化工学报 ›› 2024, Vol. 75 ›› Issue (9): 3011-3027.DOI: 10.11949/0438-1157.20240070
杨子驰1( ), 谢冰琪2(
), 谢冰琪2( ), 石瑞莘1, 雷虹1, 陈晨1, 周才金1,2(
), 石瑞莘1, 雷虹1, 陈晨1, 周才金1,2( ), 张吉松2(
), 张吉松2( )
)
收稿日期:2024-01-15
修回日期:2024-03-24
出版日期:2024-09-25
发布日期:2024-10-10
通讯作者:
周才金,张吉松
作者简介:杨子驰(1999—),男,硕士研究生,1943478473@qq.com基金资助:
Zichi YANG1( ), Bingqi XIE2(
), Bingqi XIE2( ), Ruixin SHI1, Hong LEI1, Chen CHEN1, Caijin ZHOU1,2(
), Ruixin SHI1, Hong LEI1, Chen CHEN1, Caijin ZHOU1,2( ), Jisong ZHANG2(
), Jisong ZHANG2( )
)
Received:2024-01-15
Revised:2024-03-24
Online:2024-09-25
Published:2024-10-10
Contact:
Caijin ZHOU, Jisong ZHANG
摘要:
近年来,套管膜式微反应器由于具有传质距离短、气液接触面积大和高透气性等优点,极大地提高了气液传质和反应速度,被视为加快气液传质和流动反应过程的强大工具。目前,套管膜式微反应器已被应用于建立快速准确的气液体系基本参数流动测量平台、实现高效安全的气液反应过程和强化气体介导的生物酶催化反应过程。详细介绍了套管膜式微反应器的构造、组装和操作方式,重点概述了该反应器在不同气液传质-反应过程中的最新研究进展。最后,结合当前的研究热点,展望了套管膜式微反应器的进一步开发方向和未来潜在的应用领域。
中图分类号:
杨子驰, 谢冰琪, 石瑞莘, 雷虹, 陈晨, 周才金, 张吉松. 套管膜式微反应器内高效安全的气液传质-反应过程研究进展[J]. 化工学报, 2024, 75(9): 3011-3027.
Zichi YANG, Bingqi XIE, Ruixin SHI, Hong LEI, Chen CHEN, Caijin ZHOU, Jisong ZHANG. Research progress on efficient and safe gas-liquid mass transfer and reaction processes in tube-in-tube reactor[J]. CIESC Journal, 2024, 75(9): 3011-3027.
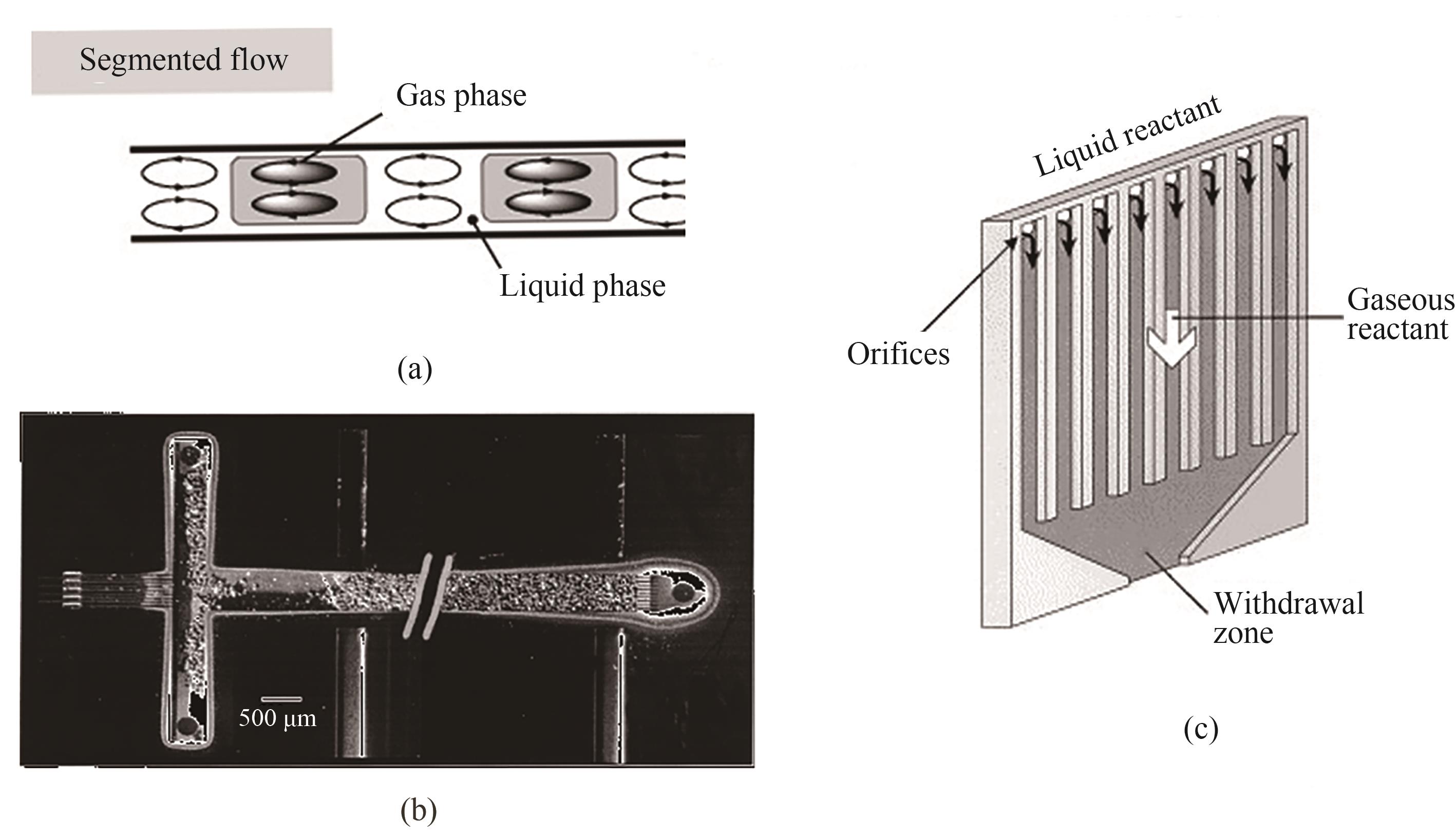
图1 常见的用于强化气液传质的反应器:(a)微通道反应器[15];(b)降膜反应器[16];(c)微填充床反应器[17]
Fig.1 The common microreactors used to enhance gas-liquid mass transfer: (a) microchannel reactor[15]; (b) falling film reactor[16]; (c) micropacked bed reactor[17]

图2 常见的套管膜式微反应器装置:(a)聚四氟乙烯外管;(b)不锈钢外管[32, 36]
Fig.2 The two typical configures of the tube-in-tube reactor: (a) polytetrafluoroethylene material outer tube; (b) stainless steel outer tube[32, 36]
图3 套管膜式微反应器内常见的气液两相流动形式:(a)气体在外管流动和液体在内管流动;(b)气体在内管流动和液体在外管流动[40]
Fig.3 The common gas-liquid two-phase flow pattern in tube-in-tube microreactors: (a) gas flows in the outer tube and liquid flows in the inner tube; (b) gas flows in the inner tube and liquid flows in the outer tube[40]
| 反应器类型 | a/(m2/m3) | kLa×102/s-1 |
|---|---|---|
| 套管膜式反应器[ | 3000~10000 | 10~100 |
| 鼓泡塔反应器[ | 50~600 | 0.5~24 |
| 填充柱反应器[ | 10~1700 | 0.04~102 |
| 管式反应器[ | 10~1000 | 0.5~100 |
| Taylor-Couette反应器[ | 50~2000 | 3~21 |
| 冲击射流吸收器[ | 200~1200 | 2.5~122 |
| 喷雾柱设备[ | 75~170 | 1.5~2.2 |
| 搅拌釜式反应器[ | 10~2000 | 0.5~20 |
| 静态混合器[ | 100~1000 | 10~250 |
| 降膜微反应器[ | 3400~9000 | 30~2100 |
| 微填充床反应器 | 1945~5464 | 1.2~50 |
| Y型微通道反应器[ | 9000 | 0.3~21 |
表1 不同反应器比表面积和气液传质系数的比较
Table 1 Comparison of specific surface area and gas-liquid mass transfer coefficient of different reactors
| 反应器类型 | a/(m2/m3) | kLa×102/s-1 |
|---|---|---|
| 套管膜式反应器[ | 3000~10000 | 10~100 |
| 鼓泡塔反应器[ | 50~600 | 0.5~24 |
| 填充柱反应器[ | 10~1700 | 0.04~102 |
| 管式反应器[ | 10~1000 | 0.5~100 |
| Taylor-Couette反应器[ | 50~2000 | 3~21 |
| 冲击射流吸收器[ | 200~1200 | 2.5~122 |
| 喷雾柱设备[ | 75~170 | 1.5~2.2 |
| 搅拌釜式反应器[ | 10~2000 | 0.5~20 |
| 静态混合器[ | 100~1000 | 10~250 |
| 降膜微反应器[ | 3400~9000 | 30~2100 |
| 微填充床反应器 | 1945~5464 | 1.2~50 |
| Y型微通道反应器[ | 9000 | 0.3~21 |
图6 周期性心形结构反应器的放大图:(a)反应器内混合单元的前视图;(b)心形结构反应器图;(c)单个流动反应器的横截面图[56]
Fig.6 The amplification process diagram of periodic heart-shaped structure reactor: (a) front view of the mixing unit inside the reactor; (b) diagram of heart-shaped reactor; (c) cross section view of a single flow reactor[56]
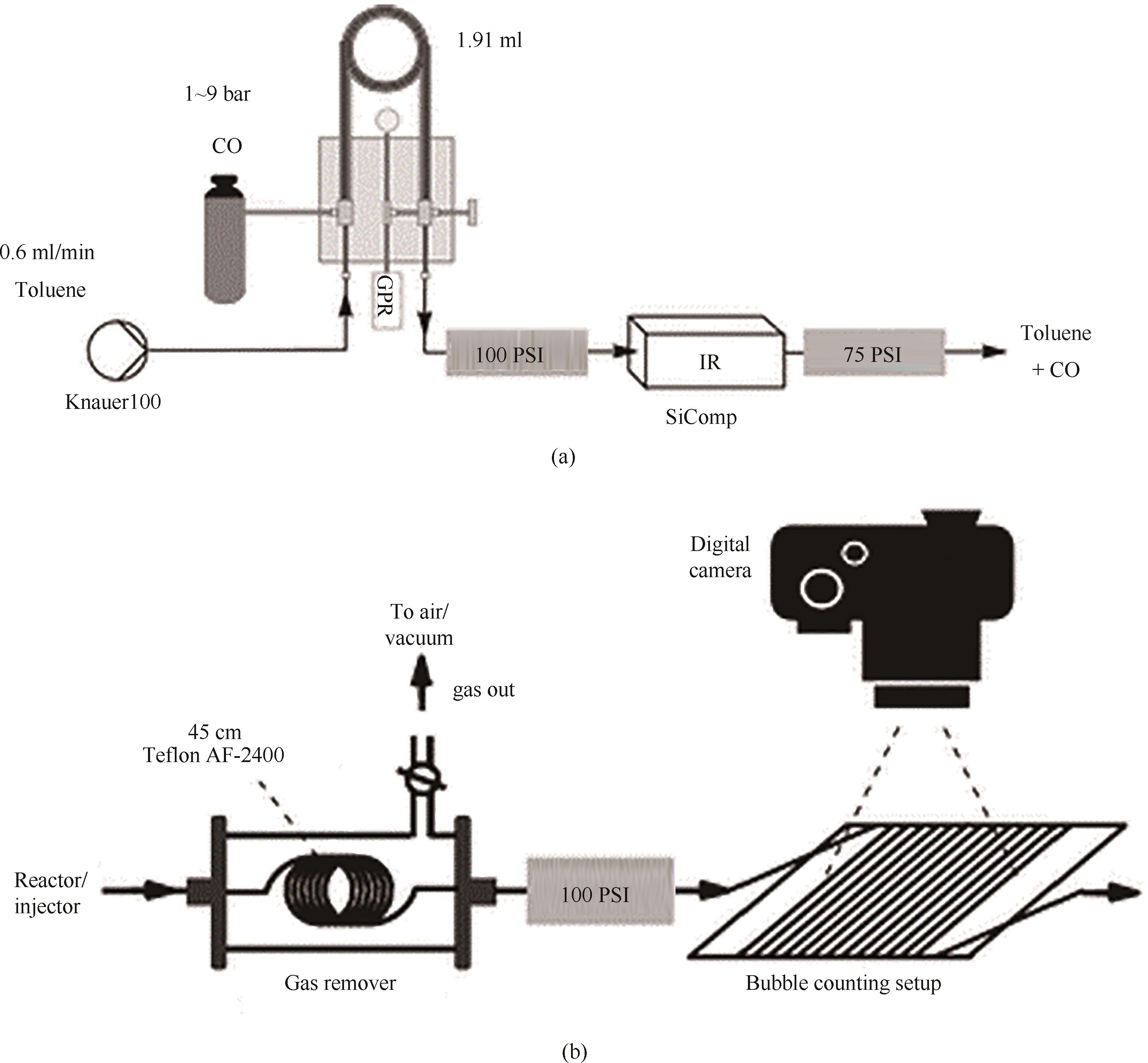
图7 基于套管膜式微反应器在线测量气体溶解度平台:(a)利用傅里叶变换红外技术在线监测溶剂中溶解的CO气体浓度变化的装置[63];(b)气泡计数法在线测定溶剂中H2气体浓度[64](1 bar=0.1 MPa)
Fig.7 An online gas solubility measurement platform based on a tube-in-tube microreactor: (a) a device for monitoring the concentration change of dissolved CO gas using Fourier transform infrared technology[63]; (b) online determination of H2 gas concentration in solvents using bubble counting method[64]
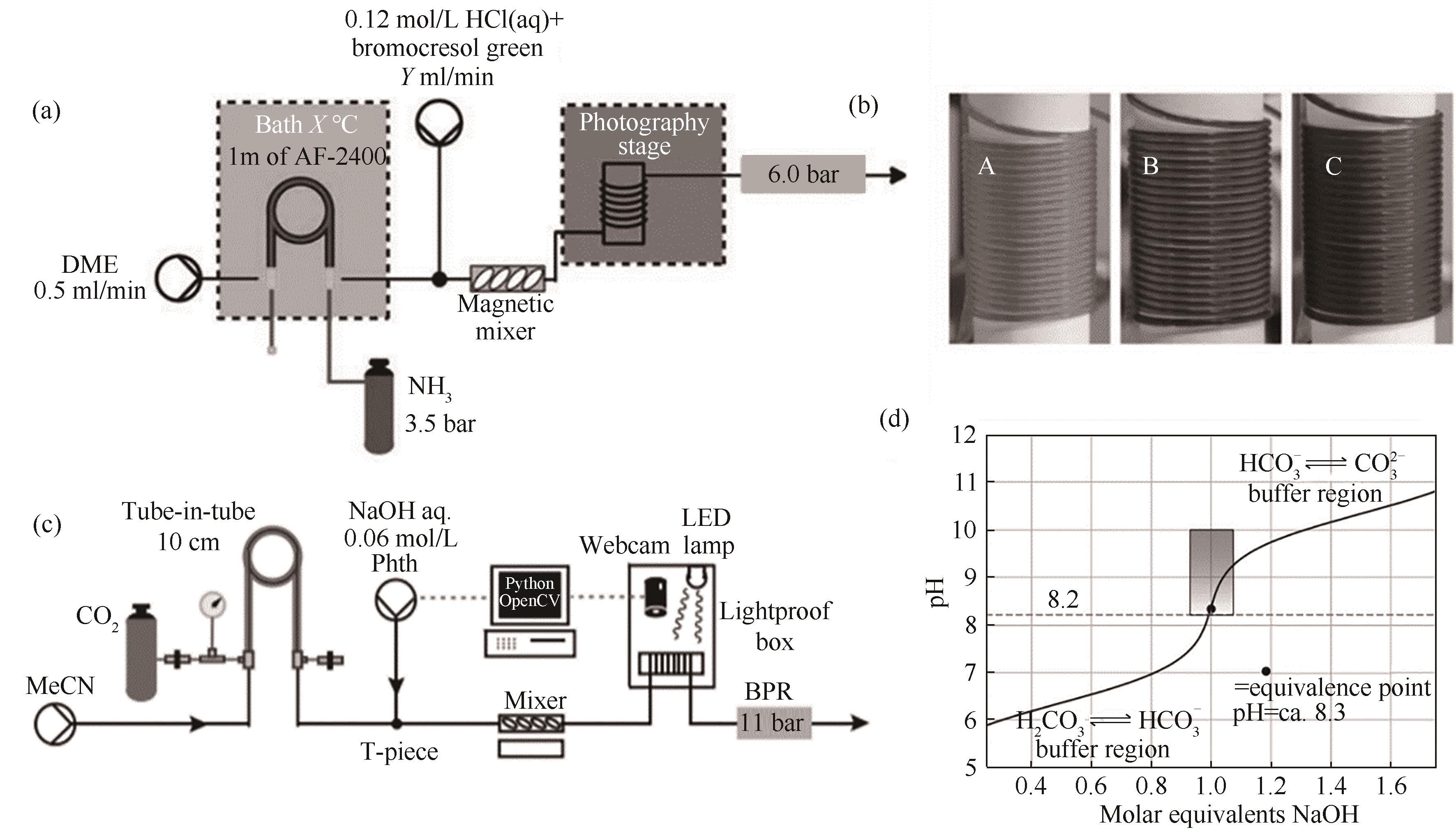
图8 套管膜式膜反应器内在线滴定法测量气体溶解度装置:(a)在线比色滴定技术测定溶剂中NH3气体浓度装置[66];(b)不同pH下反应器出口的溶液颜色变化[66];(c)比色滴定技术检测有机溶剂中CO2气体浓度[68];(d)溶液中CO2气体的滴定曲线[68]
Fig.8 The device for measuring gas solubility using online titration method in a tube-in-tube microreactor: (a) device for measuring NH3 gas concentration in solvents using online colorimetric titration technology[66]; (b) color change of the solution with different pH at the reactor outlet[66]; (c) colorimetric titration technology for detecting the concentration of CO2 gas in organic solvents[68]; (d) titration curve of CO2 gas in solution[68]
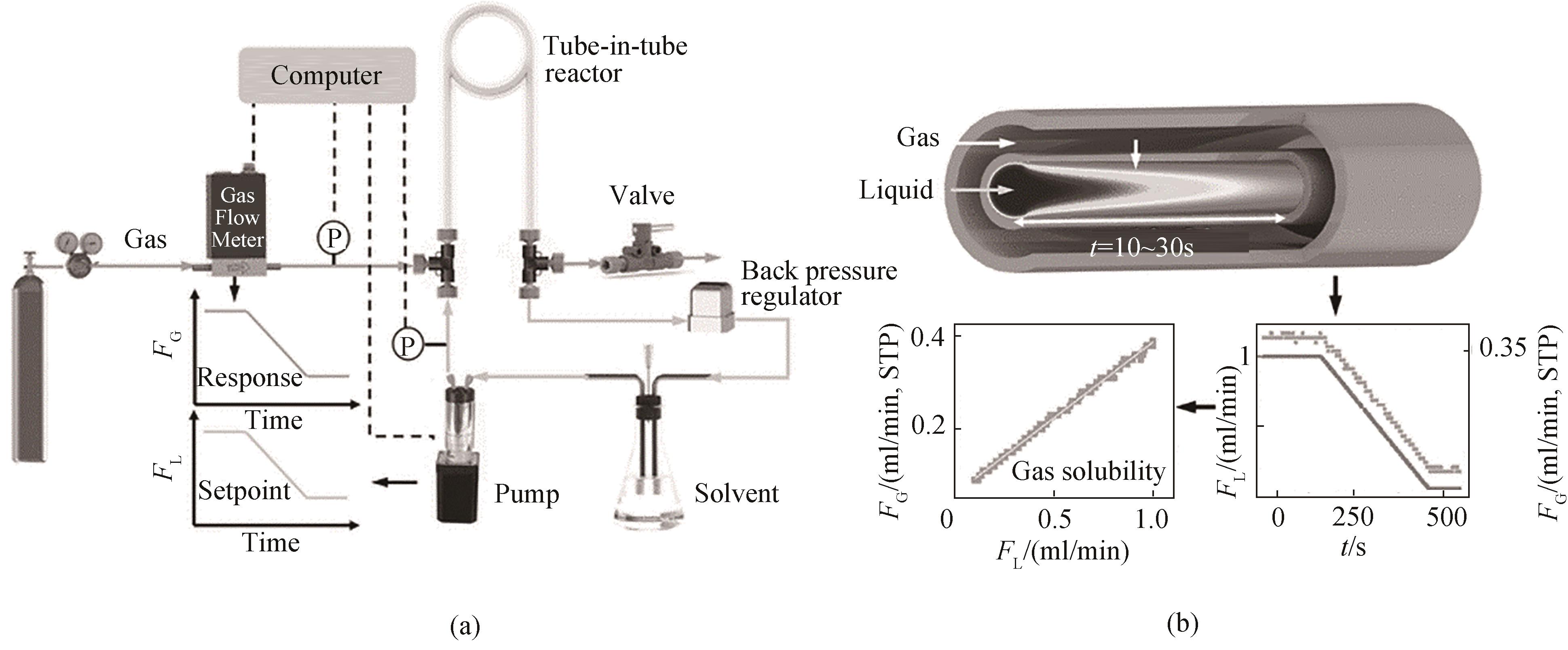
图9 套管膜式反应器内测量气体溶解度[62]:(a)实验装置;(b)液体流量和气体流量随时间的变化关系(STP表示标准状况)
Fig.9 Measurement of gas solubility in a tube-in-tube reactor[62]: (a) experimental setup; (b) the variation of liquid flow rate and gas flow rate over time (STP stands for standard temperature and pressure)
| 气体 | 液体 | 温度/℃ | KH测量值/(mol/(m2·bar)) | KH文献值/(mol/(m2·bar)) | 相对误差/% |
|---|---|---|---|---|---|
| H2 | 甲醇 | 23 | 3.83±0.02[ | 3.88[ | 1.3 |
| H2 | 甲醇 | 40 | 4.24±0.02[ | 4.26[ | 0.5 |
| H2 | 甲醇 | 60 | 4.70±0.02[ | 4.62[ | 1.7 |
| H2 | 庚烷 | 23 | 4.51±0.02[ | 4.60[ | 2.0 |
| H2 | 甲基苯乙烯 | 23 | 2.51±0.01[ | 2.56[ | 1.9 |
| N2 | 甲醇 | 23 | 6.46±0.03[ | 6.60[ | 2.0 |
| O2 | 甲醇 | 23 | 9.87±0.05[ | 9.72[ | 1.6 |
| CO2 | 甲醇 | 23 | 99.8±0.5[ | 98.7[ | 1.1 |
表2 套管膜式反应器内气体溶解度测量值与文献值的比较
Table 2 Comparison of gas solubility measurement values in tube-in-tube reactor with literature values
| 气体 | 液体 | 温度/℃ | KH测量值/(mol/(m2·bar)) | KH文献值/(mol/(m2·bar)) | 相对误差/% |
|---|---|---|---|---|---|
| H2 | 甲醇 | 23 | 3.83±0.02[ | 3.88[ | 1.3 |
| H2 | 甲醇 | 40 | 4.24±0.02[ | 4.26[ | 0.5 |
| H2 | 甲醇 | 60 | 4.70±0.02[ | 4.62[ | 1.7 |
| H2 | 庚烷 | 23 | 4.51±0.02[ | 4.60[ | 2.0 |
| H2 | 甲基苯乙烯 | 23 | 2.51±0.01[ | 2.56[ | 1.9 |
| N2 | 甲醇 | 23 | 6.46±0.03[ | 6.60[ | 2.0 |
| O2 | 甲醇 | 23 | 9.87±0.05[ | 9.72[ | 1.6 |
| CO2 | 甲醇 | 23 | 99.8±0.5[ | 98.7[ | 1.1 |
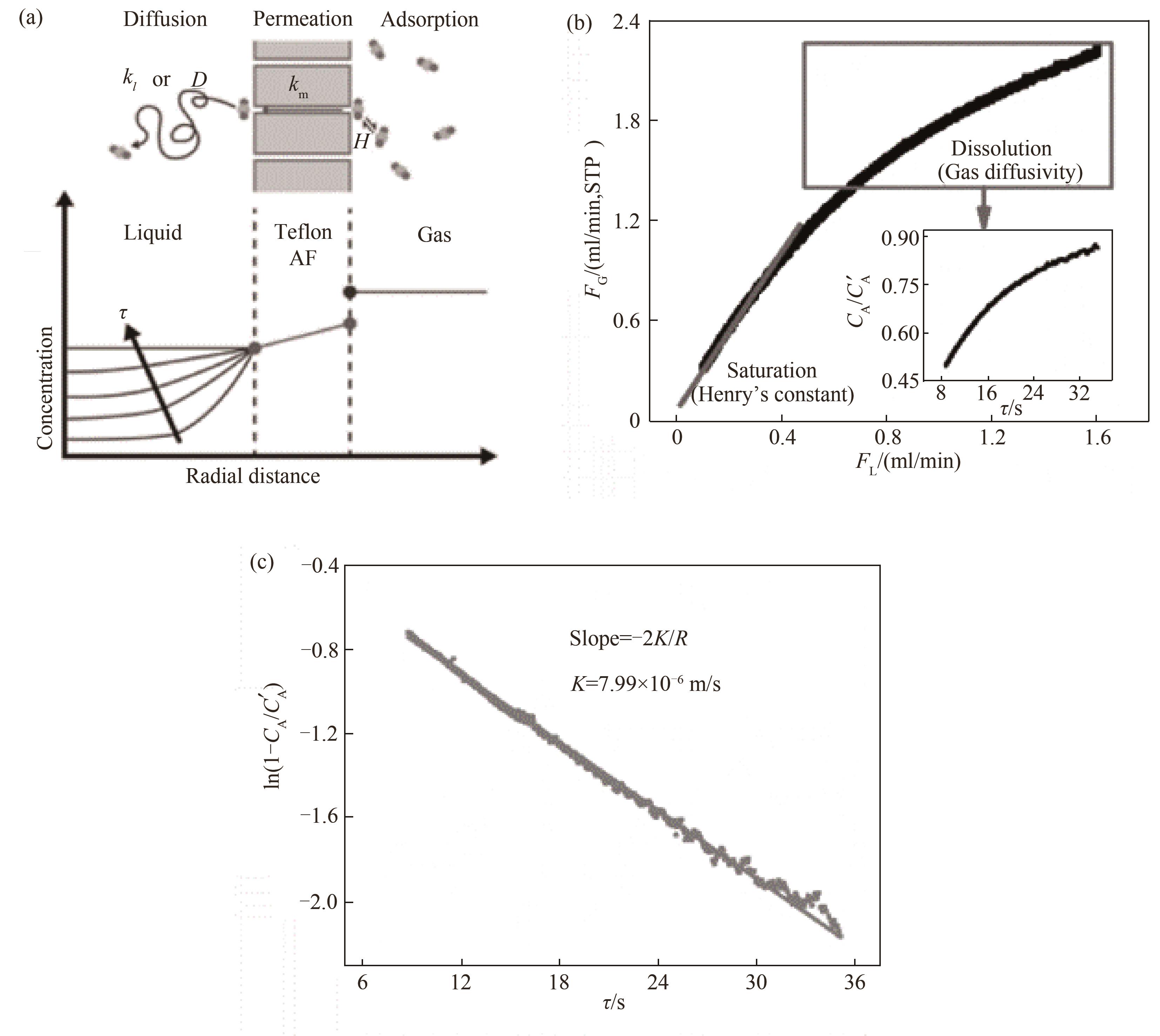
图10 套管膜式反应器用于测量气体在液体中的扩散系数:(a)反应器内气体扩散过程示意图;(b)Ramping过程中液体流速随气体流速的变化;(c)溶解气体浓度随停留时间的变化[76]
Fig.10 Measuring the diffusion coefficient of gas in liquid with tube-in-tube reactor: (a) schematic diagram of gas diffusion process in the reactor; (b) the variation of liquid flow rate with gas flow rate in the Ramping process; (c) the variation of dissolved gas concentration with residence time[76]
| Ramping时间/min | Ramping速度/ (ml/min) | 气体扩散稀释/ (10-9 m2/s) | 误差/% |
|---|---|---|---|
| 90 | 0.014 | 1.97 | 0 |
| 60 | 0.022 | 2.00 | 1.5 |
| 40 | 0.033 | 1.91 | 3.0 |
| 30 | 0.043 | 2.18 | 10.7 |
| 20 | 0.065 | 2.69 | 36.5 |
| 10 | 0.130 | 6. 00 | 204.6 |
表3 不同Ramping时间下CO2气体在水中扩散系数D的测量值[76]
Table 3 Measurement values of CO2 gas diffusion coefficient in water under different Ramping time[76]
| Ramping时间/min | Ramping速度/ (ml/min) | 气体扩散稀释/ (10-9 m2/s) | 误差/% |
|---|---|---|---|
| 90 | 0.014 | 1.97 | 0 |
| 60 | 0.022 | 2.00 | 1.5 |
| 40 | 0.033 | 1.91 | 3.0 |
| 30 | 0.043 | 2.18 | 10.7 |
| 20 | 0.065 | 2.69 | 36.5 |
| 10 | 0.130 | 6. 00 | 204.6 |
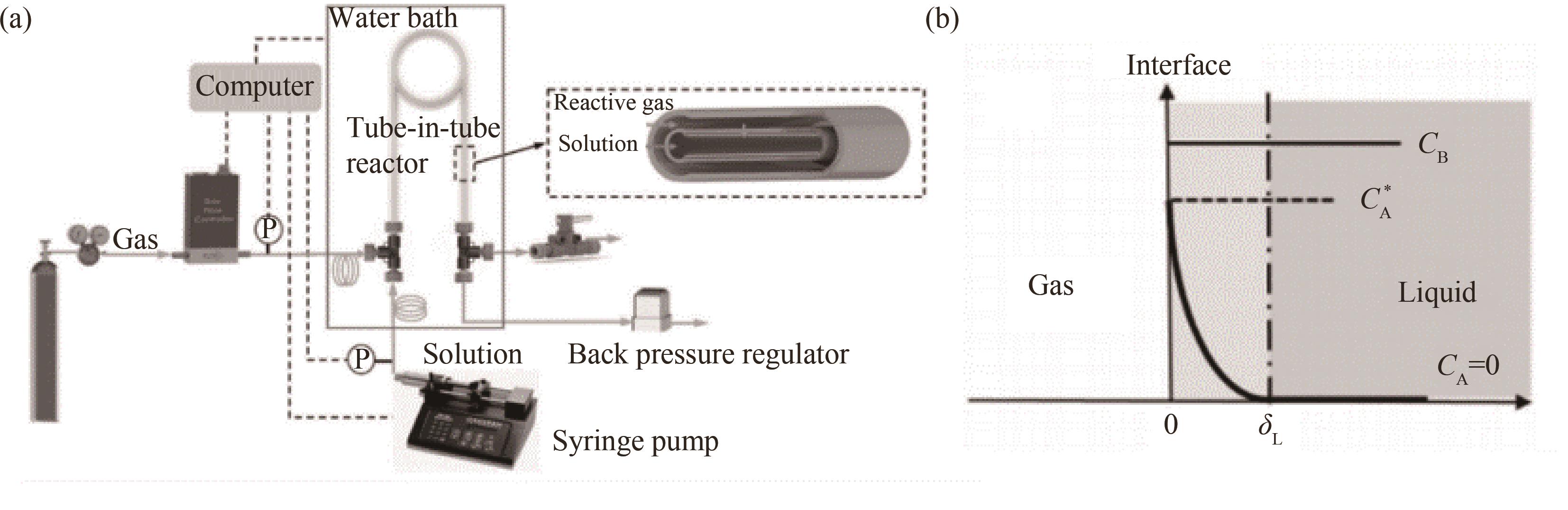
图11 套管膜式膜反应器内反应动力学测量概述:(a)气液反应动力学常数测定平台装置;(b)液膜内气体浓度的分布
Fig.11 Overview of reaction kinetics measurement in a tube-in-tube microreactor: (a) platform for measuring the kinetic constants of gas-liquid reactions; (b) the distribution of gas concentration in the liquid film
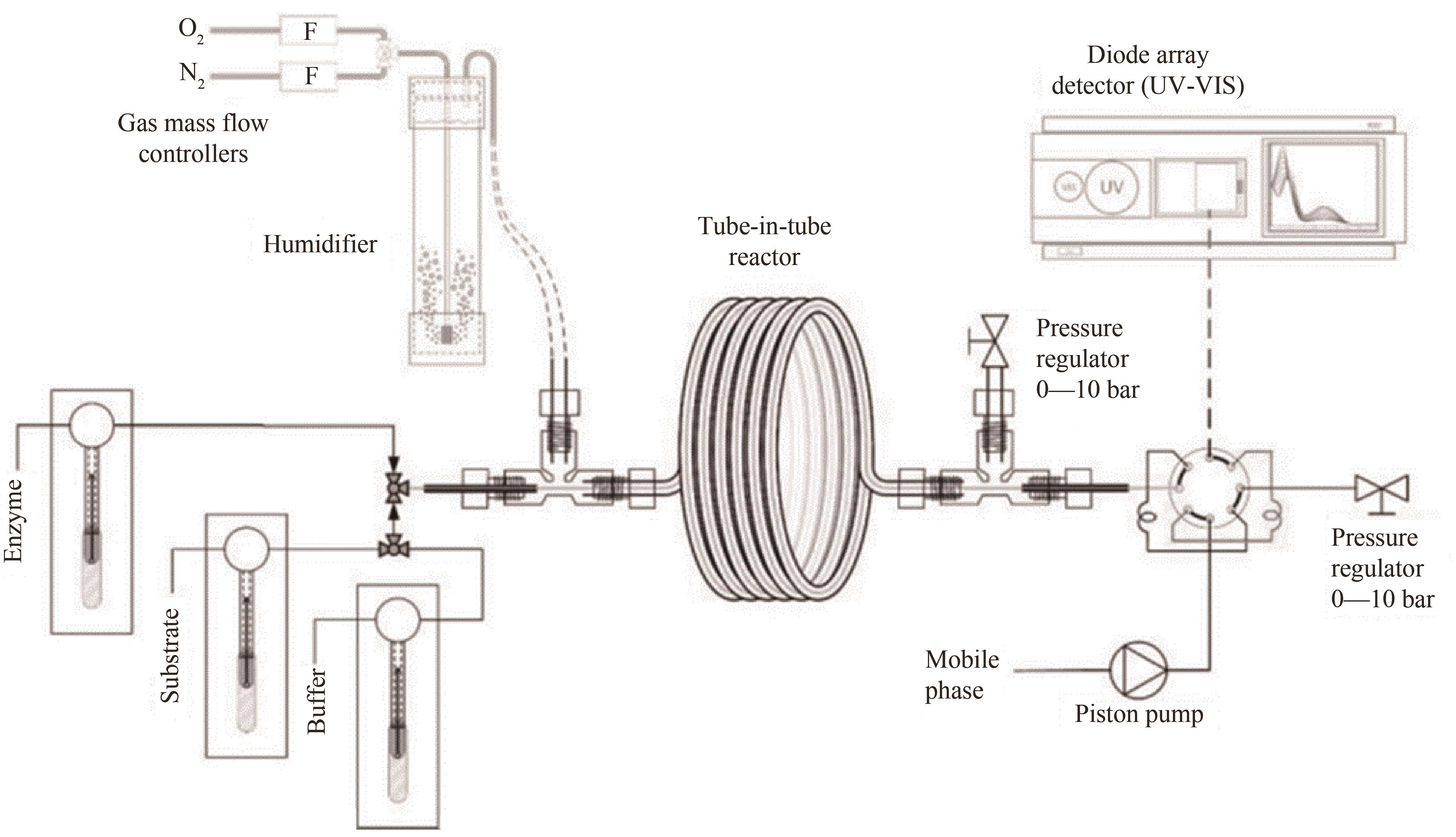
图12 套管膜式膜反应器内测定酶催化反应动力学的实验装置平台[84]
Fig.12 Experimental setup platform for measuring enzyme catalytic reaction kinetics in a tube-in-tube microreactor[84]
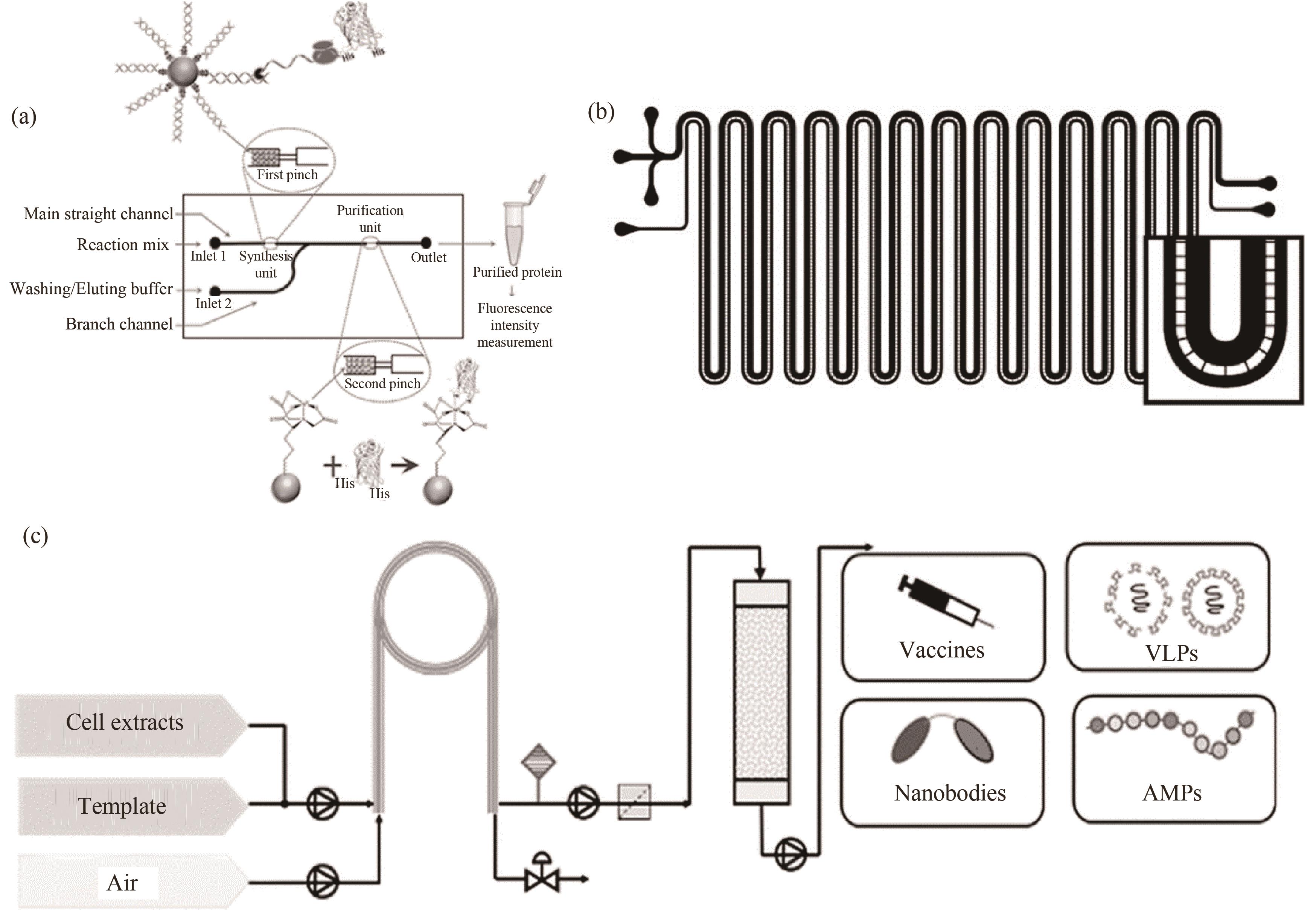
图13 基于微反应器的无细胞蛋白合成装置:(a)基于微芯片反应器开发的无细胞蛋白合成平台装置[93];(b)高透气性纳米膜微通道反应器用于蛋白质合成[87];(c)基于套管膜式微反应器的无细胞蛋白合成平台[95]
Fig.13 A cell free protein synthesis device based on a microreactor: (a) device of a developed cell-free protein synthesis platform based on a microchip reactor[93]; (b) a microchannel reactor with highly breathable nanofilms for protein synthesis[87]; (c) cell-free protein synthesis platform based on tube-in-tube microreactor[95]
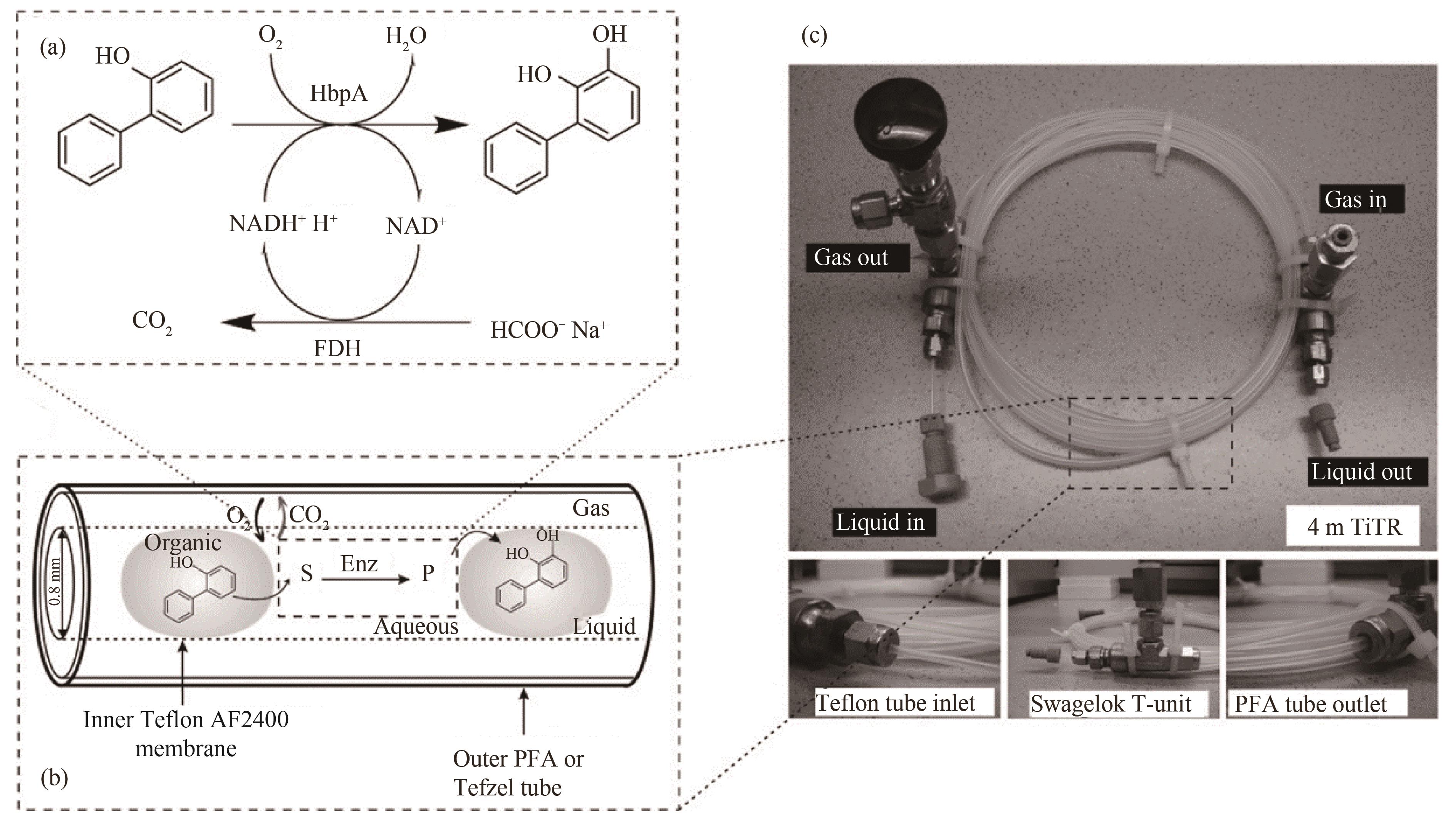
图14 套管膜式微反应器用于生物氧化过程:(a)酶催化氧化合成邻苯二酚的反应机理;(b)套管膜式微反应器内水、有机两相液体分段流动示意图;(c)连续分段流套管膜式反应器装置[96]
Fig.14 Tube-in-tube microreactor for biological oxidation process: (a) the reaction mechanism of enzymatic oxidation to synthesize catechol; (b) diagram of segmented flow of water and organic two-phase liquids in a tube in tube membrane reactor; (c) device of continuous segmented flow tube-in-tube reactor[96]
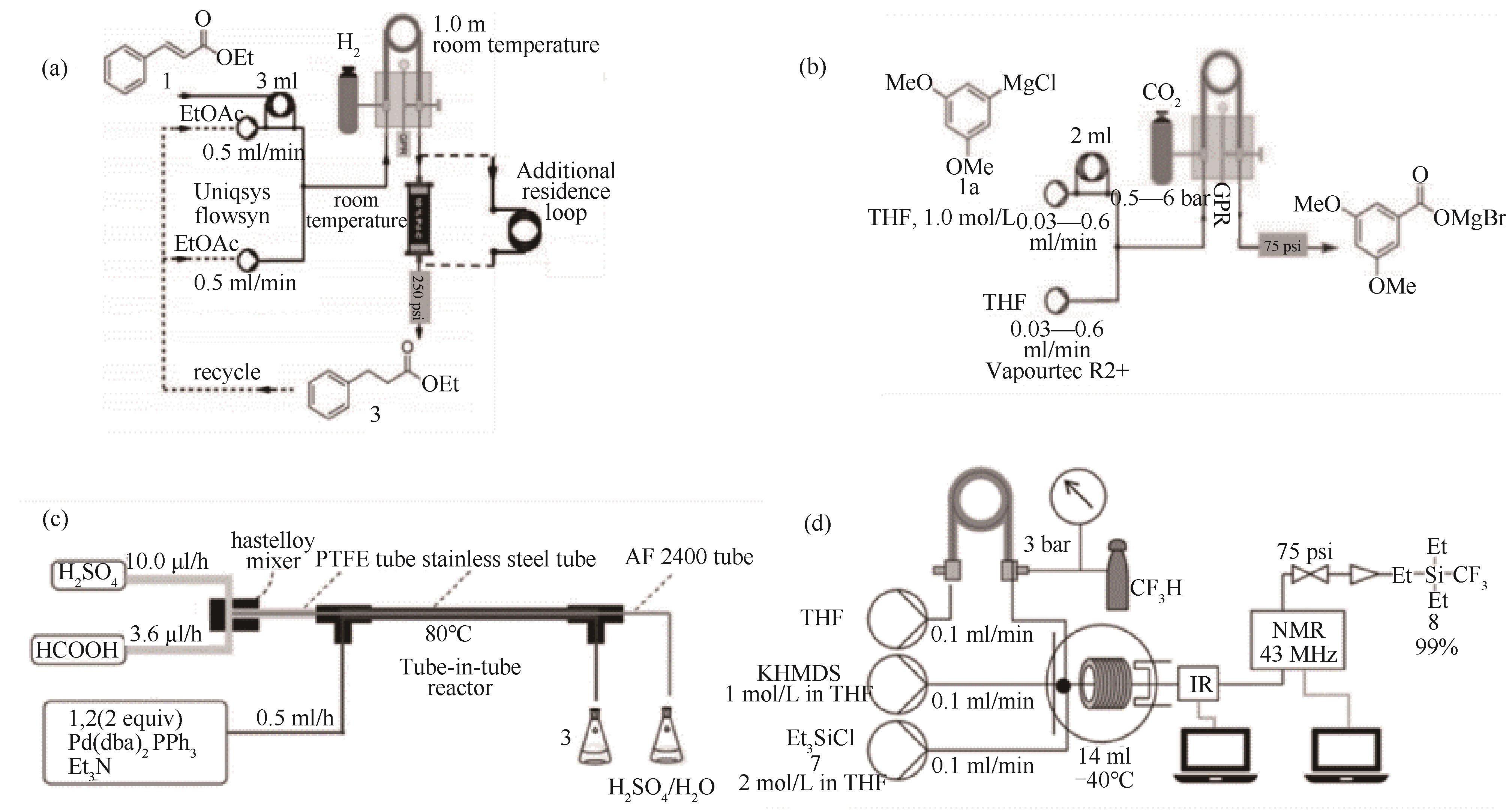
图15 基于套管膜式反应器的高效安全的气液反应平台:(a)套管膜式反应器用于加氢反应[66];(b)套管膜式反应器内利用CO2气体进行羧基化反应装置[101];(c)套管膜式微反应器用于甲酸/硫酸羰基化反应[102];(d)用于三氟甲基化的流动化学平台装置示意图[103](1 psi=6894.757 Pa)
Fig.15 Efficient and safe gas-liquid reaction platform based on tube-in-tube reactor: (a) a tube-in-tube microreactor for hydrogenation reaction[66]; (b) a tube-in-tube reactor was used for carboxylation reaction with CO2 gas[101]; (c) a tube-in-tube microreactor for formic acid/sulfuric acid carbonylation reaction[102]; (d) schematic diagram of a flow chemistry platform for trifluoromethylation[103](1 psi=6894.757 Pa)
| 1 | 娄锋炎, 尹佳滨, 段笑南, 等. 连续微反应加氢技术在脱保护反应中的应用[J]. 化工学报, 2021, 72(2): 761-771. |
| Lou F Y, Yin J B, Duan X N, et al. Application of continuous micro-reaction hydrogenation technology in deprotection reaction[J]. CIESC Journal, 2021, 72(2): 761-771. | |
| 2 | Mercadante M, Kelly C B, Leadbeater N E. Continuous flow hydrogenation using an on-demand gas delivery reactor[J]. Organic Process Research & Development, 2012, 16(5): 1064-1068. |
| 3 | 高伟, 卜宁, 卢元. 无细胞体系非天然蛋白质合成研究进展[J]. 生物工程学报, 2018, 34(9): 1371-1385. |
| Gao W, Bu N, Lu Y. Recent advances in cell-free unnatural protein synthesis[J]. Journal of Biological Engineering, 2018, 34(9): 1371-1385. | |
| 4 | Nelson J A D, Barnett R J, Hunt J P, et al. Hydrofoam and oxygen headspace bioreactors improve cell‐free therapeutic protein production yields through enhanced oxygen transport[J]. Biotechnology Progress, 2021, 37(2): e3079. |
| 5 | Tamborini L, Fernandes P, Paradisi F, et al. Flow bioreactors as complementary tools for biocatalytic process intensification[J]. Trends in Biotechnology, 2018, 36(1): 73-88. |
| 6 | Britton J, Majumdar S, Weiss G A. Continuous flow biocatalysis[J]. Chemical Society Reviews, 2018, 47(15): 5891-5918. |
| 7 | Park C P, Maurya R A, Lee J H, et al. Efficient photosensitized oxygenations in phase contact enhanced microreactors[J]. Lab on a Chip, 2011, 11(11): 1941-1945. |
| 8 | Park C P, Kim D P. Dual-channel microreactor for gas-liquid syntheses[J]. Journal of the American Chemical Society, 2010, 132(29): 10102-10106. |
| 9 | Yavorskyy A, Shvydkiv O, Limburg C, et al. Photooxygenations in a bubble column reactor[J]. Green Chemistry, 2012, 14(4): 888-892. |
| 10 | Shaikh A, Al-dahhan M. Scale-up of bubble column reactors: a review of current state-of-the-art[J]. Industrial & Engineering Chemistry Research, 2013, 52(24): 8091-8108. |
| 101 | Polyzos A, O’brien M, Petersen T P, et al. The continuous-flow synthesis of carboxylic acids using CO2 in a tube-in-tube gas permeable membrane reactor[J]. Angewandte Chemie International Edition, 2011, 50(5): 1190-1193. |
| 102 | Brancour C, Fukuyama T, Mukai Y, et al. Modernized low pressure carbonylation methods in batch and flow employing common acids as a CO source[J]. Organic Letters, 2013, 15(11): 2794-2797. |
| 103 | Musio B, Gala E, Ley S V. Real-time spectroscopic analysis enabling quantitative and safe consumption of fluoroform during nucleophilic trifluoromethylation in flow[J]. ACS Sustainable Chemistry & Engineering, 2017, 6(1): 1489-1495. |
| 104 | Dallinger D, Pinho V D, Gutmann B, et al. Laboratory-scale membrane reactor for the generation of anhydrous diazomethane[J]. The Journal of Organic Chemistry, 2016, 81(14): 5814-5823. |
| 11 | Elvira K S, Solvas X C i, Wootton R C R, et al. The past, present and potential for microfluidic reactor technology in chemical synthesis[J]. Nature Chemistry, 2013, 5(11): 905-915. |
| 12 | Günther A, Jensen K F. Multiphase microfluidics: from flow characteristics to chemical and materials synthesis[J]. Lab on a Chip, 2006, 6(12): 1487-1503. |
| 13 | Wiles C, Watts P. Continuous flow reactors: a perspective[J]. Green Chemistry, 2012, 14(1): 38-54. |
| 14 | Adamo A, Beingessner R L, Behnam M, et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system[J]. Science, 2016, 352(6281): 61-67. |
| 15 | Noël T, Hessel V. Membrane microreactors: gas-liquid reactions made easy[J]. ChemSusChem, 2013, 6(3): 405-407. |
| 16 | Losey M W, Schmidt M A, Jensen K F. Microfabricated multiphase packed-bed reactors: characterization of mass transfer and reactions[J]. Industrial & Engineering Chemistry Research, 2001, 40(12): 2555-2562. |
| 17 | Jähnisch K, Baerns M, Hessel V, et al. Direct fluorination of toluene using elemental fluorine in gas/liquid microreactors[J]. Journal of Fluorine Chemistry, 2000, 105(1): 117-128. |
| 18 | Rahman M T, Fukuyama T, Kamata N, et al. Low pressure Pd-catalyzed carbonylation in an ionic liquid using a multiphase microflow system[J]. Chemical Communications, 2006(21): 2236-2238. |
| 19 | Miller P W, Jennings L E, Demello A J, et al. A microfluidic approach to the rapid screening of palladium-catalysed aminocarbonylation reactions[J]. Advanced Synthesis & Catalysis, 2009, 351(18): 3260-3268. |
| 20 | Kobayashi J, Mori Y, Okamoto K, et al. A microfluidic device for conducting gas-liquid-solid hydrogenation reactions[J]. Science, 2004, 304(5675): 1305-1308. |
| 21 | Mehrjouei M, Müller S, Möller D. Removal of fuel oxygenates from water using advanced oxidation technologies by means of falling film reactor[J]. Chemical Engineering Journal, 2012, 211: 353-359. |
| 22 | Cantu-Perez A, Ziegenbalg D, Löb P, et al. Microstructure-based intensification of a falling film microreactor through optimal film setting with realistic profiles and in-channel induced mixing[J]. Chemical Engineering Journal, 2012, 179: 318-329. |
| 23 | Zhang J, Teixeira A R, Jensen K F. Automated measurements of gas-liquid mass transfer in micropacked bed reactors[J]. AIChE Journal, 2018, 64(2): 564-570. |
| 24 | Mo Y, Imbrogno J, Zhang H, et al. Scalable thin-layer membrane reactor for heterogeneous and homogeneous catalytic gas-liquid reactions[J]. Green Chemistry, 2018, 20(16): 3867-3874. |
| 25 | Gavriilidis A, Constantinou A, Hellgardt K, et al. Aerobic oxidations in flow: opportunities for the fine chemicals and pharmaceuticals industries[J]. Reaction Chemistry & Engineering, 2016, 1(6): 595-612. |
| 26 | Zhao C X, Middelberg A P. Two-phase microfluidic flows[J]. Chemical Engineering Science, 2011, 66(7): 1394-1411. |
| 27 | Martin L J, Marzinzik A L, Ley S V, et al. Safe and reliable synthesis of diazoketones and quinoxalines in a continuous flow reactor[J]. Organic Letters, 2011, 13(2): 320-323. |
| 28 | Müller S T, Wirth T. Diazo compounds in continuous-flow technology[J]. ChemSusChem, 2015, 8(2): 245-250. |
| 29 | Pastre J C, Browne D L, Ley S V. Flow chemistry syntheses of natural products[J]. Chemical Society Reviews, 2013, 42(23): 8849-8869. |
| 30 | Ramezani M, Kashfipour M A, Abolhasani M. Minireview: flow chemistry studies of high-pressure gas-liquid reactions with carbon monoxide and hydrogen[J]. Journal of Flow Chemistry, 2020, 10(1): 93-101. |
| 31 | Petersen T P, Polyzos A, O’brien M, et al. The oxygen-mediated synthesis of 1,3-butadiynes in continuous flow: using teflon AF-2400 to effect gas/liquid contact[J]. ChemSusChem, 2012, 5(2): 274-277. |
| 32 | Aka E C, Wimmer E, Barré E, et al. Comparing gas-liquid segmented and tube-in-tube setups for the aerobic dimerization of desmethoxycarpacine with an automated flow platform[J]. Organic Process Research & Development, 2020, 24(5): 745-751. |
| 33 | Zhou C, Xie B, Chen J, et al. Enhancement of gas-liquid mass transfer in curved membrane contactors with the generation of dean vortices[J]. Journal of Membrane Science, 2021, 636: 119592. |
| 34 | Cranwell P B, O’brien M, Browne D L, et al. Flow synthesis using gaseous ammonia in a Teflon AF-2400 tube-in-tube reactor: Paal-Knorr pyrrole formation and gas concentration measurement by inline flow titration[J]. Organic & Biomolecular Chemistry, 2012, 10(30): 5774-5779. |
| 35 | Zhu C, Raghuvanshi K, Coley C W, et al. Flow chemistry-enabled studies of rhodium-catalyzed hydroformylation reactions[J]. Chemical Communications, 2018, 54(62): 8567-8570. |
| 36 | Brzozowski M, O’brien M, Ley S V, et al. Flow chemistry: intelligent processing of gas-liquid transformations using a tube-in-tube reactor[J]. Accounts of Chemical Research, 2015, 48(2): 349-362. |
| 37 | Pinnau I, Toy L G. Gas and vapor transport properties of amorphous perfluorinated copolymer membranes based on 2,2-bistrifluoromethyl-4,5-difluoro-1,3-dioxole/tetrafluoroethylene[J]. Journal of Membrane Science, 1996, 109(1): 125-133. |
| 38 | Zhang H, Weber S G. Fluorous Chemistry: Teflon AF Materials[M]. Berlin: Springer, 2012: 307-337. |
| 39 | Zhang H, Wang S, Weber S G. Nanocomposite teflon AF 2400 films as tunable platforms for selective transport[J]. Analytical Chemistry, 2012, 84(22): 9920-9927. |
| 40 | Yang L, Jensen K F. Mass transport and reactions in the tube-in-tube reactor[J]. Organic Process Research & Development, 2013, 17(6): 927-933. |
| 41 | Mallia C J, Baxendale I R. The use of gases in flow synthesis[J]. Organic Process Research & Development, 2015, 20(2): 327-360. |
| 42 | Charpentier J C. Mass-transfer rates in gas-liquid absorbers and reactors[J]. Advances in Chemical Engineering, 1981, 11: 1-133. |
| 43 | Kies F K, Benadda B, Otterbein M. Experimental study on mass transfer of a co-current gas-liquid contactor performing under high gas velocities[J]. Chemical Engineering and Processing: Process Intensification, 2004, 43(11): 1389-1395. |
| 44 | Nieves-Remacha M J, Kulkarni A A, Jensen K F. Gas-liquid flow and mass transfer in an advanced-flow reactor[J]. Industrial & Engineering Chemistry Research, 2013, 52(26): 8996-9010. |
| 45 | Dłuska E, Wroński S, Ryszczuk T. Interfacial area in gas-liquid coquette-taylor flow reactor[J]. Experimental Thermal and Fluid Science, 2004, 28(5): 467-472. |
| 46 | Herskowits D, Herskowits V, Stephan K, et al. Characterization of a two-phase impinging jet absorber(Ⅱ): Absorption with chemical reaction of CO2 in NaOH solutions[J]. Chemical Engineering Science, 1990, 45(5): 1281-1287. |
| 47 | Yawalkar A A, Heesink A B M, Versteeg G F, et al. Gas-liquid mass transfer coefficient in stirred tank reactors[J]. The Canadian Journal of Chemical Engineering, 2008, 80(5): 840-848. |
| 48 | Heyouni A, Roustan M, Do-quang Z. Hydrodynamics and mass transfer in gas-liquid flow through static mixers[J]. Chemical Engineering Science, 2002, 57(16): 3325-3333. |
| 49 | Yue J, Chen G, Yuan Q, et al. Hydrodynamics and mass transfer characteristics in gas-liquid flow through a rectangular microchannel[J]. Chemical Engineering Science, 2007, 62(7): 2096-2108. |
| 50 | Sang L, Cao Q, Xie B, et al. Investigation of effective interfacial area in micropacked bed reactors[J]. Industrial & Engineering Chemistry Research, 2021, 60(25): 9206-9215. |
| 51 | Zhang J, Wang K, Teixeira A R, et al. Design and scaling up of microchemical systems: a review[J]. Annual Review of Chemical and Biomolecular Engineering, 2017, 8(1): 285-305. |
| 52 | Dong Z, Wen Z, Zhao F, et al. Scale-up of micro- and milli-reactors: an overview of strategies, design principles and applications[J]. Chemical Engineering Science, 2021, 10: 100097. |
| 53 | Su Y, Kuijpers K, Hessel V, et al. A convenient numbering-up strategy for the scale-up of gas-liquid photoredox catalysis in flow[J]. Reaction Chemistry & Engineering, 2016, 1(1): 73-81. |
| 54 | Wada Y, Schmidt M A, Jensen K F. Flow distribution and ozonolysis in gas-liquid multichannel microreactors[J]. Industrial & Engineering Chemistry Research, 2006, 45(24): 8036-8042. |
| 55 | Han T, Zhang L, Xu H, et al. Factory-on-chip: modularised microfluidic reactors for continuous mass production of functional materials[J]. Chemical Engineering Journal, 2017, 326: 765-773. |
| 56 | Nieves-Remacha M J, Kulkarni A A, Jensen K F. Hydrodynamics of liquid-liquid dispersion in an advanced-flow reactor[J]. Industrial & Engineering Chemistry Research, 2012, 51(50): 16251-16262. |
| 57 | Lefortier S G R, Hamersma P J, Bardow A, et al. Rapid microfluidic screening of CO2 solubility and diffusion in pure and mixed solvents[J]. Lab on a Chip, 2012, 12(18): 3387-3391. |
| 58 | Mellein B R, Scurto A M, Shiflett M B. Gas solubility in ionic liquids[J]. Chemical reviews, 2014, 114(2): 1289-1326. |
| 59 | Talebi S, Abedini A, Lele P, et al. Microfluidics-based measurement of solubility and diffusion coefficient of propane in bitumen[J]. Fuel, 2017, 210: 23-31. |
| 60 | Moganty S S, Baltus R E. Diffusivity of carbon dioxide in room-temperature ionic liquids[J]. Industrial & Engineering Chemistry Research, 2010, 49(19): 9370-9376. |
| 61 | Khodayari M, Reinsberg P, Abd‐El‐Latif A, et al. Determining solubility and diffusivity by using a flow cell coupled to a mass spectrometer[J]. ChemPhysChem, 2016, 17(11): 1647-1655. |
| 62 | Zhang J, Teixeira A R, Zhang H, et al. Automated in situ measurement of gas solubility in liquids with a simple tube-in-tube reactor[J]. Analytical Chemistry, 2017, 89(16): 8524-8530. |
| 63 | Koos P, Gross U, Polyzos A, et al. Teflon AF-2400 mediated gas-liquid contact in continuous flow methoxycarbonylations and in-line FTIR measurement of CO concentration[J]. Organic & Biomolecular Chemistry, 2011, 9(20): 6903-6908. |
| 64 | O’brien M, Taylor N, Polyzos A, et al. Hydrogenation in flow: homogeneous and heterogeneous catalysis using Teflon AF-2400 to effect gas-liquid contact at elevated pressure[J]. Chemical Science, 2011, 2(7): 1250-1257. |
| 65 | Wu G, Cao E, Kuhn S, et al. A novel approach for measuring gas solubility in liquids using a tube-in-tube membrane contactor[J]. Chemical Engineering & Technology, 2017, 40(12): 2346-2350. |
| 66 | Ley S, Browne D, O’brien M, et al. Continuous-flow processing of gaseous ammonia using a Teflon AF-2400 tube-in-tube reactor: synthesis of thioureas and in-line titrations[J]. Synlett, 2012, 23(9): 1402-1406. |
| 67 | Pastre J C, Browne D L, O’brien M, et al. Scaling up of continuous flow processes with gases using a tube-in-tube reactor: inline titrations and fanetizole synthesis with ammonia[J]. Organic Process Research & Development, 2013, 17(9): 1183-1191. |
| 68 | O’brien M. An automated colorimetric inline titration of CO2 concentrations in solvent flow streams using a Teflon AF-2400 tube-in-tube device[J]. Journal of CO2 Utilization, 2017, 21: 580-588. |
| 69 | Lan M, Zhao Z, Zeng Q, et al. Rapid measurement of gas solubility in ionic liquids with a simple tube-in-tube reactor[J]. Industrial & Engineering Chemistry Research, 2019, 58(16): 6696-6703. |
| 70 | Radhakrishnan K, Ramachandran P A, Brahme P H, et al. Solubility of hydrogen in methanol, nitrobenzene, and their mixtures experimental data and correlation[J]. Journal of Chemical & Engineering Data, 1983, 28(1): 1-4. |
| 71 | Lachowicz S K, Newitt d M, Weale K E. The solubility of hydrogen and deuterium in n-heptane and n-octane at high pressures[J]. Transactions of the Faraday Society, 1955, 51: 1198. |
| 72 | Herskowitz M, Morita S, Smith J M. Solubility of hydrogen in alpha-methylstyrene[J]. Journal of Chemical and Engineering Data, 1978, 23(3): 227-228. |
| 73 | Battino R, Rettich T R, Tominaga T. The solubility of nitrogen and air in liquids[J]. Journal of Physical & Chemical Reference Data, 1984, 13(2): 563-600. |
| 74 | Fischer K, Wilken M. Experimental determination of oxygen and nitrogen solubility in organic solvents up to 10 MPa at temperatures between 298 K and 398 K[J]. The Journal of Chemical Thermodynamics, 2001, 33(10): 1285-1308. |
| 75 | Schüler N, Hecht K, Kraut M, et al. On the solubility of carbon dioxide in binary water-methanol mixtures[J]. Journal of Chemical & Engineering Data, 2012, 57(8): 2304-2308. |
| 76 | Zhang J, Teixeira A R, Zhang H, et al. Flow toolkit for measuring gas diffusivity in liquids[J]. Analytical Chemistry, 2019, 91(6): 4004-4009. |
| 77 | Deen W M. Analysis of Transport Phenomena[M]. New York: Oxford University Press, 1998. |
| 78 | Littel R J, Versteeg G F, Van Swaaij W. Diffusivity measurements in some organic solvents by a gas-liquid diaphragm cell[J]. Journal of Chemical & Engineering Data, 2002, 37(1): 42-45. |
| 79 | Hogendoorn J A, van Swaaij W P M, Versteeg G F. Experimental study of the absorption of acid gases in porous particles impregnated with aqueous alkanolamide solutions[J]. Chemical Engineering Science, 1994, 49(20): 3421-3438. |
| 80 | Zhang J, Teixeira A R, Zhang H, et al. Determination of fast gas-liquid reaction kinetics in flow[J]. Reaction Chemistry & Engineering, 2020, 5(1): 51-57. |
| 81 | Lewis W K, Whitman W G. Principles of gas absorption[J]. Industrial & Engineering Chemistry, 2002, 16(12): 1215-1220. |
| 82 | Kierzkowska-Pawlak H. Determination of kinetics in gas-liquid reaction systems. An overview[J]. Ecological Chemistry and Engineering S, 2012, 19(2): 175-196. |
| 83 | Hoigné J, Bader H, Haag W R, et al. Rate constants of reactions of ozone with organic and inorganic compounds in water (Ⅲ): Inorganic compounds and radicals[J]. Water Research, 1985, 19(8): 993-1004. |
| 84 | Ringborg R H, Toftgaard Pedersen A, Woodley J M. Automated determination of oxygen-dependent enzyme kinetics in a tube-in-tube flow reactor[J]. ChemCatChem, 2017, 9(17): 3285-3288. |
| 85 | Liu Y, Sun L, Zhang H, et al. Microfluidics for drug development: from synthesis to evaluation[J]. Chemical Reviews, 2021, 121(13): 7468-7529. |
| 86 | Siuti P, Retterer S T, Doktycz M J. Continuous protein production in nanoporous, picolitre volume containers[J]. Lab on a Chip, 2011, 11(20): 3523-3529. |
| 87 | Timm A C, Shankles P G, Foster C M, et al. Toward microfluidic reactors for cell-free protein synthesis at the point-of-care[J]. Small, 2016, 12(6): 810-817. |
| 88 | Katzen F, Chang G, Kudlicki W. The past, present and future of cell-free protein synthesis[J]. Trends in Biotechnology, 2005, 23(3): 150-156. |
| 89 | Dondapati S K, Stech M, Zemella A, et al. Cell-free protein synthesis: a promising option for future drug development[J]. BioDrugs, 2020, 34(3): 327-348. |
| 90 | Carlson E D, Gan R, Hodgman C E, et al. Cell-free protein synthesis: applications come of age[J]. Biotechnology Advances, 2012, 30(5): 1185-1194. |
| 91 | Mei Q, Fredrickson C K, Simon A, et al. Cell-free protein synthesis in microfluidic array devices[J]. Biotechnology Progress, 2007, 23(6): 1305-1311. |
| 92 | Damiati S, Mhanna R, Kodzius R, et al. Cell-free approaches in synthetic biology utilizing microfluidics[J]. Genes, 2018, 9: 144. |
| 93 | Xiao X, Zhou Y, Sun Y, et al. Integration of cell-free protein synthesis and purification in one microfluidic chip for on-demand production of recombinant protein[J]. Biomicrofluidics, 2018, 12(5):054102. |
| 94 | Kim T W, Kim D M, Choi C Y. Rapid production of milligram quantities of proteins in a batch cell-free protein synthesis system[J]. Journal of Biotechnology, 2006, 124(2): 373-380. |
| 95 | Zhou C, Lin X, Lu Y, et al. Flexible on-demand cell-free protein synthesis platform based on a tube-in-tube reactor[J]. Reaction Chemistry & Engineering, 2020, 5(2): 270-277. |
| 96 | Tomaszewski B, Schmid A, Buehler K. Biocatalytic production of catechols using a high pressure tube-in-tube segmented flow microreactor[J]. Organic Process Research & Development, 2014, 18(11): 1516-1526. |
| 97 | Tomaszewski B, Lloyd R C, Warr A J, et al. Regioselective biocatalytic aromatic hydroxylation in a gas-liquid multiphase tube-in-tube reactor[J]. ChemCatChem, 2014, 6(9): 2567-2576. |
| 98 | Gutmann B, Cantillo D, Kappe C O. Continuous-flow technology-a tool for the safe manufacturing of active pharmaceutical ingredients[J]. Angewandte Chemie International Edition, 2015, 54(23): 6688-6728. |
| 99 | Brzozowski M, Forni J A, Paul Savage G, et al. The direct α-C(sp3)—H functionalisation of N-aryl tetrahydroisoquinolines via an iron-catalysed aerobic nitro-mannich reaction and continuous flow processing[J]. Chemical Communications, 2015, 51(2): 334-337. |
| 100 | Wernik M, Poechlauer P, Schmoelzer C, et al. Design and optimization of a continuous stirred tank reactor cascade for membrane-based diazomethane production: synthesis of α-chloroketones[J]. Organic Process Research & Development, 2019, 23(7): 1359-1368. |
| [1] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| [2] | 王冉, 王焕, 熊晓云, 关慧敏, 郑云锋, 陈彩琳, 秦玉才, 宋丽娟. FCC催化剂传质强化活性位利用效率的可视化分析[J]. 化工学报, 2024, 75(9): 3198-3209. |
| [3] | 赵帅琪, 张瑞, 黄瀚, 赵昆鹏, 白博峰. 水气转化对超临界水煤气化的抑制特性[J]. 化工学报, 2024, 75(8): 2960-2969. |
| [4] | 杨锦蕊, 郑宏飞, 马兴龙, 金日辉, 梁深. 两级叠置式加湿除湿海水淡化装置性能研究[J]. 化工学报, 2024, 75(7): 2446-2454. |
| [5] | 王金山, 王世学, 朱禹. 冷却表面温差对高温质子交换膜燃料电池性能的影响[J]. 化工学报, 2024, 75(5): 2026-2035. |
| [6] | 冯彬彬, 卢明佳, 黄志宏, 常译文, 崔志明. 碳载体在质子交换膜燃料电池中的应用及优化[J]. 化工学报, 2024, 75(4): 1469-1484. |
| [7] | 徐安冉, 刘凯, 王娜, 赵振宇, 李洪, 高鑫. 强吸波催化剂协同微波能强化果糖脱水制5-羟甲基糠醛[J]. 化工学报, 2024, 75(4): 1565-1577. |
| [8] | 薛潇, 商敏静, 苏远海. 微反应器内药物连续流合成的研究进展[J]. 化工学报, 2024, 75(4): 1439-1454. |
| [9] | 董霄, 白志山, 杨晓勇, 殷伟, 刘宁普, 于启凡. CHPPO工艺氧化液耦合除杂技术的研究与工业应用[J]. 化工学报, 2024, 75(4): 1630-1641. |
| [10] | 何宇航, 谢丹, 吕阳成. 微反应器内阳离子聚合研究进展[J]. 化工学报, 2024, 75(4): 1302-1316. |
| [11] | 王成秀, 宋大山, 李之辉, 杨潇, 蓝兴英, 高金森, 徐春明. Geldart C类脱硫灰颗粒在环流耦合提升管内稳定流动特性[J]. 化工学报, 2024, 75(4): 1485-1496. |
| [12] | 蒋方涛, 钱刚, 周兴贵, 段学志, 张晶. 基于[bmim][BF4]相转移催化的氟代碳酸乙烯酯高效合成[J]. 化工学报, 2024, 75(4): 1543-1551. |
| [13] | 程婷, 焦纬洲, 刘有智. 功能性填料在超重力旋转填料床中的应用和研究进展[J]. 化工学报, 2024, 75(4): 1414-1428. |
| [14] | 陈饶, 赵鑫, 陈戴欣, 姜圣坤, 廉应江, 王金波, 杨梅, 陈光文. 微反应器内甲苯连续二硝化制备二硝基甲苯[J]. 化工学报, 2024, 75(3): 867-876. |
| [15] | 王娟, 李秀明, 邵炜涛, 丁续, 霍莹, 付连超, 白云宇, 李迪. 多孔板鼓泡塔流动与传质特性数值模拟[J]. 化工学报, 2024, 75(3): 801-814. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号