化工学报 ›› 2022, Vol. 73 ›› Issue (2): 730-738.DOI: 10.11949/0438-1157.20210683
收稿日期:2021-05-19
修回日期:2021-10-26
出版日期:2022-02-05
发布日期:2022-02-18
通讯作者:
周欣
作者简介:王洒(1996—),女,硕士研究生,基金资助:
Sa WANG( ),Yijing WEN,Danyu GUO,Xin ZHOU(
),Yijing WEN,Danyu GUO,Xin ZHOU( ),Zhong LI
),Zhong LI
Received:2021-05-19
Revised:2021-10-26
Online:2022-02-05
Published:2022-02-18
Contact:
Xin ZHOU
摘要:
从天然气中回收C2/C3轻烃组分具有重要的工业价值,吸附分离技术可在常温常压下实现轻烃的回收。对MOF材料进行次级结构单元(SBU)调控,可在继承其晶体结构和发达孔道的同时,优化孔道化学微环境并引入新的吸附位点。使用三嗪(TZ)取代Zr-TBAPy(NU-1000)SBU中的配位水分子,在其孔道内构筑对轻烃吸附质具有更强限域作用的碱性表面化学微环境,得到了高选择性的新型TZ@Zr-TBAPy吸附剂。TZ的引入在分子尺度上提高了孔道的表面粗糙度,同时强化对轻烃吸附质的限域作用,提高材料对烷烃的吸附容量和选择性。常温常压下,TZ@Zr-TBAPy对丙烷和乙烷的吸附容量分别为10.08和4.19 mmol?g-1,比Zr-TBAPy提高了27%和9%,是目前国际上已报道的丙烷吸附容量最高的吸附剂之一。此外,丙烷/甲烷的IAST选择性为1518,是原材料的6.27倍;乙烷/甲烷的IAST选择性为11.7,比原材料提高了22%。更为重要的是,以TZ@Zr-TBAPy吸附剂为核心的固定床吸附过程可实现在常温常压天然气中乙烷和丙烷的一步分离回收。
中图分类号:
王洒, 温怡静, 郭丹煜, 周欣, 李忠. 锆基MOF次级结构单元调控及轻烃吸附分离性能增强[J]. 化工学报, 2022, 73(2): 730-738.
Sa WANG, Yijing WEN, Danyu GUO, Xin ZHOU, Zhong LI. Tuning secondary building unit of zirconium-based MOF for enhanced separation of light hydrocarbons[J]. CIESC Journal, 2022, 73(2): 730-738.
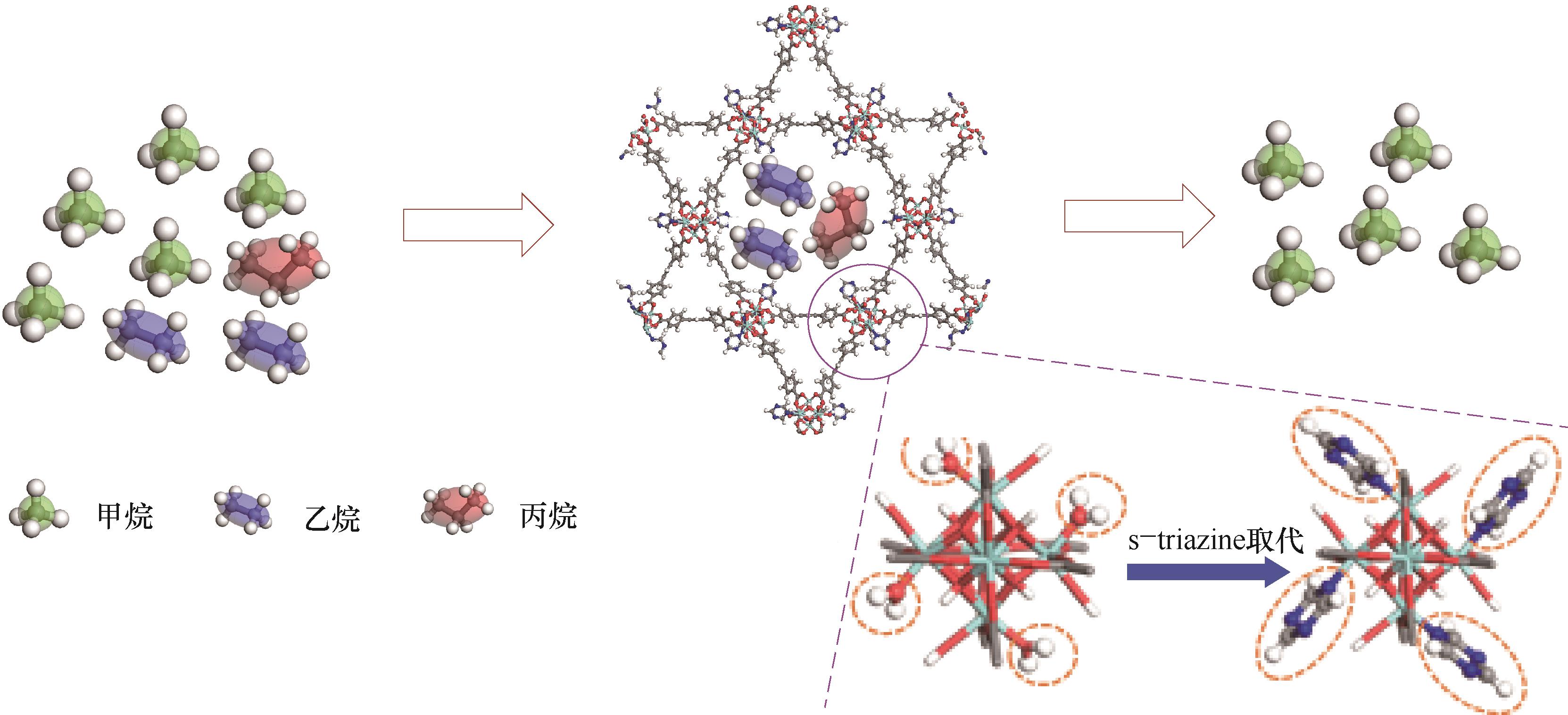
图1 TZ@Zr-TBAPy从天然气中分离乙烷和丙烷的示意图(放大图:TZ取代Zr-TBAPy的SBU上水分子形成TZ@Zr-TBAPy)
Fig.1 Structure of TZ@Zr-TBAPy for the separation of ethane and propane from natural gas(Enlarged: water molecules on the SBU of Zr-TBAPy were replaced by TZ to yield TZ@Zr-TBAPy)
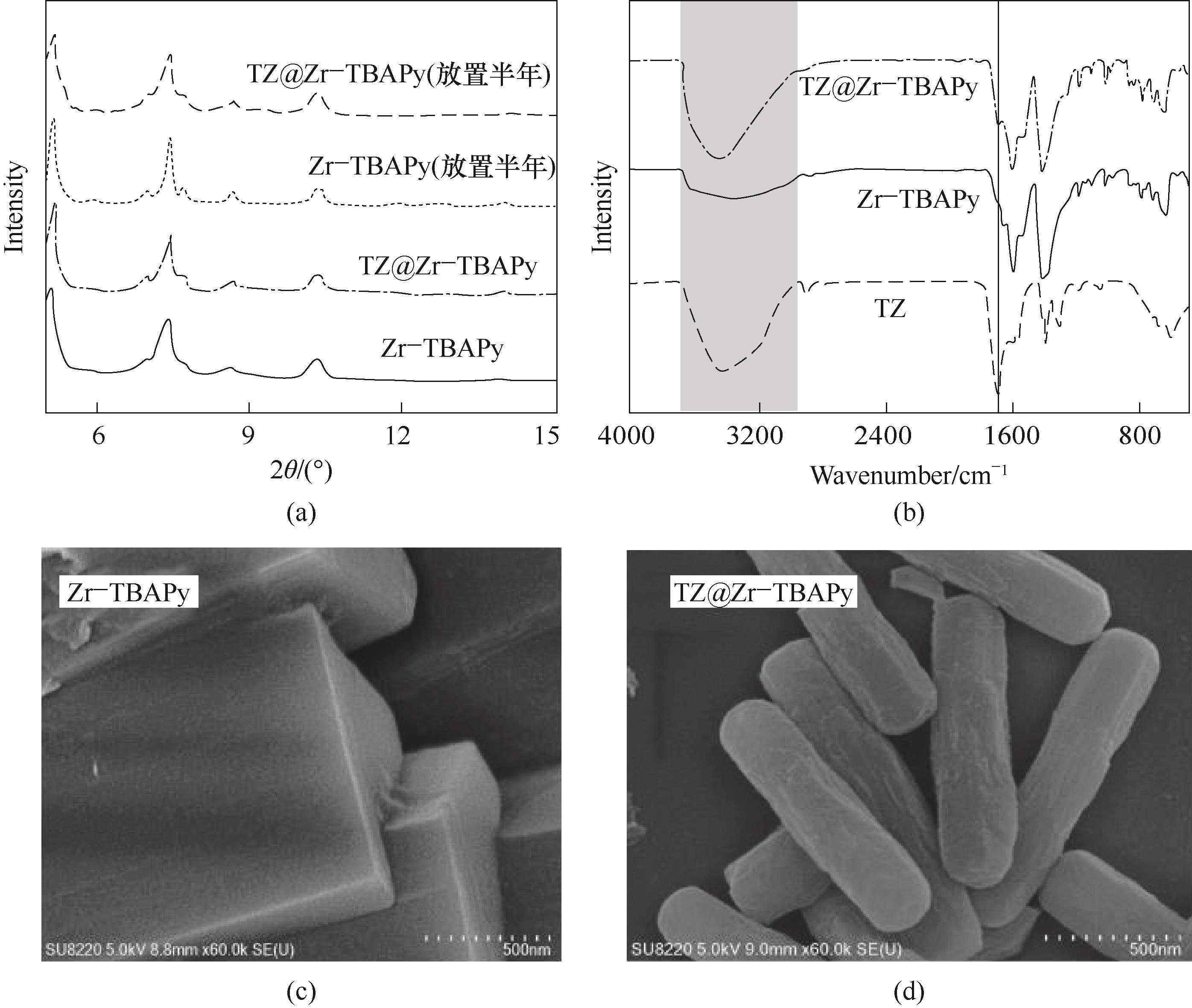
图3 Zr-TBAPy和TZ@Zr-TBAPy的表征:(a) XRD; (b) FT-IR; (c),(d) SEM
Fig.3 XRD pattern (a), FT-IR spectra (b), and SEM images[(c),(d)] of Zr-TBAPy and TZ@Zr-TBAPy
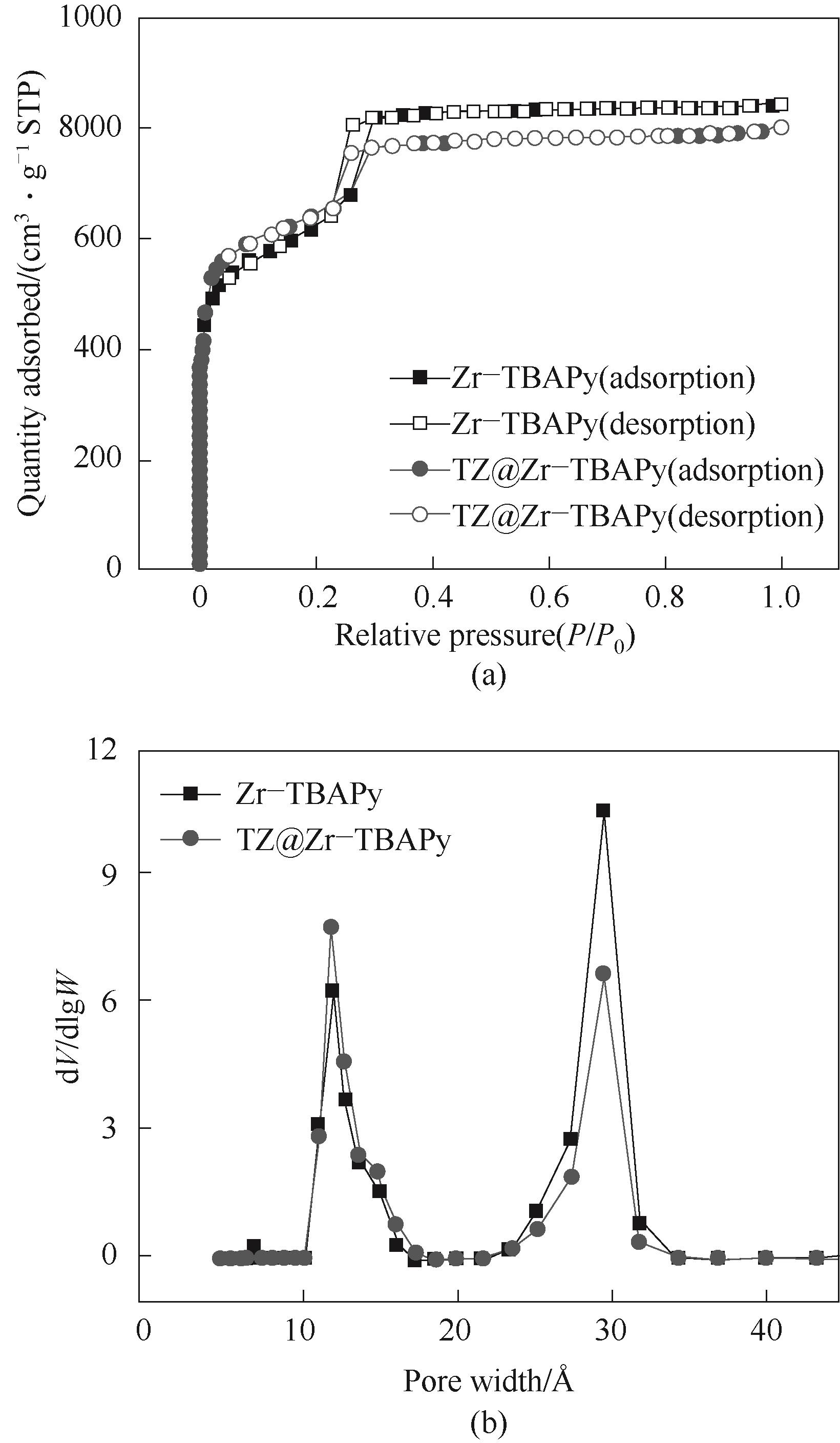
图4 Zr-TBAPy和TZ@Zr-TBAPy材料上77 K下的N2吸附脱附等温线(a)和孔径分布曲线(b)
Fig.4 N2 adsorption-desorption isotherms at 77 K (a) and pore size distribution (b) of Zr-TBAPy and TZ@Zr-TBAPy
| 吸附剂 | BET比表面积/(m2·g-1) | 总孔容,Vt/(cm3·g-1) | 微孔孔容,Vmicro/(cm3·g-1) | 介孔孔容,Vmeso/(cm3·g-1) |
|---|---|---|---|---|
| Zr-TBAPy | 2283 | 1.30 | 0.31 | 0.99 |
| TZ@Zr-TBAPy | 2441 | 1.24 | 0.30 | 0.94 |
表1 Zr-TBAPy和TZ@Zr-TBAPy的BET比表面积和孔径
Table 1 BET surface area and pore volume of Zr-TBAPy and TZ@Zr-TBAPy
| 吸附剂 | BET比表面积/(m2·g-1) | 总孔容,Vt/(cm3·g-1) | 微孔孔容,Vmicro/(cm3·g-1) | 介孔孔容,Vmeso/(cm3·g-1) |
|---|---|---|---|---|
| Zr-TBAPy | 2283 | 1.30 | 0.31 | 0.99 |
| TZ@Zr-TBAPy | 2441 | 1.24 | 0.30 | 0.94 |
| 参数 | Zr-TBAPy | TZ@Zr-TBAPy | ||||
|---|---|---|---|---|---|---|
| C3H8 | C2H6 | CH4 | C3H8 | C2H6 | CH4 | |
| qm,1/(mmol·g-1) | 8.04 | 4.49 | 1.48 | 9.49 | 6.03 | 1.66 |
| b1 | 0.036 | 0.0043 | 0.0023 | 0.037 | 0.0043 | 0.0018 |
| n1 | 1.08 | 0.88 | 0.89 | 1.38 | 0.88 | 0.81 |
| qm,2/(mmol·g-1) | 4.87 | 3.30 | 1.34 | 6.21 | 3.15 | 0.60 |
| b2 | 0.010 | 0.0032 | 0.0016 | 0.021 | 0.0030 | 0.0010 |
| n2 | 1.02 | 0.82 | 1.10 | 0.85 | 0.82 | 1.04 |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
表2 DSLF拟合模型的参数以及相应的决定系数
Table 2 The fitting parameters of DSLF model and the corresponding correlation coefficients
| 参数 | Zr-TBAPy | TZ@Zr-TBAPy | ||||
|---|---|---|---|---|---|---|
| C3H8 | C2H6 | CH4 | C3H8 | C2H6 | CH4 | |
| qm,1/(mmol·g-1) | 8.04 | 4.49 | 1.48 | 9.49 | 6.03 | 1.66 |
| b1 | 0.036 | 0.0043 | 0.0023 | 0.037 | 0.0043 | 0.0018 |
| n1 | 1.08 | 0.88 | 0.89 | 1.38 | 0.88 | 0.81 |
| qm,2/(mmol·g-1) | 4.87 | 3.30 | 1.34 | 6.21 | 3.15 | 0.60 |
| b2 | 0.010 | 0.0032 | 0.0016 | 0.021 | 0.0030 | 0.0010 |
| n2 | 1.02 | 0.82 | 1.10 | 0.85 | 0.82 | 1.04 |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 | 0.999 |
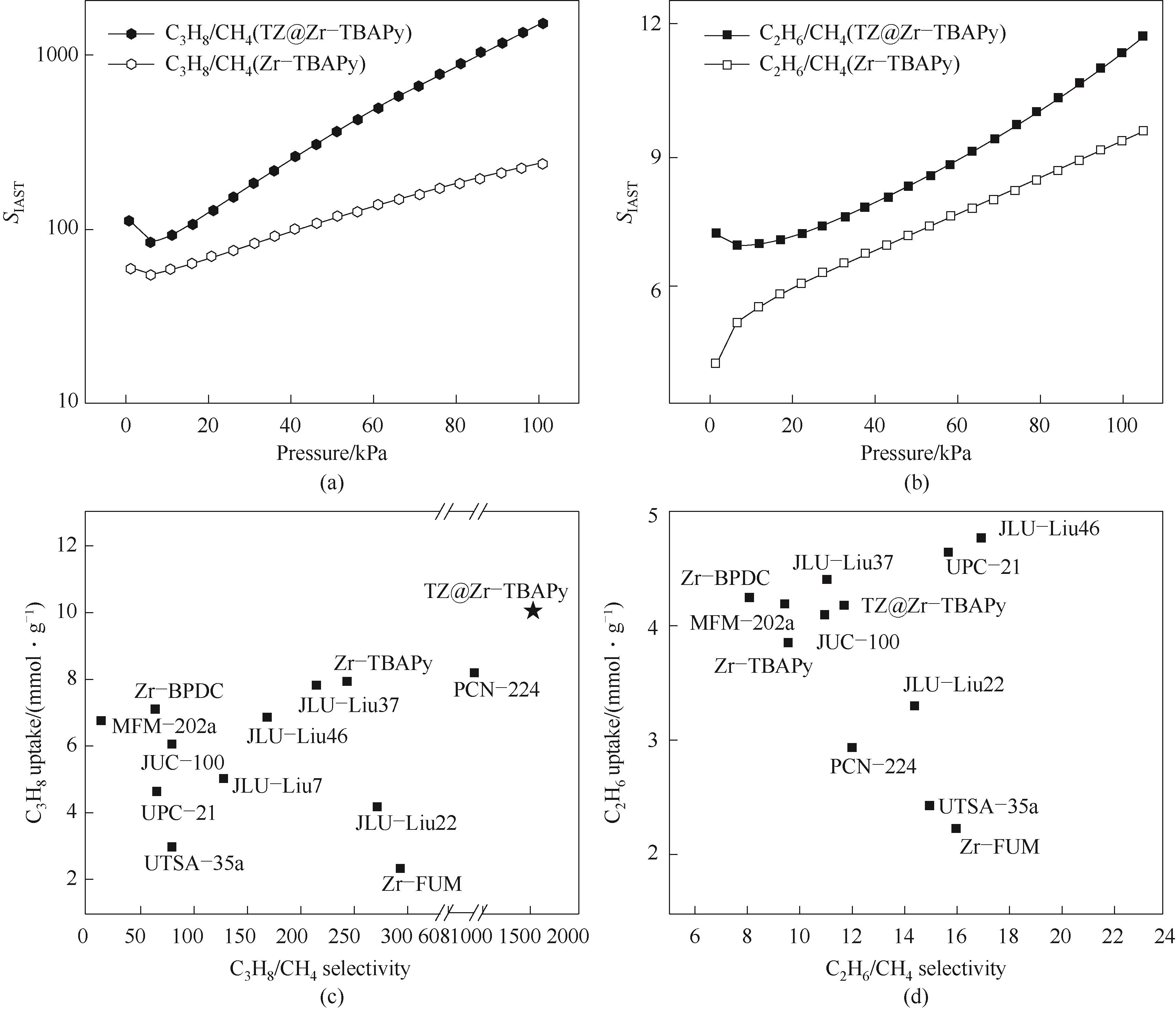
图7 Zr-TBAPy和TZ@Zr-TBAPy对丙烷/甲烷(a)和乙烷/甲烷(b)的IAST选择性;丙烷/甲烷吸附选择性与丙烷吸附量比较(c);乙烷/甲烷吸附选择性与乙烷吸附量比较(d) (UTSA-35a和MFM-202a的测试温度分别为296和293 K)
Fig.7 IAST selectivity of C3H8/CH4 (a) and C2H6/CH4 (b) for Zr-TBAPy and TZ@Zr-TBAPy; C3H8/CH4 adsorption selectivity vs C3H8 absorption curve (c); C2H6/CH4 adsorption selectivity vs C2H6 adsorption (d) (The test temperatures of UTSA-35a and MFM-202a are 296 K and 293 K, respectively)
| 材料 | GCD/? | PLD/? | LCD/? |
|---|---|---|---|
| Zr-TBAPy | 28.84 | 27.74 | 28.84 |
| TZ@Zr-TBAPy | 26.13 | 21.70 | 26.12 |
表3 分子模型的孔道参数
Table 3 Pore parameters of molecular model
| 材料 | GCD/? | PLD/? | LCD/? |
|---|---|---|---|
| Zr-TBAPy | 28.84 | 27.74 | 28.84 |
| TZ@Zr-TBAPy | 26.13 | 21.70 | 26.12 |
| 1 | Khan M I, Yasmin T, Shakoor A. Technical overview of compressed natural gas (CNG) as a transportation fuel[J]. Renewable and Sustainable Energy Reviews, 2015, 51: 785-797. |
| 2 | Shen J M, Dailly A, Beckner M. Natural gas sorption evaluation on microporous materials[J]. Microporous and Mesoporous Materials, 2016, 235: 170-177. |
| 3 | Duan J G, Jin W Q, Krishna R. Natural gas purification using a porous coordination polymer with water and chemical stability[J]. Inorganic Chemistry, 2015, 54(9): 4279-4284. |
| 4 | Ma Y X, Cui P Z, Wang Y K, et al. A review of extractive distillation from an azeotropic phenomenon for dynamic control[J]. Chinese Journal of Chemical Engineering, 2019, 27(7): 1510-1522. |
| 5 | Zhang S H, Taylor M K, Jiang L C, et al. Light hydrocarbon separations using porous organic framework materials[J]. Chemistry - A European Journal, 2020, 26(15): 3205-3221. |
| 6 | Furukawa H, Cordova K E, O'Keeffe M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. |
| 7 | 崔希利, 邢华斌. 金属有机框架材料分离低碳烃的研究进展[J]. 化工学报, 2018, 69(6): 2339-2352. |
| Cui X L, Xing H B. Separation of light hydrocarbons with metal-organic frameworks[J]. CIESC Journal, 2018, 69(6): 2339-2352. | |
| 8 | Yuan S, Feng L, Wang K C, et al. Stable metal-organic frameworks: design, synthesis, and applications[J]. Advanced Materials, 2018, 30(37): 1704303. |
| 9 | Ji P, Manna K, Lin Z, et al. Single-site cobalt catalysts at new Zr12(μ3-O)8(μ3-OH)8(μ2-OH)6 metal-organic framework nodes for highly active hydrogenation of nitroarenes, nitriles, and isocyanides [J]. Journal of the American Chemical Society, 2017, 139(20): 7004-7011. |
| 10 | Zhang Y F, Xiao H Y, Zhou X, et al. Selective adsorption performances of UiO-67 for separation of light hydrocarbons C1, C2, and C3[J]. Industrial & Engineering Chemistry Research, 2017, 56(30): 8689-8696. |
| 11 | Shi R F, Lv D, Chen Y W, et al. Highly selective adsorption separation of light hydrocarbons with a porphyrinic zirconium metal-organic framework PCN-224[J]. Separation and Purification Technology, 2018, 207: 262-268. |
| 12 | Li H, Lin Z D, Zhou X, et al. Ultrafast room temperature synthesis of novel composites Imi@Cu-BTC with improved stability against moisture[J]. Chemical Engineering Journal, 2017, 307: 537-543. |
| 13 | Lin Z D, Lv Z, Zhou X, et al. Postsynthetic strategy to prepare ACN@Cu-BTCs with enhanced water vapor stability and CO2/CH4 separation selectivity[J]. Industrial & Engineering Chemistry Research, 2018, 57(10): 3765-3772. |
| 14 | Wu Y F, Lv Z, Zhou X, et al. Tuning secondary building unit of Cu-BTC to simultaneously enhance its CO2 selective adsorption and stability under moisture[J]. Chemical Engineering Journal, 2019, 355: 815-821. |
| 15 | Mondloch J E, Bury W, Fairen-Jimenez D, et al. Vapor-phase metalation by atomic layer deposition in a metal-organic framework[J]. Journal of the American Chemical Society, 2013, 135(28): 10294-10297. |
| 16 | Feng D W, Gu Z Y, Li J R, et al. Zirconium-metalloporphyrin PCN-222: mesoporous metal-organic frameworks with ultrahigh stability as biomimetic catalysts[J]. Angewandte Chemie, 2012, 124(41): 10453-10456. |
| 17 | Planas N, Mondloch J E, Tussupbayev S, et al. Defining the proton topology of the Zr6-based metal–organic framework NU-1000[J]. The Journal of Physical Chemistry Letters, 2014, 5(21): 3716-3723. |
| 18 | Rappe A K, Casewit C J, Colwell K S, et al. UFF, a full periodic table force field for molecular mechanics and molecular dynamics simulations[J]. Journal of the American Chemical Society, 1992, 114(25): 10024-10035. |
| 19 | Rappe A K, Goddard W A. Charge equilibration for molecular dynamics simulations[J]. The Journal of Physical Chemistry, 1991, 95(8): 3358-3363. |
| 20 | 王磊, 方桂英, 阳庆元. 金属-有机骨架材料CO2捕获性能的大规模计算筛选[J]. 化工学报, 2019, 70(3): 1135-1143. |
| Wang L, Fang G Y, Yang Q Y. Performance of metal-organic frameworks for CO2 capture from large-scale computational screening[J]. CIESC Journal, 2019, 70(3): 1135-1143. | |
| 21 | Thommes M, Kaneko K, Neimark A V, et al. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)[J]. Pure and Applied Chemistry, 2015, 87(9/10): 1051-1069. |
| 22 | Myers A L, Prausnitz J M. Thermodynamics of mixed-gas adsorption[J]. AIChE Journal, 1965, 11(1): 121-127. |
| 23 | Férey G, Mellot-Draznieks C, Serre C, et al. A chromium terephthalate-based solid with unusually large pore volumes and surface area[J]. Science, 2005, 309(5743): 2040-2042. |
| 24 | He Y, Zhang Z, Xiang S, et al. A robust doubly interpenetrated metal-organic framework constructed from a novel aromatic tricarboxylate for highly selective separation of small hydrocarbons[J]. Chemical Communications, 2012, 48(52): 6493-6495. |
| 25 | Zhang M H, Xin X L, Xiao Z Y, et al. A multi-aromatic hydrocarbon unit induced hydrophobic metal–organic framework for efficient C2/C1 hydrocarbon and oil/water separation[J]. Journal of Materials Chemistry A, 2017, 5(3): 1168-1175. |
| 26 | Luo J H, Wang J, Cao Y, et al. Assembly of an indium–porphyrin framework JLU-Liu7: a mesoporous metal–organic framework with high gas adsorption and separation of light hydrocarbons[J]. Inorganic Chemistry Frontiers, 2017, 4(1): 139-143. |
| 27 | Jia J T, Wang L, Sun F X, et al. The adsorption and simulated separation of light hydrocarbons in isoreticular metal-organic frameworks based on dendritic ligands with different aliphatic side chains[J]. Chemistry - A European Journal, 2014, 20(29): 9073-9080. |
| 28 | Gao S, Morris C G, Lu Z Z, et al. Selective hysteretic sorption of light hydrocarbons in a flexible metal–organic framework material[J]. Chemistry of Materials, 2016, 28(7): 2331-2340. |
| 29 | Li J T, Luo X L, Zhao N, et al. Two finite binuclear [M2(μ2-OH)(COO)2] (M = Co, Ni) based highly porous metal–organic frameworks with high performance for gas sorption and separation[J]. Inorganic Chemistry, 2017, 56(7): 4141-4147. |
| 30 | Wang D M, Liu B, Yao S, et al. A polyhedral metal-organic framework based on the supermolecular building block strategy exhibiting high performance for carbon dioxide capture and separation of light hydrocarbons[J]. Chemical Communications, 2015, 51(83): 15287-15289. |
| 31 | Han G P, Wang K K, Peng Y G, et al. Enhancing higher hydrocarbons capture for natural gas upgrading by tuning van der Waals interactions in fcu-type Zr-MOFs[J]. Industrial & Engineering Chemistry Research, 2017, 56(49): 14633-14641. |
| [1] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [2] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [3] | 朱兴驰, 郭志远, 纪志永, 汪婧, 张盼盼, 刘杰, 赵颖颖, 袁俊生. 选择性电渗析镁锂分离过程模拟优化[J]. 化工学报, 2023, 74(6): 2477-2485. |
| [4] | 顾浩, 张福建, 刘珍, 周文轩, 张鹏, 张忠强. 力电耦合作用下多孔石墨烯膜时间维度的脱盐性能及机理研究[J]. 化工学报, 2023, 74(5): 2067-2074. |
| [5] | 张正, 何永平, 孙海东, 张荣子, 孙正平, 陈金兰, 郑一璇, 杜晓, 郝晓刚. 蛇形流场电控离子交换装置用于选择性提锂[J]. 化工学报, 2023, 74(5): 2022-2033. |
| [6] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [7] | 张家庆, 蒋榕培, 史伟康, 武博翔, 杨超, 刘朝晖. 煤基/石油基火箭煤油高参数黏温特性与组分特性研究[J]. 化工学报, 2023, 74(2): 653-665. |
| [8] | 许万, 陈振斌, 张慧娟, 牛昉昉, 火婷, 刘兴盛. 线性温敏性聚合物嵌段调控的 |
| [9] | 孙嘉辰, 裴春雷, 陈赛, 赵志坚, 何盛宝, 巩金龙. 化学链低碳烷烃氧化脱氢技术进展[J]. 化工学报, 2023, 74(1): 205-223. |
| [10] | 李沐紫, 贾国伟, 赵砚珑, 张鑫, 李建荣. 金属有机框架材料对非二氧化碳温室气体捕捉研究进展[J]. 化工学报, 2023, 74(1): 365-379. |
| [11] | 闫军营, 王皝莹, 李瑞瑞, 符蓉, 蒋晨啸, 汪耀明, 徐铜文. 选择性电渗析:机遇与挑战[J]. 化工学报, 2023, 74(1): 224-236. |
| [12] | 余后川, 任腾, 张宁, 姜晓滨, 代岩, 张晓鹏, 鲍军江, 贺高红. 二维氧化石墨烯膜离子选择性传递调控的研究进展[J]. 化工学报, 2023, 74(1): 303-312. |
| [13] | 席国君, 刘子涵, 雷广平. FeTPPs-CuBTC协同强化低浓度煤层气吸附分离[J]. 化工学报, 2022, 73(9): 3940-3949. |
| [14] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| [15] | 郭丹, 方雨洁, 许一寒, 李致远, 黄守莹, 王胜平, 马新宾. 乙烷和二氧化碳催化转化的研究进展[J]. 化工学报, 2022, 73(8): 3406-3416. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号