化工学报 ›› 2020, Vol. 71 ›› Issue (11): 5035-5042.DOI: 10.11949/0438-1157.20200790
张雅婷1( ),熊文杰1(
),熊文杰1( ),赵天翔2,3,姚晨飞1,丁宇宬1,张效敏1(
),赵天翔2,3,姚晨飞1,丁宇宬1,张效敏1( ),吴有庭1,胡兴邦1(
),吴有庭1,胡兴邦1( )
)
收稿日期:2020-06-22
修回日期:2020-09-11
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
张效敏,胡兴邦
作者简介:张雅婷(1998—),女,硕士研究生,基金资助:
Yating ZHANG1( ),Wenjie XIONG1(
),Wenjie XIONG1( ),Tianxiang ZHAO2,3,Chenfei YAO1,Yucheng DING1,Xiaomin ZHANG1(
),Tianxiang ZHAO2,3,Chenfei YAO1,Yucheng DING1,Xiaomin ZHANG1( ),Youting WU1,Xingbang HU1(
),Youting WU1,Xingbang HU1( )
)
Received:2020-06-22
Revised:2020-09-11
Online:2020-11-05
Published:2020-11-05
Contact:
Xiaomin ZHANG,Xingbang HU
摘要:
开发易制备、价格便宜、面向SO2气体高效分离的离子液体(ILs),是当前ILs从实验探索迈向工业应用的难点与重大挑战。合成了不同摩尔比(3∶1、2∶1、1∶1、1∶2和1∶3)的1-乙基-3-甲基咪唑氯盐([Emim][Cl])和1-乙基-3-甲基咪唑乙酸盐([Emim][OAc])的离子液体混合物[Emim][Cl]x[OAc]1-x, 在测定其密度、黏度、热稳定性等基本物性数据的基础上,研究了[Emim][Cl]x[OAc]1-x混合物在不同温度和SO2分压下的SO2吸收能力。结果表明,[Emim][Cl]x[OAc]1-x能够有效地捕获SO2。[Emim][Cl]与[Emim][OAc]之间存在协同促进作用,有利于实现SO2高效吸收。[Emim][Cl]0.33[OAc]0.66混合液在1.0和0.2 atm(1 atm=101325 Pa)下捕获SO2量分别为(1.34±0.08)和(0.74±0.05) g/g,与现有结果相比,混合物在SO2捕获方面有明显优势。此外,这些离子液体混合物对二氧化硫的吸收和解吸具有良好的可逆性。
中图分类号:
张雅婷,熊文杰,赵天翔,姚晨飞,丁宇宬,张效敏,吴有庭,胡兴邦. 咪唑类离子液体混合物用于二氧化硫高效吸收[J]. 化工学报, 2020, 71(11): 5035-5042.
Yating ZHANG,Wenjie XIONG,Tianxiang ZHAO,Chenfei YAO,Yucheng DING,Xiaomin ZHANG,Youting WU,Xingbang HU. High capacity absorption of SO2 using imidazole ionic liquid mixtures[J]. CIESC Journal, 2020, 71(11): 5035-5042.
| 离子液体 | ρ - T | η - T | |||||
|---|---|---|---|---|---|---|---|
| A | B | R2 | a | b | c | R2 | |
| [Emim][OAc] | 1.124 | -6.16×10-4 | 0.9989 | 73.35 | -0.34 | 4.58×10-4 | 0.9992 |
| [Emim][Cl]0.75[OAc]0.25 | 1.151 | -5.94×10-4 | 0.9999 | 83.00 | -0.44 | 5.97×10-4 | 0.9978 |
| [Emim][Cl]0.66[OAc]0.33 | 1.145 | -5.93×10-4 | 0.9999 | 65.79 | -0.34 | 4.60×10-4 | 0.9999 |
| [Emim][Cl]0.5[OAc]0.5 | 1.140 | -6.06×10-4 | 0.9998 | 64.66 | -0.40 | 5.51×10-5 | 0.9999 |
| [Emim][Cl]0.33[OAc]0.66 | 1.334 | -6.12×10-4 | 0.9999 | 78.22 | -0.42 | 5.89×10-6 | 0.9992 |
| [Emim][Cl]0.25[OAc]0.75 | 1.133 | -6.09×10-4 | 0.9999 | 69.07 | -0.37 | 5.06×10-4 | 0.9998 |
表1 密度-温度和黏度-温度模型参数
Table 1 Density-temperature and viscosity-temperature model parameters
| 离子液体 | ρ - T | η - T | |||||
|---|---|---|---|---|---|---|---|
| A | B | R2 | a | b | c | R2 | |
| [Emim][OAc] | 1.124 | -6.16×10-4 | 0.9989 | 73.35 | -0.34 | 4.58×10-4 | 0.9992 |
| [Emim][Cl]0.75[OAc]0.25 | 1.151 | -5.94×10-4 | 0.9999 | 83.00 | -0.44 | 5.97×10-4 | 0.9978 |
| [Emim][Cl]0.66[OAc]0.33 | 1.145 | -5.93×10-4 | 0.9999 | 65.79 | -0.34 | 4.60×10-4 | 0.9999 |
| [Emim][Cl]0.5[OAc]0.5 | 1.140 | -6.06×10-4 | 0.9998 | 64.66 | -0.40 | 5.51×10-5 | 0.9999 |
| [Emim][Cl]0.33[OAc]0.66 | 1.334 | -6.12×10-4 | 0.9999 | 78.22 | -0.42 | 5.89×10-6 | 0.9992 |
| [Emim][Cl]0.25[OAc]0.75 | 1.133 | -6.09×10-4 | 0.9999 | 69.07 | -0.37 | 5.06×10-4 | 0.9998 |
| ILs | SO2 absorption at 20℃/(g/g) | Ref. |
|---|---|---|
| [Emim][Cl]0.75[OAc]0.25 | 1.32±0.08 | this work |
| [Emim][Cl]0.66[OAc]0.33 | 1.33±0.08 | this work |
| [Emim][Cl]0.5[OAc]0.5 | 1.20±0.07 | this work |
| [Emim][Cl]0.33[OAc]0.66 | 1.34±0.08 | this work |
| [DMDEEH][MOAc] | 1.02 | [ |
| [DMDEEH][EOAc] | 1.06 | [ |
| [DMDEEH][MEAAc] | 1.04 | [ |
| [EDBEAH][MOAc] | 0.34 | [ |
| [TMPDAH][BAc] | 1.23 | [ |
| EminCl-AA(2∶1) | 1.39 | [ |
| [NH2Emim][OAc]-[Bmim][OH] (1∶1) | 0.36① | [ |
| [N2222][FA]/PEG200 | 0.46② | [ |
| [Ch][FA]/PEG200 | 0.30② | [ |
| [TMG][SUC] | 0.88① | [ |
| [TMG][SUB] | 0.95 | [ |
| [TMG][DOD] | 0.83 | [ |
| [Emim][SCN] | 1.13 | [ |
| [Emim][C(CN)3] | 0.74 | [ |
| [Emim][Cl] | 1.20③ | [ |
| [C4Py][SCN] | 0.44 | [ |
| [C4Py][BF4] | 0.84 | [ |
| [Emim][Cl][SCN](1∶1) | 1.22 | [ |
| ChCl-EG (1∶2) | 0.70 | [ |
| ChCl-thiourea (1∶1) | 0.88 | [ |
表2 不同体系对二氧化硫的吸收能力
Table 2 SO2 absorption capacities of different systems
| ILs | SO2 absorption at 20℃/(g/g) | Ref. |
|---|---|---|
| [Emim][Cl]0.75[OAc]0.25 | 1.32±0.08 | this work |
| [Emim][Cl]0.66[OAc]0.33 | 1.33±0.08 | this work |
| [Emim][Cl]0.5[OAc]0.5 | 1.20±0.07 | this work |
| [Emim][Cl]0.33[OAc]0.66 | 1.34±0.08 | this work |
| [DMDEEH][MOAc] | 1.02 | [ |
| [DMDEEH][EOAc] | 1.06 | [ |
| [DMDEEH][MEAAc] | 1.04 | [ |
| [EDBEAH][MOAc] | 0.34 | [ |
| [TMPDAH][BAc] | 1.23 | [ |
| EminCl-AA(2∶1) | 1.39 | [ |
| [NH2Emim][OAc]-[Bmim][OH] (1∶1) | 0.36① | [ |
| [N2222][FA]/PEG200 | 0.46② | [ |
| [Ch][FA]/PEG200 | 0.30② | [ |
| [TMG][SUC] | 0.88① | [ |
| [TMG][SUB] | 0.95 | [ |
| [TMG][DOD] | 0.83 | [ |
| [Emim][SCN] | 1.13 | [ |
| [Emim][C(CN)3] | 0.74 | [ |
| [Emim][Cl] | 1.20③ | [ |
| [C4Py][SCN] | 0.44 | [ |
| [C4Py][BF4] | 0.84 | [ |
| [Emim][Cl][SCN](1∶1) | 1.22 | [ |
| ChCl-EG (1∶2) | 0.70 | [ |
| ChCl-thiourea (1∶1) | 0.88 | [ |
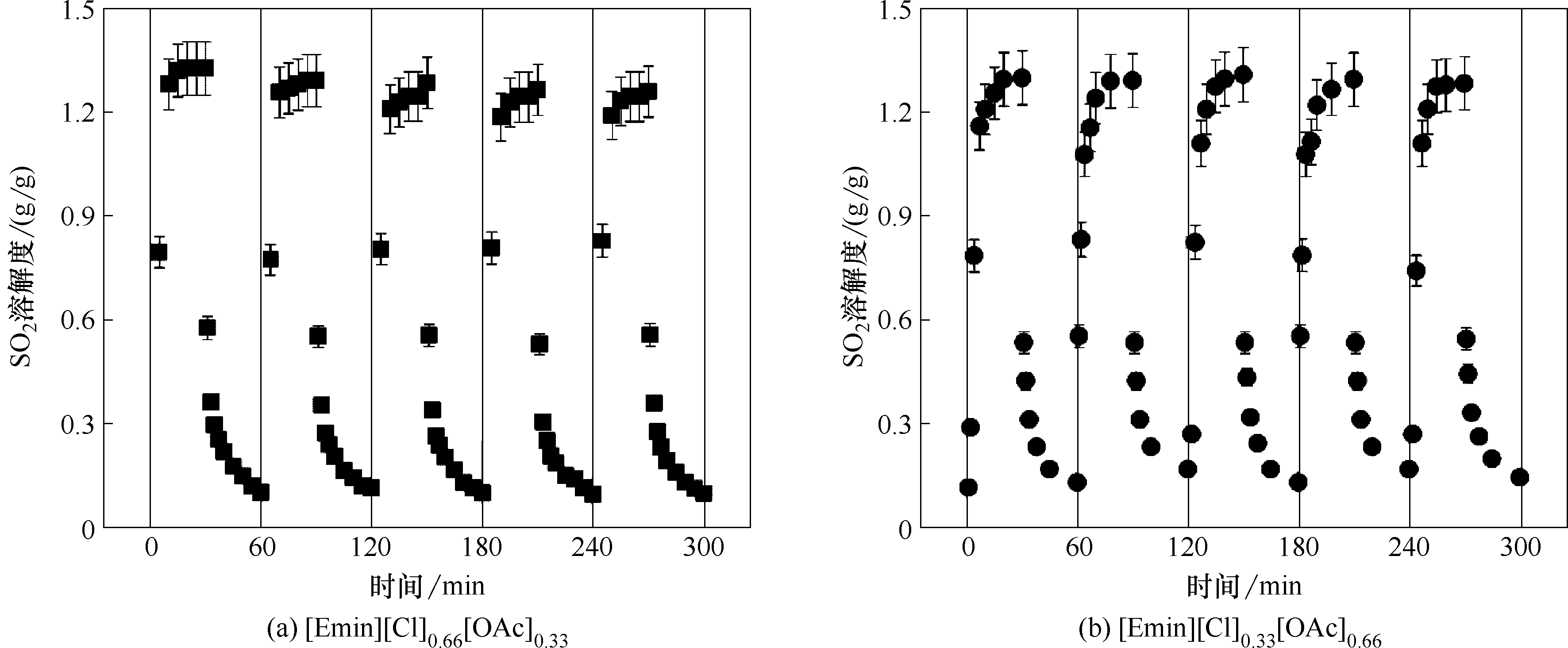
图8 [Emim][Cl]0.66[OAc]0.33和[Emim][Cl]0.33[OAc]0.66的SO2吸收-解吸循环
Fig.8 SO2 absorption-desorption cycles in [Emim][Cl]0.66[OAc]0.33 and [Emim][Cl]0.33[OAc]0.66
| 1 | Solarin S A, Tiwari A. Convergence in sulphur dioxide (SO2) emissions since 1850 in OECD countries: evidence from a new panel unit root test[J]. Environmental Modeling & Assessment, 2020, 25(5): 665-675. |
| 2 | Tang L, Xue X D, Jia M. Iron and steel industry emissions and contribution to the air quality in China[J].Atmospheric Environment, 2020, 237: 117668. |
| 3 | Fang D, Liao X, Zhang X F, et al. A novel resource utilization of the calcium-based semi-dry flue gas desulfurization ash: as a reductant to remove chromium and vanadium from vanadium industrial waste water[J]. Journal of Hazardous Materials, 2018, 34: 436-445. |
| 4 | Córdoba P. Status of flue gas desulphurisation (FGD) systems from coal-fired power plants: overview of the physic-chemical control processes of wet limestone FGDs[J]. Fuel, 2015, 144: 274-286. |
| 5 | Duo Y K, Wang X P, He J J, et al. Simultaneous removal of SO2 and NO by Fe-II(EDTA) solution: promotion of Mn powder and mechanism of reduction[J]. Environmental Science and Pollution Research, 2019, 26(28): 28808-28816. |
| 6 | 韩天义, 姚远, 徐珺, 等.吸湿剂、表面活性剂及催化剂对烟气循环流化床脱硫的增效机制[J]. 化工学报, 2018, 69(9): 4044-4050. |
| Han T Y, Yao Y, Xu J, et al. Synergetic mechanism of hygroscopic agent, surfactant and catalyst on desulfurization of flue gas circulating fluidized bed[J]. CIESC Journal, 2018, 69(9): 4044-4050. | |
| 7 | Lei Z, Dai C, Chen B. Gas solubility in ionic liquids[J]. Chemical Reviews, 2014, 114(2): 1289-1326. |
| 8 | Cui G, Wang J, Zhang S. Active chemisorption sites in functionalized ionic liquids for carbon capture[J]. Chemical Society Reviews, 2016, 45(15): 4307-4339. |
| 9 | Zhao T, Li Y, Zhang Y, et al. Efficient SO2 capture and fixation to cyclic sulfites by dual ether-functionalized protic ionic liquids without any additives[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(8): 10886-10895. |
| 10 | Zhao T, Liang J, Zhang Y, et al. Unexpectedly efficient SO2 capture and conversion to sulfur in novel imidazole-based deep eutectic solvents[J]. Chemical Communications, 2018, 54(65): 8964-8967. |
| 11 | Li W, Liu Y, Wang L, et al. Using ionic liquid mixtures to improve the SO2 absorption performance in flue gas[J]. Energy & Fuels, 2017, 31(2): 1771-1777. |
| 12 | Jiang Y, Liu X, Deng D. Absorption of SO2 in furoate ionic liquids/PEG200 mixtures and thermodynamic analysis[J]. Journal of Chemical & Engineering Data, 2018, 63(2): 259-268. |
| 13 | Yang D, Han Y, Qi H, et al. Efficient absorption of SO2 by EmimCl-EG deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(8): 6382-6386. |
| 14 | Wu W, Han B, Gao H, et al. Desulfurization of flue gas: SO2 absorption by an ionic liquid[J]. Angewandte Chemie-International Edition, 2004, 43: 2415-2417. |
| 15 | 邓晓霞, 龚磊, 刘小棒, 等. 咪唑类三元低共熔溶剂捕集低压SO2的实验研究[J]. 化工学报, 2020, 71(1): 368-375. |
| Deng X X, Gong L, Liu X B, et al. Study on the capture of low pressure SO2 by imidazole-based ternary deep eutectic solvents[J]. CIESC Journal, 2020, 71(1): 368-375. | |
| 16 | Meng X, Wang J, Xie P, et al. Structure and SO2 absorption properties of guanidinium-based dicarboxylic acid ionic liquids[J]. Energy & Fuels, 2018, 32(2): 1956-1962. |
| 17 | Li Z L, Zhou L S, Wei Y H, et al. Highly efficient, reversible, and selective absorption of SO2 in 1-ethyl-3-methylimidazolium chloride plus imidazole deep eutectic solvents[J]. Industrial & Engineering Chemistry Research, 2020, 59(30): 13696-13705. |
| 18 | Wang C, Zheng J, Cui G, et al. Highly efficient SO2 capture through tuning the interaction between anion-functionalized ionic liquids and SO2[J]. Chemical Communications, 2013, 49(12): 1166-1168. |
| 19 | Zhang H M, Jiang B, Yang N, et al. Highly efficient and reversible absorption of SO2 from flue gas using diamino polycarboxylate protic ionic liquid aqueous solutions[J]. Energy & Fuels, 2019, 33(9): 8937-8945. |
| 20 | Xing H, Liao C, Yang Q, et al. Ambient lithium-SO2 batteries with ionic liquids as electrolytes[J]. Angewandte Chemie-International Edition, 2014, 53(8): 2099-2103. |
| 21 | Zeng S, Gao H, Zhang X, et al. Efficient and reversible capture of SO2 by pyridinium-based ionic liquids[J]. Chemical Engineering Journal, 2014, 251: 248-256. |
| 22 | Yang D, Cui G, Lv M. Efficient absorption of SO2 by [Emim][Cl]-[Emim][SCN] ionic liquid mixtures[J]. Energy & Fuels, 2018, 32(10): 10796-10800. |
| 23 | Sun S, Niu Y, Xu Q, et al. Efficient SO2 absorptions by four kinds of deep eutectic solvents based on choline chloride[J]. Industrial & Engineering Chemistry Research, 2015, 54(33): 8019-8024. |
| 24 | Yang D, Zhang S, Jiang D, et al. SO2 absorption in EmimCl-TEG deep eutectic solvents[J]. Physical Chemistry Chemical Physics, 2018, 20: 15168-15173. |
| 25 | Wang L, Zhang Y, Liu Y, et al. SO2 absorption in pure ionic liquids: solubility and functionalization [J]. Journal of Hazardous Materials, 2020, 392: 122504. |
| 26 | Mondal A, Balasubramanian S. Understanding SO2 capture by ionic liquids [J]. The Journal of Physical Chemistry B, 2016, 120(19): 4457-4466. |
| 27 | Zhu J, Xu Y, Feng X, et al. A detailed study of physicochemical properties and microstructure of EmimCl-EG deep eutectic solvents: their influence on SO2 absorption behavior[J]. Journal of Industrial and Engineering Chemistry, 2018, 67: 148-155. |
| 28 | Souckova M, Klomfar J, Patek J. Group contribution and parachor analysis of experimental data on density and surface tension for members of the homologous series of 1-Cn-3-methylimidazolium chlorides [J]. Fluid Phase Equilibria, 2017, 454: 43-56. |
| 29 | Freire M G, Teles A R, Rocha M A, et al. Thermophysical characterization of ionic liquids able to dissolve biomass [J]. Journal of Chemical & Engineering Data, 2011, 56: 4813-4822. |
| 30 | Araujo J M, Pereiro A B, Alves F, et al. Nucleic acid bases in 1-alkyl-3-methylimidazolium acetate ionic liquids: a thermophysical and ionic conductivity analysis [J]. The Journal of Chemical Thermodynamics, 2013, 57: 1-8. |
| 31 | Vitz E, Erdmenger T, Haensch C, et al. Extended dissolution studies of cellulose in imidazolium based ionic liquids [J]. Green Chemistry, 2009, 11: 417-424. |
| 32 | Huang K, Wu Y T, Hu X B. Effect of alkalinity on absorption capacity and selectivity of SO2 and H2S over CO2: substituted benzoate-based ionic liquids as the study platform [J]. Chemical Engineering Journal, 2016, 297: 265-276. |
| 33 | Zheng W T, Huang K, Wu Y T, et al. Protic ionic liquid as excellent shuttle of MDEA for fast capture of CO2 [J]. AIChE Journal, 2018, 64: 209-219. |
| 34 | 崔国凯, 赵宁, 张峰涛, 等. 离子液体捕集二氧化硫气体的研究进展[J]. 科学通报, 2016, 61: 3115-3126. |
| Cui G K, Zhao N, Zhang F T, et al. Progress in SO2 capture by ionic liquids[J]. Chinese Science Bulletin, 2016, 61: 3115-3126. | |
| 35 | Ando R A, Siqueira L J A, Bazito F C, et al. The sulfur dioxide-1-butyl-3-methylimidazolium bromide interaction: drastic changes in structural and physical properties[J]. The Journal of Physical Chemistry B, 2007, 111(30): 8717-8719. |
| 36 | Ren S, Hou Y, Wu W, et al. Properties of ionic liquids absorbing SO2 and the mechanism of the absorption[J]. The Journal of Physical Chemistry B, 2010, 114(6): 2175-2179. |
| 37 | Zhao Q, Zhao W, Chai M, et al. Phase-change absorption of SO2 by N, N, N′, N′-tetramethyl-p-phenylenediamine in organic solvents and utilization of absorption product[J]. Energy & Fuels, 2018, 32(2): 2073-2080. |
| 38 | Chen Y, Jiang B, Dou H, et al. Highly efficient and reversible capture of low partial pressure SO2 by functional deep eutectic solvents[J]. Energy & Fuels, 2018, 32(10): 10737-10744. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [5] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [6] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [7] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [11] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [12] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [13] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [14] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [15] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号