化工学报 ›› 2022, Vol. 73 ›› Issue (10): 4448-4460.DOI: 10.11949/0438-1157.20220857
收稿日期:2022-06-21
修回日期:2022-09-07
出版日期:2022-10-05
发布日期:2022-11-02
通讯作者:
姜召
作者简介:龚翔(1994—),男,博士研究生,gx2017@stu.xjtu.edu.cn
基金资助:
Xiang GONG( ), Linsen LI, Zhao JIANG(
), Linsen LI, Zhao JIANG( )
)
Received:2022-06-21
Revised:2022-09-07
Online:2022-10-05
Published:2022-11-02
Contact:
Zhao JIANG
摘要:
乙基咔唑/十二氢乙基咔唑(N-ethylcarbazole/dodecahydro-N-ethylcarbazole,NECZ/12H-NECZ)体系被认为在有机液体储氢领域具有较大的开发应用前景,但高活性、选择性的脱氢催化剂的设计开发制约着其工业应用。基于此,设计开发出一种具有高脱氢活性和高选择性的双金属催化剂Pd1Co3/ SiO2(Pd质量分数为1.25%),对其结构进行了XPS、XRD、HRTEM等表征分析,并评价了其催化十二氢乙基咔唑的脱氢性能。与5.0%(质量分数)Pd/SiO2相比,表明引入一定量的Co金属形成的PdCo合金可提高12H-NECZ脱氢反应效率和NECZ选择性,动力学与DFT计算发现双金属催化剂可以有效降低三步基元反应的能垒,大幅提升第二步基元反应(8H-NECZ到4H-NECZ)的脱氢反应速率。研究结果为揭示12H-NECZ脱氢反应机理和高效脱氢反应催化剂的设计与开发提供了新的思路。
中图分类号:
龚翔, 李林森, 姜召. PdCo/SiO2双金属催化剂用于杂环储氢载体的高效脱氢[J]. 化工学报, 2022, 73(10): 4448-4460.
Xiang GONG, Linsen LI, Zhao JIANG. Employing PdCo/SiO2 catalyst in high activity dehydrogenation reaction of heterocyclic H2 storage carrier[J]. CIESC Journal, 2022, 73(10): 4448-4460.
| 催化剂 | Pd0 /eV | Pd δ+ /eV | Co0 /eV | Co δ+ /eV | Pd0/Pd δ+ | Co0/Co δ+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3d5/2 | 3d3/2 | 3d5/2 | 3d3/2 | 3d3/2 | 3d1/2 | 3d3/2 | 3d1/2 | |||
| Pd/SiO2 | 335.1 | 340.4 | 336.6 | 341.9 | — | — | — | — | 84%/16% | — |
| Co/SiO2 | — | — | — | — | 778.3 | 793.3 | 792.1 | 798.4 | 81%/19% | — |
| Pd3Co1/SiO2 | 335.3 | 340.6 | 336.6 | 341.9 | 777.5 | 792.7 | 783.2 | 798.4 | 82%/18% | 77%/23% |
| Pd1Co1/SiO2 | 335.5 | 340.7 | 336.8 | 342.1 | 777.8 | 793.0 | 783.6 | 798.8 | 85%/15% | 76%/24% |
| Pd1Co3/SiO2 | 335.8 | 341.1 | 337.2 | 342.5 | 778.0 | 793.1 | 783.9 | 799.1 | 81%/19% | 76%/24% |
表1 双金属催化剂XPS表征结果
Table 1 XPS results of bimetal catalysts
| 催化剂 | Pd0 /eV | Pd δ+ /eV | Co0 /eV | Co δ+ /eV | Pd0/Pd δ+ | Co0/Co δ+ | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3d5/2 | 3d3/2 | 3d5/2 | 3d3/2 | 3d3/2 | 3d1/2 | 3d3/2 | 3d1/2 | |||
| Pd/SiO2 | 335.1 | 340.4 | 336.6 | 341.9 | — | — | — | — | 84%/16% | — |
| Co/SiO2 | — | — | — | — | 778.3 | 793.3 | 792.1 | 798.4 | 81%/19% | — |
| Pd3Co1/SiO2 | 335.3 | 340.6 | 336.6 | 341.9 | 777.5 | 792.7 | 783.2 | 798.4 | 82%/18% | 77%/23% |
| Pd1Co1/SiO2 | 335.5 | 340.7 | 336.8 | 342.1 | 777.8 | 793.0 | 783.6 | 798.8 | 85%/15% | 76%/24% |
| Pd1Co3/SiO2 | 335.8 | 341.1 | 337.2 | 342.5 | 778.0 | 793.1 | 783.9 | 799.1 | 81%/19% | 76%/24% |
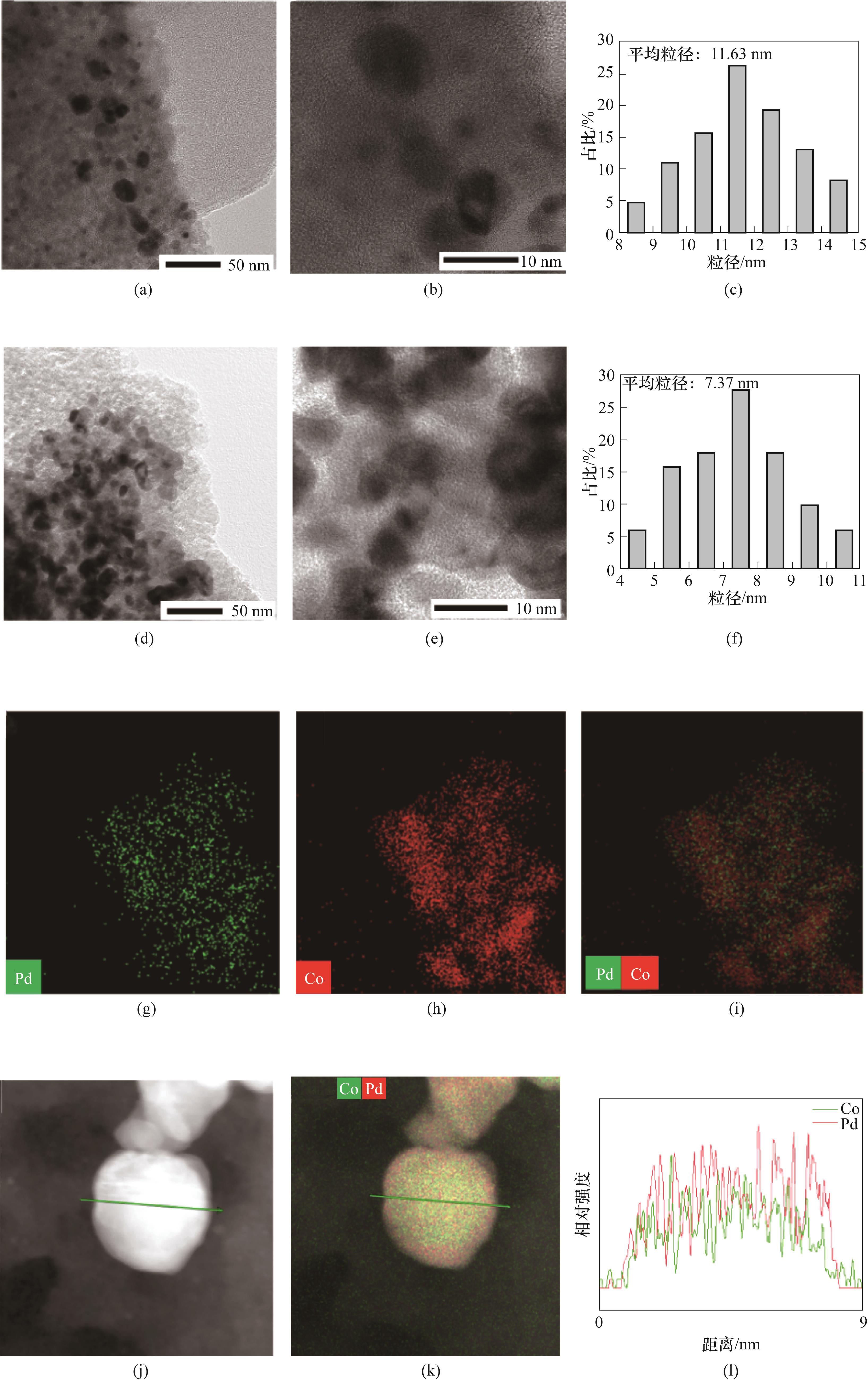
图6 Pd/SiO2的HRTEM图[(a),(b)]和粒径分布(c);Pd1Co3/SiO2的HRTEM图[(d),(e)]、粒径分布(f)、mapping图[(g)~(i)]和线扫图[(j)~(l)]
Fig.6 HRTEM images [(a), (b)] and particle size distributions (c) of Pd/SiO2; HRTEM images [(d), (e)], particle size distributions (f), mapping images [(g)—(i)] and line scanning images [(j)—(l)] of Pd1Co3/SiO2 catalyst

图7 8 h反应脱氢量(a)和双金属催化剂8h脱氢反应产物分布[(b)~(f)]
Fig.7 Hydrogen release amount (a) and product distributions [(b)—(f)] of 12H-NECZ dehydrogenation process on bimetal catalysts over 8 h
| 样品 | 脱氢量/%(质量) | 12H-NECZ转化率/% | 4H-NECZ选择性/% | NECZ选择性/% | ||
|---|---|---|---|---|---|---|
| 1 h | 4 h | 8 h | ||||
| Pd/SiO2 | 2.18 | 4.33 | 5.20 | 100 | 30.82 | 69.18 |
| Pd3Co1/SiO2 | 0.92 | 2.81 | 3.78 | 100 | 66.70 | 14.60 |
| Pd1Co1/SiO2 | 0.89 | 2.51 | 3.17 | 100 | 43.70 | 10.21 |
| Pd1Co3/SiO2 | 2.93 | 4.67 | 5.25 | 100 | 28.34 | 71.66 |
| Pd1Co4/SiO2 | 1.10 | 2.70 | 3.70 | 100 | 36.20 | 9.72 |
| Pd1Co5/SiO2 | 0.73 | 1.87 | 2.47 | 38.68 | 23.27 | 10.09 |
| Co/SiO2 | 0 | 0.07 | 0.12 | 4.10 | 1.05 | 0.0055 |
表2 不同催化剂在453 K温度下反应脱氢量和选择性
Table 2 H2 release amount and selectivity data of mentioned catalysts for 12H-NECZ dehydrogenation at 453 K
| 样品 | 脱氢量/%(质量) | 12H-NECZ转化率/% | 4H-NECZ选择性/% | NECZ选择性/% | ||
|---|---|---|---|---|---|---|
| 1 h | 4 h | 8 h | ||||
| Pd/SiO2 | 2.18 | 4.33 | 5.20 | 100 | 30.82 | 69.18 |
| Pd3Co1/SiO2 | 0.92 | 2.81 | 3.78 | 100 | 66.70 | 14.60 |
| Pd1Co1/SiO2 | 0.89 | 2.51 | 3.17 | 100 | 43.70 | 10.21 |
| Pd1Co3/SiO2 | 2.93 | 4.67 | 5.25 | 100 | 28.34 | 71.66 |
| Pd1Co4/SiO2 | 1.10 | 2.70 | 3.70 | 100 | 36.20 | 9.72 |
| Pd1Co5/SiO2 | 0.73 | 1.87 | 2.47 | 38.68 | 23.27 | 10.09 |
| Co/SiO2 | 0 | 0.07 | 0.12 | 4.10 | 1.05 | 0.0055 |
| 样品 | k1/min-1 | k2/min-1 | k3/min-1 | R2 |
|---|---|---|---|---|
Pd/SiO2 Pd3Co1/SiO2 Pd1Co1/SiO2 Pd1Co3/SiO2 | 0.0276 0.0104 0.0096 0.0523 | 0.0192 0.0045 0.0026 0.0486 | 0.0031 0.0028 0.0013 0.0034 | 0.979 0.994 0.975 0.991 |
表3 脱氢反应动力学分析结果
Table 3 Kinetic fitting results of 12H-NECZ sequential dehydrogenation process
| 样品 | k1/min-1 | k2/min-1 | k3/min-1 | R2 |
|---|---|---|---|---|
Pd/SiO2 Pd3Co1/SiO2 Pd1Co1/SiO2 Pd1Co3/SiO2 | 0.0276 0.0104 0.0096 0.0523 | 0.0192 0.0045 0.0026 0.0486 | 0.0031 0.0028 0.0013 0.0034 | 0.979 0.994 0.975 0.991 |
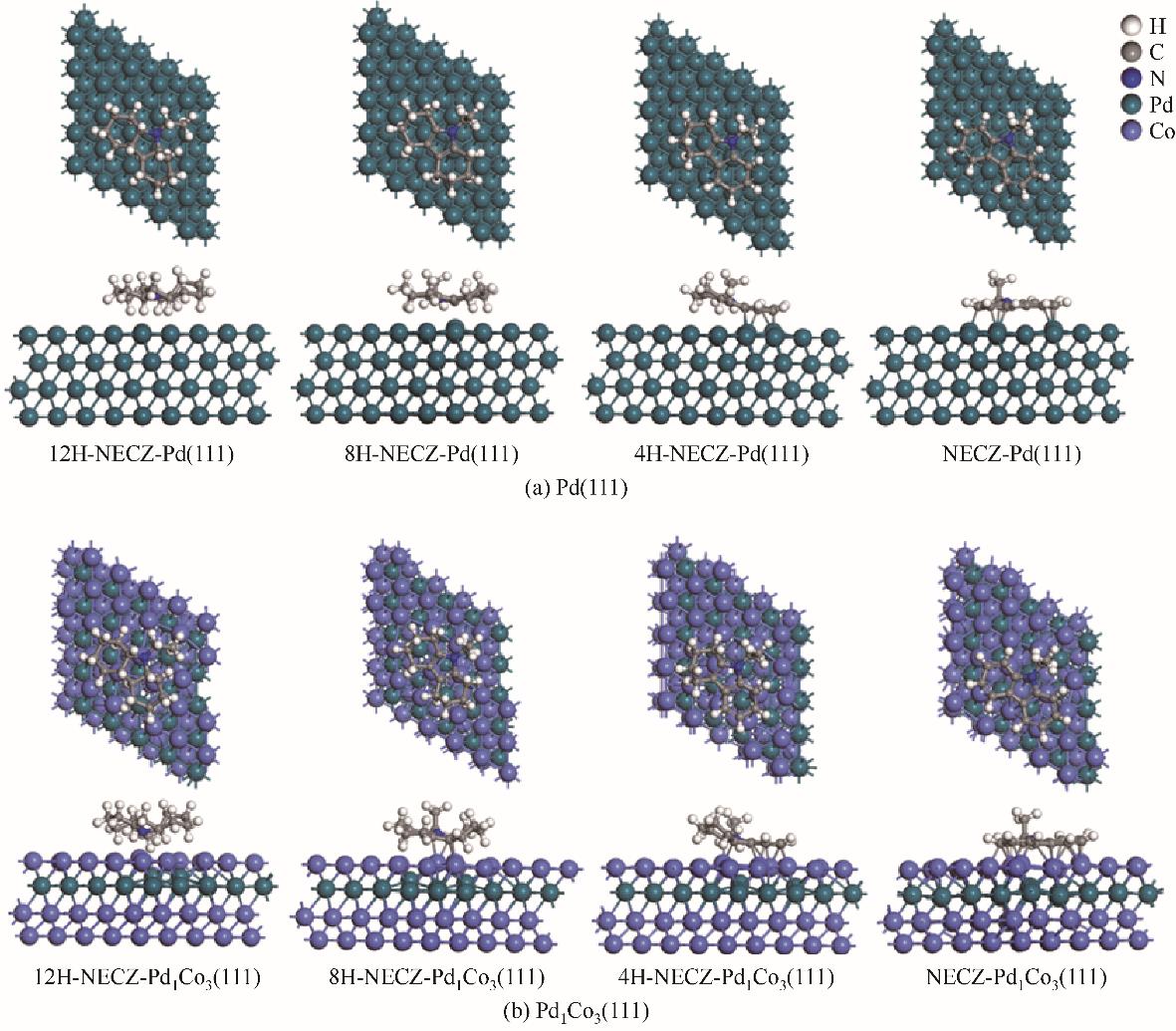
图9 12H-NECZ、8H-NECZ、4H-NECZ和NECZ在Pd(111)和Pd1Co3(111)表面稳定吸附结构
Fig.9 Optimized stable adsorption geometries of 12H-NECZ, 8H-NECZ, 4H-NECZ, and NECZ on the Pd (111) and Pd1Co3 (111) surfaces
| 表面 | 结合能/eV | |||
|---|---|---|---|---|
| 0H-NECZ | 4H-NECZ | 8H-NECZ | 12H-NECZ | |
| Pd (111) | -2.89 | -2.66 | -2.37 | -2.16 |
| Pd1Co3(111) | -5.44 | -4.99 | -3.98 | -3.68 |
表4 12H-NECZ、8H-NECZ、4H-NECZ和NECZ在Pd(111)和Pd1Co3(111)表面的结合能
Table 4 DFT calculated binding energies of 12H-NECZ, 8H-NECZ, 4H-NECZ, and NECZ on Pd (111) and Pd1Co3 (111) surfaces
| 表面 | 结合能/eV | |||
|---|---|---|---|---|
| 0H-NECZ | 4H-NECZ | 8H-NECZ | 12H-NECZ | |
| Pd (111) | -2.89 | -2.66 | -2.37 | -2.16 |
| Pd1Co3(111) | -5.44 | -4.99 | -3.98 | -3.68 |
| 反应路径 | ΔG/eV | |
|---|---|---|
| Pd(111) | Pd1Co3(111) | |
12H 8H+2H2 8H+2H2 | 0.96 | 0.85 |
8H 4H+2H2 4H+2H2 | 1.25 | 0.54 |
4H 0H+2H2 0H+2H2 | 1.36 | 1.14 |
表5 12H-NECZ脱氢三步基元反应在Pd(111)和Pd1Co3(111)表面的Gibbs自由能变化
Table 5 Change of Gibbs free energies of three elementary reactions from 12H-NECZ dehydrogenation process to NECZ on the Pd (111) and Pd1Co3 (111) surfaces
| 反应路径 | ΔG/eV | |
|---|---|---|
| Pd(111) | Pd1Co3(111) | |
12H 8H+2H2 8H+2H2 | 0.96 | 0.85 |
8H 4H+2H2 4H+2H2 | 1.25 | 0.54 |
4H 0H+2H2 0H+2H2 | 1.36 | 1.14 |

图10 12H-NECZ在Pd(111)和Pd1Co3(111)表面脱氢至NECZ的反应势能
Fig.10 DFT-calculated energy profile of 12H-NECZ dehydrogenation process to NECZ on the Pd (111) and Pd1Co3 (111) surfaces
| 1 | Barreto L, Makihira A, Riahi K. The hydrogen economy in the 21st century: a sustainable development scenario[J]. International Journal of Hydrogen Energy, 2003, 28(3): 267-284. |
| 2 | Tarhan C, Cil M A. A study on hydrogen, the clean energy of the future: hydrogen storage methods[J]. Journal of Energy Storage, 2021, 40: 102676. |
| 3 | Schlapbach L, Züttel A. Hydrogen-storage materials for mobile applications[J]. Nature, 2001, 414(6861): 353-358. |
| 4 | Momirlan M, Veziroglu T N. Current status of hydrogen energy[J]. Renewable and Sustainable Energy Reviews, 2002, 6(1/2): 141-179. |
| 5 | Chalk S G, Miller J F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems[J]. Journal of Power Sources, 2006, 159(1): 73-80. |
| 6 | 童海航, 石德智, 刘嘉宇, 等. 金属纳米颗粒辅助木质纤维素暗发酵生物制氢的研究进展[J]. 化工学报, 2022, 73(4): 1417-1435. |
| Tong H H, Shi D Z, Liu J Y, et al. Research progress on dark fermentative bio-hydrogen production from lignocellulose assisted by metal nanoparticles[J]. CIESC Journal, 2022, 73(4): 1417-1435. | |
| 7 | 陈晨, 王明明, 王志刚, 等. 镍基非对称中空纤维膜用于乙醇自热重整制氢[J]. 化工学报, 2021, 72(S1): 482-493. |
| Chen C, Wang M M, Wang Z G, et al. Hydrogen production by ethanol autothermal reforming using nickel-based asymmetric hollow fiber membranes[J]. CIESC Journal, 2021, 72(S1): 482-493. | |
| 8 | Jiang Z, Pan Q, Xu J, et al. Current situation and prospect of hydrogen storage technology with new organic liquid[J]. International Journal of Hydrogen Energy, 2014, 39(30): 17442-17451. |
| 9 | Zheng J Y, Liu X X, Xu P, et al. Development of high pressure gaseous hydrogen storage technologies[J]. International Journal of Hydrogen Energy, 2012, 37(1): 1048-1057. |
| 10 | Zhang M, Lv H, Kang H R, et al. A literature review of failure prediction and analysis methods for composite high-pressure hydrogen storage tanks[J]. International Journal of Hydrogen Energy, 2019, 44(47): 25777-25799. |
| 11 | Peschka W, Carpetis C. Cryogenic hydrogen storage and refueling for automobiles[J]. International Journal of Hydrogen Energy, 1980, 5(6): 619-625. |
| 12 | Sadaghiani M S, Mehrpooya M. Introducing and energy analysis of a novel cryogenic hydrogen liquefaction process configuration[J]. International Journal of Hydrogen Energy, 2017, 42(9): 6033-6050. |
| 13 | Dillon A C, Jones K M, Bekkedahl T A, et al. Storage of hydrogen in single-walled carbon nanotubes[J]. Nature, 1997, 386(6623): 377-379. |
| 14 | He Q F, Zeng L P, Han L H, et al. Electrochemical hydrogen-storage capacity of graphene can achieve a carbon-hydrogen atomic ratio of 1∶1[J]. Science China Chemistry, 2022, 65(2): 318-321. |
| 15 | Wang D, Wang Y Q, Huang Z N, et al. Design optimization and sensitivity analysis of the radiation mini-channel metal hydride reactor[J]. Energy, 2019, 173: 443-456. |
| 16 | Zhang X L, Wang K, Zhang X, et al. Synthesis process and catalytic activity of Nb2O5 hollow spheres for reversible hydrogen storage of MgH2 [J]. International Journal of Energy Research, 2021, 45(2): 3129-3141. |
| 17 | Schüth F, Bogdanovi B, Felderhoff M. Light metal hydrides and complex hydrides for hydrogen storage[J]. Chemical Communications, 2004, 36(20): 2249-2258. |
| 18 | Shevlin S A, Kerkeni B, Guo Z X. Dehydrogenation mechanisms and thermodynamics of MNH2BH3 (M = Li, Na) metal amidoboranes as predicted from first principles[J]. Physical Chemistry Chemical Physics, 2011, 13(17): 7649. |
| 19 | Gao C, Qi X B, Zhang Z W, et al. Fabrication of monodisperse precursor gel microspheres for hollow glass microspheres by combining the sol-microemulsion-gel process with a T-shaped microfluidic device[J]. International Journal of Hydrogen Energy, 2011, 36(16): 9758-9766. |
| 20 | Sotoodeh F, Smith K J. An overview of the kinetics and catalysis of hydrogen storage on organic liquids[J]. The Canadian Journal of Chemical Engineering, 2013, 91(9): 1477-1490. |
| 21 | Kariya N, Fukuoka A, Ichikawa M. Efficient evolution of hydrogen from liquid cycloalkanes over Pt-containing catalysts supported on active carbons under “wet-dry multiphase conditions”[J]. Applied Catalysis A: General, 2002, 233(1/2): 91-102. |
| 22 | Dong Y, Yang M, Yang Z H, et al. Catalytic hydrogenation and dehydrogenation of N-ethylindole as a new heteroaromatic liquid organic hydrogen carrier[J]. International Journal of Hydrogen Energy, 2015, 40(34): 10918-10922. |
| 23 | Cacciola G, Giordano N, Restuccia G. Cyclohexane as a liquid phase carrier in hydrogen storage and transport[J]. International Journal of Hydrogen Energy, 1984, 9(5): 411-419. |
| 24 | Shi L J, Liu X J, Tuo Y X, et al. Graphene-CNT composite as catalyst support for microwave-assisted hydrogen releasing from liquid organic hydride[J]. International Journal of Hydrogen Energy, 2017, 42(27): 17403-17413. |
| 25 | 齐随涛, 李迎迎, 岳佳琪, 等. 活性炭负载Pt-Ni双金属催化剂上十氢化萘脱氢[J]. 催化学报, 2014, 35(11): 1833-1839. |
| Qi S T, Li Y Y, Yue J Q, et al. Hydrogenation kinetics of N-ethylindole on a supported Ru catalyst [J]. Chinese Journal of Catalysis, 2014, 35(11): 1833-1839. | |
| 26 | Dong Y, Yang M, Zhu T, et al. Hydrogenation kinetics of N-ethylindole on a supported Ru catalyst[J]. Energy Technology, 2018, 6(3): 558-562. |
| 27 | Yang M, Dong Y, Fei S X, et al. A comparative study of catalytic dehydrogenation of perhydro-N-ethylcarbazole over noble metal catalysts[J]. International Journal of Hydrogen Energy, 2014, 39(33): 18976-18983. |
| 28 | Jiang Z, Gong X, Wang B, et al. A experimental study on the dehydrogenation performance of dodecahydro-N-ethylcarbazole on M/TiO2 catalysts[J]. International Journal of Hydrogen Energy, 2019, 44(5): 2951-2959. |
| 29 | Wang B, Chang T Y, Jiang Z, et al. Catalytic dehydrogenation study of dodecahydro-N-ethylcarbazole by noble metal supported on reduced graphene oxide[J]. International Journal of Hydrogen Energy, 2018, 43(15): 7317-7325. |
| 30 | Jorschick H, Geißelbrecht M, Eßl M, et al. Benzyltoluene/dibenzyltoluene-based mixtures as suitable liquid organic hydrogen carrier systems for low temperature applications[J]. International Journal of Hydrogen Energy, 2020, 45(29): 14897-14906. |
| 31 | Shi L B, Zhou Y M, Qi S T, et al. Pt catalysts supported on H2 and O2 plasma-treated Al2O3 for hydrogenation and dehydrogenation of the liquid organic hydrogen carrier pair dibenzyltoluene and perhydrodibenzyltoluene[J]. ACS Catalysis, 2020, 10(18): 10661-10671. |
| 32 | Pez G P, Scott A R, Cooper A C, et al. Hydrogen storage by reversible hydrogenation of pi-conjugated substrates: US7351395[P]. 2008-04-01. |
| 33 | Stark K, Emel'yanenko V N, Zhabina A A, et al. Liquid organic hydrogen carriers: thermophysical and thermochemical studies of carbazole partly and fully hydrogenated derivatives[J]. Industrial & Engineering Chemistry Research, 2015, 54(32): 7953-7966. |
| 34 | Emel'yanenko V N, Varfolomeev M A, Verevkin S P, et al. Hydrogen storage: thermochemical studies of N-alkylcarbazoles and their derivatives as a potential liquid organic hydrogen carriers[J]. The Journal of Physical Chemistry C, 2015, 119(47): 26381-26389 |
| 35 | Wan C, An Y, Xu G H, et al. Study of catalytic hydrogenation of N-ethylcarbazole over ruthenium catalyst[J]. International Journal of Hydrogen Energy, 2012, 37(17): 13092-13096. |
| 36 | Morawa Eblagon K, Tam K, Yu K M K, et al. Study of catalytic sites on ruthenium for hydrogenation of N-ethylcarbazole: implications of hydrogen storage via reversible catalytic hydrogenation[J]. The Journal of Physical Chemistry C, 2010, 114(21): 9720-9730. |
| 37 | Fei S, Han B, Li L, et al. A study on the catalytic hydrogenation of N-ethylcarbazole on the mesoporous Pd/MoO3 catalyst[J]. International Journal of Hydrogen Energy, 2017, 42(41): 25942-25950. |
| 38 | Ye X F, An Y, Xu G H. Kinetics of 9-ethylcarbazole hydrogenation over Raney-Ni catalyst for hydrogen storage[J]. Journal of Alloys and Compounds, 2011, 509(1): 152-156. |
| 39 | Yu H E, Yang X, Jiang X J, et al. LaNi5.5 particles for reversible hydrogen storage in N-ethylcarbazole[J]. Nano Energy, 2021, 80: 105476. |
| 40 | Yang X, Wu Y M, Yu H E, et al. A YH3 promoted palladium catalyst for reversible hydrogen storage of N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2020, 45(58): 33657-33662. |
| 41 | Gong X, Jiang Z, Fang T. Enhancing selectivity and reducing cost for dehydrogenation of dodecahydro-N-ethylcarbazole by supporting platinum on titanium dioxide[J]. International Journal of Hydrogen Energy, 2020, 45(11): 6838-6847. |
| 42 | Jiang Z, Gong X, Guo S, et al. Engineering PdCu and PdNi bimetallic catalysts with adjustable alloying degree for the dehydrogenation reaction of dodecahydro-N-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2021, 46(2): 2376-2389. |
| 43 | Yang M, Dong Y, Cheng H S. Hydrogenation kinetics of N-ethylcarbaozle as a heteroaromatic liquid organic hydrogen carrier [J]. Advanced Materials Research, 2014, 953/954: 981-984. |
| 44 | Wang B, Chen Y T, Chang T Y, et al. Facet-dependent catalytic activities of Pd/rGO: exploring dehydrogenation mechanism of dodecahydro-N-ethylcarbazole[J]. Applied Catalysis B: Environmental, 2020, 266: 118658. |
| 45 | Wang B, Chang T Y, Gong X, et al. One-pot synthesis of Au/Pd core/shell nanoparticles supported on reduced graphene oxide with enhanced dehydrogenation performance for dodecahydro-N-ethylcarbazole[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(1): 1760-1768. |
| 46 | Wang B, Chang T Y, Jiang Z, et al. Component controlled synthesis of bimetallic PdCu nanoparticles supported on reduced graphene oxide for dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Applied Catalysis B: Environmental, 2019, 251: 261-272. |
| 47 | Sotoodeh F, Huber B J M, Smith K J. Dehydrogenation kinetics and catalysis of organic heteroaromatics for hydrogen storage[J]. International Journal of Hydrogen Energy, 2012, 37(3): 2715-2722. |
| 48 | Sotoodeh F, Smith K J. Kinetics of hydrogen uptake and release from heteroaromatic compounds for hydrogen storage[J]. Industrial & Engineering Chemistry Research, 2010, 49(3): 1018-1026. |
| 49 | Sotoodeh F, Smith K J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts[J]. Journal of Catalysis, 2011, 279(1): 36-47. |
| 50 | Sotoodeh F, Zhao L, Smith K J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst[J]. Applied Catalysis A: General, 2009, 362(1/2): 155-162. |
| 51 | Sobota M, Nikiforidis I, Amende M, et al. Dehydrogenation of dodecahydro-N-ethylcarbazole on Pd//Al2O3 model catalysts [J]. Chemistry - A European Journal, 2011, 17(41): 11542-11552. |
| 52 | Amende M, Gleichweit C, Werner K, et al. Model catalytic studies of liquid organic hydrogen carriers: dehydrogenation and decomposition mechanisms of dodecahydro-N-ethylcarbazole on Pt(111)[J]. ACS Catalysis, 2014, 4(2): 657-665. |
| 53 | Yang M, Han C Q, Ni G, et al. Temperature controlled three-stage catalytic dehydrogenation and cycle performance of perhydro-9-ethylcarbazole[J]. International Journal of Hydrogen Energy, 2012, 37(17): 12839-12845. |
| 54 | Wang B, Yan T, Chang T Y, et al. Palladium supported on reduced graphene oxide as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Carbon, 2017, 122: 9-18. |
| 55 | Kustov L M, Tarasov A L, Kirichenko O A. Microwave-activated dehydrogenation of perhydro-N-ethylcarbazol over bimetallic Pd-M/TiO2 catalysts as the second stage of hydrogen storage in liquid substrates[J]. International Journal of Hydrogen Energy, 2017, 42(43): 26723-26729. |
| 56 | Gong X, Guo S Y, Jiang Z, et al. Tuning the alloy degree for Pd-M/Al2O3 (M=Co/ Ni /Cu) bimetallic catalysts to enhance the activity and selectivity of dodecahydro-N-ethylcarbazole dehydrogenation[J]. International Journal of Hydrogen Energy, 2021, 46(68): 33835-33848. |
| 57 | Jiang Z, Guo S Y, Fang T. Enhancing the catalytic activity and selectivity of PdAu/SiO2 bimetallic catalysts for dodecahydro-N-ethylcarbazole dehydrogenation by controlling the particle size and dispersion[J]. ACS Applied Energy Materials, 2019, 2(10): 7233-7243. |
| 58 | Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Physical Review B, Condensed Matter, 1996, 54(16): 11169-11186. |
| 59 | Kresse G. Ab initio molecular dynamics for liquid metals[J]. Journal of Non-Crystalline Solids, 1995, 192/193: 222-229. |
| 60 | Perdew J P, Burke K, Ernzerhof M. Generalized gradient approximation made simple[J]. Physical Review Letters, 1996, 77(18): 3865-3868. |
| 61 | Kresse G, Joubert D. From ultrasoft pseudopotentials to the projector augmented-wave method[J]. Physical Review B, 1999, 59(3): 1758-1775. |
| 62 | Lu S L, Menning C A, Zhu Y X, et al. Correlating benzene hydrogenation activity with binding energies of hydrogen and benzene on co-based bimetallic catalysts[J]. ChemPhysChem, 2009, 10(11): 1763-1765 |
| 63 | Chen S T, Jenkins S V, Tao J, et al. Anisotropic seeded growth of Cu-M (M = Au, Pt, or Pd) bimetallic nanorods with tunable optical and catalytic properties[J]. The Journal of Physical Chemistry C, 2013, 117(17): 8924-8932. |
| 64 | Leppert L, Albuquerque R Q, Küemmel S. Gold-platinum alloys and Vegard's law on the nanoscale[J]. Physical Review B, Condensed Matter, 2012, 86(24): 241401-241403. |
| 65 | Zhang J, Dong Y N, Liu Q X, et al. Hierarchically alloyed Pd-Cu microarchitecture with tunable shapes: morphological engineering, and catalysis for hydrogen evolution reaction of ammonia borane[J]. International Journal of Hydrogen Energy, 2019, 44(57): 30226-30236. |
| 66 | Stefanov P, Todorova S, Naydenov A, et al. On the development of active and stable Pd-Co/γ-Al2O3 catalyst for complete oxidation of methane[J]. Chemical Engineering Journal, 2015, 266: 329-338. |
| 67 | Yuan E X, Wu C, Hou X, et al. Synergistic effects of second metals on performance of (Co, Ag, Cu)-doped Pd/Al2O3 catalysts for 2-ethyl-anthraquinone hydrogenation[J]. Journal of Catalysis, 2017, 347: 79-88. |
| 68 | Shukla A K, Neergat M, Bera P, et al. An XPS study on binary and ternary alloys of transition metals with platinized carbon and its bearing upon oxygen electroreduction in direct methanol fuel cells[J]. Journal of Electroanalytical Chemistry, 2001, 504(1): 111-119. |
| 69 | Aricò A S, Cretì P, Modica E, et al. Investigation of direct methanol fuel cells based on unsupported Pt-Ru anode catalysts with different chemical properties[J]. Electrochimica Acta, 2000, 45(25/26): 4319-4328. |
| 70 | Li Y B, Zhang C B, Ma J Z, et al. High temperature reduction dramatically promotes Pd/TiO2 catalyst for ambient formaldehyde oxidation[J]. Applied Catalysis B: Environmental, 2017, 217: 560-569. |
| 71 | Sotoodeh F, Smith K J. Analysis of H2 release from organic polycyclics over Pd catalysts using DFT[J]. The Journal of Physical Chemistry C, 2013, 117(1): 194-204. |
| 72 | Zhu M Y, Xu L X, Du L, et al. Palladium supported on carbon nanotubes as a high-performance catalyst for the dehydrogenation of dodecahydro-N-ethylcarbazole[J]. Catalysts, 2018, 8(12): 638. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [3] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [4] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [8] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [9] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [10] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [11] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [12] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [13] | 李科, 文键, 忻碧平. 耦合蒸气冷却屏的真空多层绝热结构对液氢储罐自增压过程的影响机制研究[J]. 化工学报, 2023, 74(9): 3786-3796. |
| [14] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [15] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号