zhang J B, et al. A low-cost biomimetic heterostructured multilayer membrane with geopolymer microparticles for broad-spectrum water purification [J]. ACS Applied Materials & Interfaces, 2020, 12(10): 12133-12142. |
| 14 | Chen X, He Y, Fan Y, et al. Nature-inspired polyphenol chemistry to fabricate halloysite nanotubes decorated PVDF membrane for the removal of wastewater [J]. Separation and Purification Technology, 2019, 212: 326-336. |
| 15 | Xu S J, Wang Z Y, Li S X, et al. Fabrication of polyimide-based hollow fiber membrane by synergetic covalent-crosslinking strategy for organic solvent nanofiltration (OSN) application [J]. Separation and Purification Technology, 2020, 241: 116751. |
| 16 | Urban N R, Ernst K, Bernasconi S. Addition of sulfur to organic matter during early diagenesis of lake sediments [J]. Geochimica et Cosmochimica Acta, 1999, 63(6): 837-853. |
| 17 | Zheng Y M, Zou S W, Nanayakkara K G N, et al. Adsorptive removal of arsenic from aqueous solution by a PVDF/zirconia blend flat sheet membrane [J]. Journal of Membrane Science, 2011, 374(1/2): 1-11. |
| 18 | Gohari R J, Lau W J, Matsuura T, et al. Fabrication and characterization of novel PES/Fe-Mn binary oxide UF mixed matrix membrane for adsorptive removal of As(Ⅲ) from contaminated water solution [J]. Separation and Purification Technology, 2013, 118: 64-72. |
| 19 | Hernández S, Islam M S, Thompson S, et al. Thiol-functionalized membranes for mercury capture from water [J]. Industrial & Engineering Chemistry Research, 2020, 59(12): 5287-5295. |
| 20 | Delavar M, Bakeri G, Hosseini M. Fabrication of polycarbonate mixed matrix membranes containing hydrous manganese oxide and alumina nanoparticles for heavy metal decontamination: characterization and comparative study [J]. Chemical Engineering Research and Design, 2017, 120: 240-253. |
| 21 | Mukherjee R, Bhunia P, De S. Impact of graphene oxide on removal of heavy metals using mixed matrix membrane [J]. Chemical Engineering Journal, 2016, 292: 284-297. |
| 22 | Abdullah N, Gohari R J, Yusof N, et al. Polysulfone/hydrous ferric oxide ultrafiltration mixed matrix membrane: preparation, characterization and its adsorptive removal of lead (Ⅱ) from aqueous solution [J]. Chemical Engineering Journal, 2016, 289: 28-37. |
| 23 | Mondal M, Dutta M, De S. A novel ultrafiltration grade nickel iron oxide doped hollow fiber mixed matrix membrane: spinning, characterization and application in heavy metal removal [J]. Separation and Purification Technology, 2017, 188: 155-166. |
| 24 | Zhao D D, Yu Y, Chen J P. Treatment of lead contaminated water by a PVDF membrane that is modified by zirconium, phosphate and PVA [J]. Water Research, 2016, 101: 564-573. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [4] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 张澳, 罗英武. 低模量、高弹性、高剥离强度丙烯酸酯压敏胶[J]. 化工学报, 2023, 74(7): 3079-3092. |
| [7] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [8] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [9] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [10] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [11] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [12] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [13] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [14] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [15] | 孙永尧, 高秋英, 曾文广, 王佳铭, 陈艺飞, 周永哲, 贺高红, 阮雪华. 面向含氮油田伴生气提质利用的膜耦合分离工艺设计优化[J]. 化工学报, 2023, 74(5): 2034-2045. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号