化工学报 ›› 2022, Vol. 73 ›› Issue (6): 2669-2676.DOI: 10.11949/0438-1157.20211795
李丽媛1( ),王建强1,陈奕1,郭友娣1,周健1,刘志成1(
),王建强1,陈奕1,郭友娣1,周健1,刘志成1( ),王仰东1,谢在库2
),王仰东1,谢在库2
收稿日期:2021-12-21
修回日期:2022-02-18
出版日期:2022-06-05
发布日期:2022-06-30
通讯作者:
刘志成
作者简介:李丽媛(1984—),女,博士,高工,基金资助:
Liyuan LI1( ),Jianqiang WANG1,Yi CHEN1,Youdi GUO1,Jian ZHOU1,Zhicheng LIU1(
),Jianqiang WANG1,Yi CHEN1,Youdi GUO1,Jian ZHOU1,Zhicheng LIU1( ),Yangdong WANG1,Zaiku XIE2
),Yangdong WANG1,Zaiku XIE2
Received:2021-12-21
Revised:2022-02-18
Online:2022-06-05
Published:2022-06-30
Contact:
Zhicheng LIU
摘要:
甲醇制丙烯(MTP)是当前煤化工领域亟需发展的关键催化技术,积炭被认为是导致催化剂失活的重要原因之一。以积炭分子筛为研究对象,通过IGA、FTIR及TG等多种表征手段,考察甲醇的吸附行为、分子筛表面酸性、积炭成分与MTP反应中甲醇反应活性之间的构效关系。研究结果表明,甲醇的吸附量随催化剂的失活而降低,其下降速率与甲醇转化率成正比。催化剂上滞留的碳物种的主要成分为轻烃、BTX芳烃、活性结焦和积炭,而其中积炭是引起分子筛失活的主要原因。完全失活的催化剂与新鲜催化剂相比仍保留一定的甲醇吸附能力,推测积炭主要存在于酸活性中心周围。积炭首先覆盖的是B酸中心的羟基和桥式羟基,随后是非骨架Al—OH;而催化剂的甲醇转化率与分子筛中可接触的B酸和L酸数量成正比。另外,基于催化剂的失活速率与转化率存在的正比关系,结合反应动力学,推导出了失活曲线的数学表达式,理论上解释了MTP反应过程中的积炭失活介尺度机制。
中图分类号:
李丽媛, 王建强, 陈奕, 郭友娣, 周健, 刘志成, 王仰东, 谢在库. 甲醇制丙烯反应中ZSM-5分子筛催化剂积炭失活介尺度机制研究[J]. 化工学报, 2022, 73(6): 2669-2676.
Liyuan LI, Jianqiang WANG, Yi CHEN, Youdi GUO, Jian ZHOU, Zhicheng LIU, Yangdong WANG, Zaiku XIE. Study on the mesoscale mechanism of coking and deactivation of ZSM-5 catalyst in methanol to propylene reaction[J]. CIESC Journal, 2022, 73(6): 2669-2676.
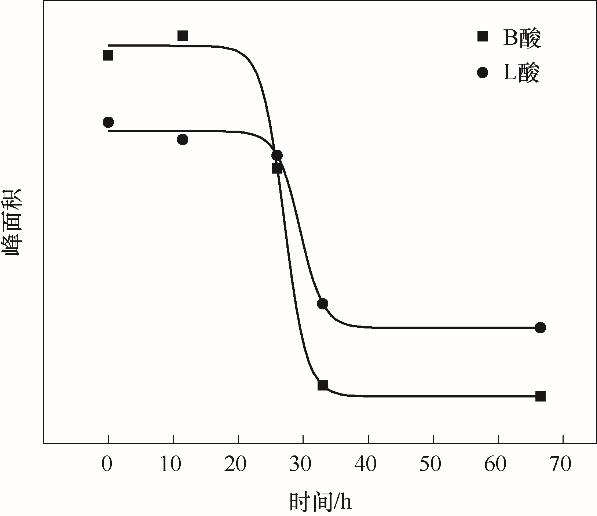
图8 不同反应时间分子筛样品的B酸(1540 cm-1)和L酸(1450 cm-1)的含量变化
Fig.8 Content of Br?nsted acid sites (1540 cm-1) and Lewis acid sites (1450 cm-1) for coked zeolites changed with time
| 1 | Martínez C, Corma A. Inorganic molecular sieves: preparation, modification and industrial application in catalytic processes[J]. Coordination Chemistry Reviews, 2011, 255(13/14): 1558-1580. |
| 2 | Guisnet M, Magnoux P, Martin D. Roles of acidity and pore structure in the deactivation of zeolites by carbonaceous deposits[J]. Studies in Surface Science and Catalysis, 1997, 111: 1-19. |
| 3 | Olsbye U, Svelle S, Bjørgen M, et al. Conversion of methanol to hydrocarbons: how zeolite cavity and pore size controls product selectivity[J]. Angewandte Chemie (International Ed. in English), 2012, 51(24): 5810-5831. |
| 4 | Bleken F, Skistad W, Barbera K, et al. Conversion of methanol over 10-ring zeolites with differing volumes at channel intersections: comparison of TNU-9, IM-5, ZSM-11 and ZSM-5[J]. Physical Chemistry Chemical Physics: PCCP, 2011, 13(7): 2539-2549. |
| 5 | Hereijgers B P C, Bleken F, Nilsen M H, et al. Product shape selectivity dominates the methanol-to-olefins (MTO) reaction over H-SAPO-34 catalysts[J]. Journal of Catalysis, 2009, 264(1): 77-87. |
| 6 | Zakaria Z Y, Amin N A S, Linnekoski J. A perspective on catalytic conversion of glycerol to olefins[J]. Biomass and Bioenergy, 2013, 55: 370-385. |
| 7 | Khanmohammadi M, Amani S, Garmarudi A B, et al. Methanol-to-propylene process: perspective of the most important catalysts and their behavior[J]. Chinese Journal of Catalysis, 2016, 37(3): 325-339. |
| 8 | 郭春垒, 方向晨, 贾立明, 等. 分子筛催化剂积炭失活行为探讨[J]. 工业催化, 2011, 19(12): 15-20. |
| Guo C L, Fang X C, Jia L M, et al. Investigation on coking deactivation behavior of molecular sieve catalysts[J]. Industrial Catalysis, 2011, 19(12): 15-20. | |
| 9 | Riaz A, Zahedi G, Klemeš J J. A review of cleaner production methods for the manufacture of methanol[J]. Journal of Cleaner Production, 2013, 57: 19-37. |
| 10 | Liu Z C, Wang Y D, Xie Z K. Thoughts on the future development of zeolitic catalysts from an industrial point of view[J]. Chinese Journal of Catalysis, 2012, 33(1): 22-38. |
| 11 | Asplund S. Coke formation and its effect on internal mass transfer and selectivity in Pd-catalysed acetylene hydrogenation[J]. Journal of Catalysis, 1996, 158(1): 267-278. |
| 12 | García-Ochoa F, Santos A. Coke effect in mass transport and morphology of Pt-Al2O3 and Ni-Mo-Al2O3 catalysts[J]. AIChE Journal, 1996, 42(2): 524-531. |
| 13 | Park J W, Lee J Y, Kim K S, et al. Effects of cage shape and size of 8-membered ring molecular sieves on their deactivation in methanol-to-olefin (MTO) reactions[J]. Applied Catalysis A: General, 2008, 339(1): 36-44. |
| 14 | Schulz H. “Coking” of zeolites during methanol conversion: basic reactions of the MTO-, MTP- and MTG processes[J]. Catalysis Today, 2010, 154(3/4): 183-194. |
| 15 | Wood J, Gladden L F. Effect of coke deposition upon pore structure and self-diffusion in deactivated industrial hydroprocessing catalysts[J]. Applied Catalysis A: General, 2003, 249(2): 241-253. |
| 16 | Mores D, Stavitski E, Kox M, et al. Space- and time-resolved in situ spectroscopy on the coke formation in molecular sieves: methanol-to-olefin conversion over H-ZSM-5 and H-SAPO-34[J]. Chemistry — A European Journal, 2008, 14(36): 11320-11327. |
| 17 | Chung Y M, Mores D, Weckhuysen B M. Spatial and temporal mapping of coke formation during paraffin and olefin aromatization in individual H-ZSM-5 crystals[J]. Applied Catalysis A: General, 2011, 404(1/2): 12-20. |
| 18 | Zhou F, Gao Y, Wu G, et al. Improved catalytic performance and decreased coke formation in post-treated ZSM-5 zeolites for methanol aromatization[J]. Microporous and Mesoporous Materials, 2017, 240: 96-107. |
| 19 | Valle B, Castaño P, Olazar M, et al. Deactivating species in the transformation of crude bio-oil with methanol into hydrocarbons on a HZSM-5 catalyst[J]. Journal of Catalysis, 2012, 285(1): 304-314. |
| 20 | 刘中民, 陈国权, 王清遐, 等. 分子筛催化剂的失活与积炭[J]. 催化学报, 1994(4):301-303. |
| Liu Z M, Chen G Q, Wang Q X, et al. Deactivation and coke formation on zeolite catalysts[J]. Chinese Journal of Catalysis, 1994(4):301-303. | |
| 21 | Ducarme V, Vedrine J C. ZSM-5 and ZSM-11 zeolites: influence of morphological and chemical parameters on catalytic selectivity and deactivation[J]. Applied Catalysis, 1985, 17(1): 175-184. |
| 22 | Li C, Stair P C. Ultraviolet Raman spectroscopy characterization of coke formation in zeolites[J]. Catalysis Today, 1997, 33(1/2/3): 353-360. |
| 23 | Mores D, Kornatowski J, Olsbye U, et al. Coke formation during the methanol-to-olefin conversion: in situ microspectroscopy on individual H-ZSM-5 crystals with different brønsted acidity[J]. Chemistry — A European Journal, 2011, 17(10): 2874-2884. |
| 24 | 李丽媛, 陈奕, 许中强, 等. 烃类分子在分子筛中扩散行为研究进展[J]. 化工进展, 2014, 33(3): 655-659, 688. |
| Li L Y, Chen Y, Xu Z Q, et al. Research advances in the diffusion of hydrocarbons in zeolites[J]. Chemical Industry and Engineering Progress, 2014, 33(3): 655-659, 688. | |
| 25 | 李丽媛, 陈奕, 许中强, 等. 均三甲苯在MCM-22和MCM-56分子筛上的吸附和扩散[J]. 工业催化, 2013, 21(7): 30-34. |
| Li L Y, Chen Y, Xu Z Q, et al. Adsorption and diffusion of mesitylene on MCM-22 and MCM-56 molecular sieves[J]. Industrial Catalysis, 2013, 21(7): 30-34. | |
| 26 | Zhou J, Liu Z C, Li L Y, et al. Hierarchical mesoporous ZSM-5 zeolite with increased external surface acid sites and high catalytic performance in o-xylene isomerization[J]. Chinese Journal of Catalysis, 2013, 34(7): 1429-1433. |
| 27 | Zhou J, Liu Z C, Wang Y D, et al. Enhanced accessibility and utilization efficiency of acid sites in hierarchical MFI zeolite catalyst for effective diffusivity improvement[J]. RSC Adv., 2014, 4(82): 43752-43755. |
| 28 | Zhou J, Wang Y D, Zou W, et al. Mass transfer advantage of hierarchical zeolites promotes methanol converting into para-methyl group in toluene methylation[J]. Industrial & Engineering Chemistry Research, 2017, 56(33): 9310-9321. |
| 29 | Zhai M, Li L Y, Ba Y L, et al. Fabricating ZSM-23 with reduced aspect ratio through ball-milling and recrystallization: synthesis, structure and catalytic performance in n-heptane hydroisomerization[J]. Catalysis Today, 2019, 329: 82-93. |
| 30 | Sun M H, Zhou J, Hu Z Y, et al. Hierarchical zeolite single-crystal reactor for excellent catalytic efficiency[J]. Matter, 2020, 3(4): 1226-1245. |
| 31 | Zhu W, Kapteijn F, van der Linden B, et al. Equilibrium adsorption of linear and branched C6 alkanes on silicalite-1 studied by the tapered element oscillating microbalance[J]. Physical Chemistry Chemical Physics, 2001, 3(9): 1755-1761. |
| 32 | Schmidt F, Hoffmann C, Giordanino F, et al. Coke location in microporous and hierarchical ZSM-5 and the impact on the MTH reaction[J]. Journal of Catalysis, 2013, 307: 238-245. |
| 33 | Rostamizadeh M, Yaripour F. Dealumination of high silica H-ZSM-5 as long-lived nanocatalyst for methanol to olefin conversion[J]. Journal of the Taiwan Institute of Chemical Engineers, 2017, 71: 454-463. |
| 34 | 忻睦迪, 邢恩会. 三甲基膦和金属氧化物复合改性ZSM-5分子筛及其裂解性能研究[J]. 化工学报, 2021, 72(5): 2657-2668. |
| Xin M D, Xing E H. Researches on trimethylphosphine and metal oxide modification on ZSM-5 and their influence on catalytic cracking[J]. CIESC Journal, 2021, 72(5): 2657-2668. | |
| 35 | Karge H G, Nießen W, Bludau H. In-situ FTIR measurements of diffusion in coking zeolite catalysts[J]. Applied Catalysis A: General, 1996, 146(2): 339-349. |
| 36 | Park J W, Seo G. IR study on methanol-to-olefin reaction over zeolites with different pore structures and acidities[J]. Applied Catalysis A: General, 2009, 356(2): 180-188. |
| 37 | Kerssens M M, Sprung C, Whiting G T, et al. Selective staining of zeolite acidity: recent progress and future perspectives on fluorescence microscopy[J]. Microporous and Mesoporous Materials, 2014, 189: 136-143. |
| 38 | Bjørgen M, Olsbye U, Kolboe S. Coke precursor formation and zeolite deactivation: mechanistic insights from hexamethylbenzene conversion[J]. Journal of Catalysis, 2003, 215(1): 30-44. |
| 39 | Janssens T V W, Svelle S, Olsbye U. Kinetic modeling of deactivation profiles in the methanol-to-hydrocarbons (MTH) reaction: a combined autocatalytic-hydrocarbon pool approach[J]. Journal of Catalysis, 2013, 308: 122-130. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [4] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [5] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [6] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [9] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [10] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [11] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [12] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [13] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [14] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [15] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号