化工学报 ›› 2021, Vol. 72 ›› Issue (3): 1264-1274.DOI: 10.11949/0438-1157.20200894
张盈盈( ),郭淑娜,宋帅龙,杨许召,吴诗德,田俊峰,韩光鲁,张静静,李亚坤,张建强
),郭淑娜,宋帅龙,杨许召,吴诗德,田俊峰,韩光鲁,张静静,李亚坤,张建强
收稿日期:2020-07-06
修回日期:2020-11-12
出版日期:2021-03-05
发布日期:2021-03-05
通讯作者:
张盈盈
作者简介:张盈盈(1985—),女,博士,讲师,基金资助:
ZHANG Yingying( ),GUO Shuna,SONG Shuailong,YANG Xuzhao,WU Shide,TIAN Junfeng,HAN Guanglu,ZHANG Jingjing,LI Yakun,ZHANG Jianqiang
),GUO Shuna,SONG Shuailong,YANG Xuzhao,WU Shide,TIAN Junfeng,HAN Guanglu,ZHANG Jingjing,LI Yakun,ZHANG Jianqiang
Received:2020-07-06
Revised:2020-11-12
Online:2021-03-05
Published:2021-03-05
Contact:
ZHANG Yingying
摘要:
氨气(NH3)作为一种有害气体,其传统吸收技术存在诸多缺陷,亟需开发性能优越的NH3吸收剂,以开发新型NH3分离技术。离子液体和低共熔溶剂作为气体分离过程的潜在吸收剂,因低挥发性、良好的热稳定性以及灵活的可调控性等特点受到越来越多的关注。但离子液体和低共熔溶剂数量众多,筛选困难。采用热力学分析方法分析离子液体和低共熔溶剂用于NH3分离过程,基于Gibbs自由能变,拟合出分离过程的最佳操作条件,将总能耗和离子液体用量作为筛选标准,筛选出性能良好的[Omim][BF4]。将[Omim][BF4]与传统NH3吸收剂水对比,发现[Omim][BF4]具有更低的能耗。最后,拟合出筛选标准与离子液体/低共熔溶剂的临界性质间的规律,为开发新的NH3吸收剂和新的分离技术提供依据。
中图分类号:
张盈盈, 郭淑娜, 宋帅龙, 杨许召, 吴诗德, 田俊峰, 韩光鲁, 张静静, 李亚坤, 张建强. 离子液体/低共熔溶剂用于NH3分离过程的热力学分析[J]. 化工学报, 2021, 72(3): 1264-1274.
ZHANG Yingying, GUO Shuna, SONG Shuailong, YANG Xuzhao, WU Shide, TIAN Junfeng, HAN Guanglu, ZHANG Jingjing, LI Yakun, ZHANG Jianqiang. Thermodynamic analysis for NH3 separation using ionic liquids/deep eutectic solvents[J]. CIESC Journal, 2021, 72(3): 1264-1274.
| 氨气(NH3) | 氢气(H2) | 氮气(N2) | 甲烷(CH4) |
|---|---|---|---|
| 43.8%~50% | 25%~36.3% | 9.5%~15.8% | 3.6%~9.2% |
表1 合成氨驰放气的组成[15-16]
Table 1 The composition of synthetic ammonia purge gas[15-16]
| 氨气(NH3) | 氢气(H2) | 氮气(N2) | 甲烷(CH4) |
|---|---|---|---|
| 43.8%~50% | 25%~36.3% | 9.5%~15.8% | 3.6%~9.2% |
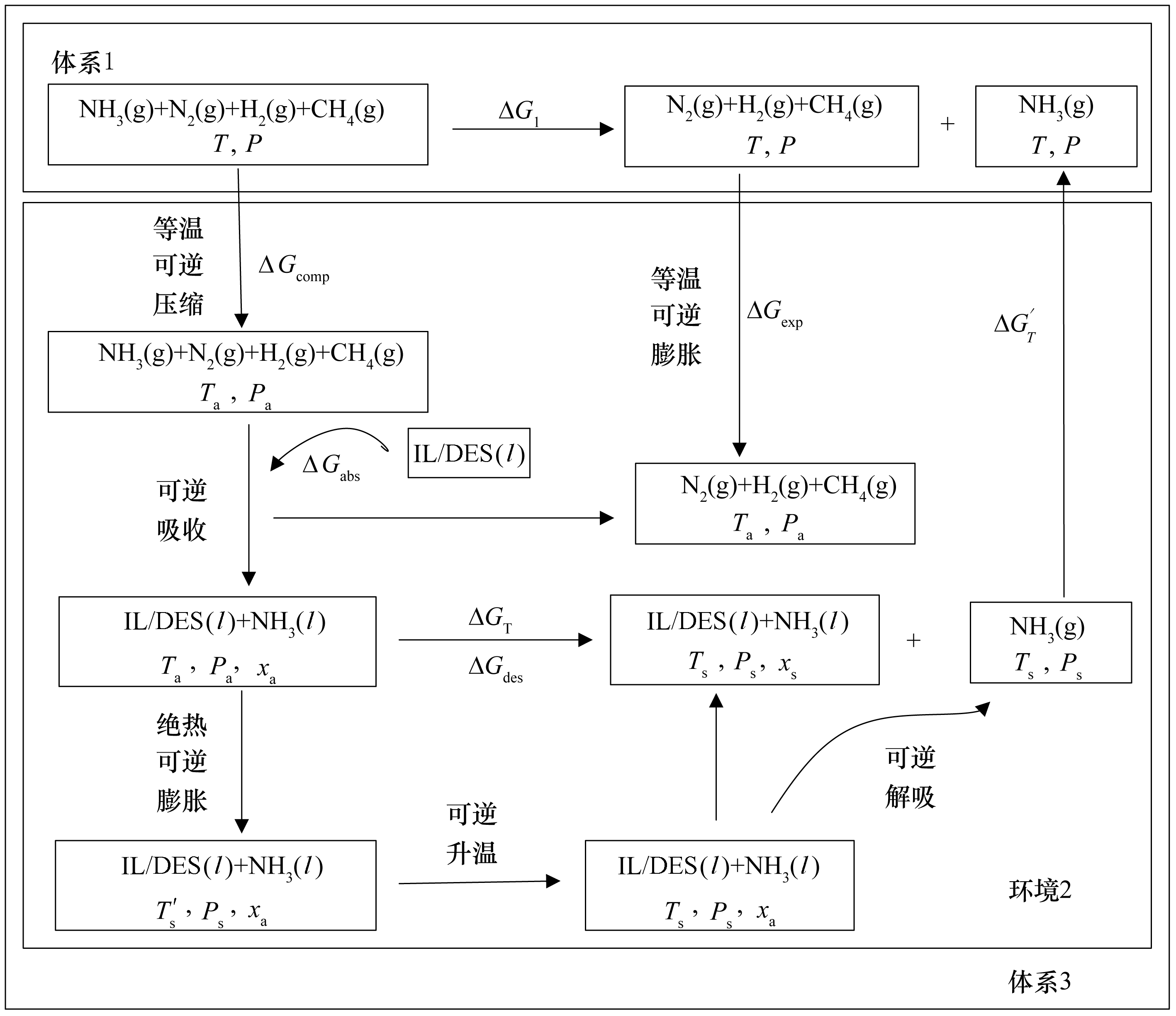
图3 采用离子液体/低共熔溶剂分离合成氨驰放气中NH3的热力学分析及过程耦合图
Fig.3 The process coupling thermodynamic analysis of NH3 separation from synthetic ammonia purge gas with ILs/DESs
| 离子液体/低共熔溶剂 | M/ (g?mol-1) | ρ/(g?cm-3) | cp/(J?mol-1?K-1) | Tc/K | Pc/bar | |||
|---|---|---|---|---|---|---|---|---|
| T/K | P/bar | |||||||
| [Emim][BF4] | 197.97 | 313.15~333.15 | 1.2~6.3 | 0.084~0.526[ | 1.280[ | 311.5~316.8[ | 585.3[ | 23.60[ |
| [Bmim][BF4] | 226.02 | 313.15~333.15 | 0.7~8.3 | 0.061~0.562[ | 1.201[ | 371.7~377.7[ | 632.3[ | 20.40[ |
| [Hmim][BF4] | 254.08 | 313.15~333.15 | 1.4~7.1 | 0.128~0.624[ | 1.145[ | 436.2~444.0[ | 679.1[ | 17.90[ |
| [Omim][BF4] | 282.13 | 313.15~333.15 | 1.0~6.0 | 0.132~0.644[ | 1.104[ | 505.4~514.2[ | 726.1[ | 16.00[ |
| [Bmim][PF6] | 284.18 | 298.60~347.20 | 1.8~7.7 | 0.253~0.737 [ | 1.368[ | 402.0~409.0[ | 708.9[ | 17.30[ |
| ChCl/urea(1∶2) | 86.58 | 313.20~333.20 | 0.1~3.0 | 0.017~0.241[ | 1.206[ | 182.2~184.5[ | 644.4[ | 49.54[ |
| ChCl/gly(1∶2) | 107.94 | 313.15~333.15 | 0.1~5.7 | 0.030~0.556[ | 1.192[ | 239.1~243.5[ | 680.7[ | 33.46[ |
| ChCl/EG(1∶2) | 87.92 | 313.15~333.15 | 0.1~5.5 | 0.035~0.511[ | 1.117[ | 192.2~196.4[ | 602.0[ | 40.99[ |
表2 8种离子液体/低共熔溶剂的分子量、NH3溶解度、密度、比定压热容和临界性质
Table 2 The molecular weight, NH3 solubility, density, isobaric heat capacity and critical properties of 8 ILs/DESs
| 离子液体/低共熔溶剂 | M/ (g?mol-1) | ρ/(g?cm-3) | cp/(J?mol-1?K-1) | Tc/K | Pc/bar | |||
|---|---|---|---|---|---|---|---|---|
| T/K | P/bar | |||||||
| [Emim][BF4] | 197.97 | 313.15~333.15 | 1.2~6.3 | 0.084~0.526[ | 1.280[ | 311.5~316.8[ | 585.3[ | 23.60[ |
| [Bmim][BF4] | 226.02 | 313.15~333.15 | 0.7~8.3 | 0.061~0.562[ | 1.201[ | 371.7~377.7[ | 632.3[ | 20.40[ |
| [Hmim][BF4] | 254.08 | 313.15~333.15 | 1.4~7.1 | 0.128~0.624[ | 1.145[ | 436.2~444.0[ | 679.1[ | 17.90[ |
| [Omim][BF4] | 282.13 | 313.15~333.15 | 1.0~6.0 | 0.132~0.644[ | 1.104[ | 505.4~514.2[ | 726.1[ | 16.00[ |
| [Bmim][PF6] | 284.18 | 298.60~347.20 | 1.8~7.7 | 0.253~0.737 [ | 1.368[ | 402.0~409.0[ | 708.9[ | 17.30[ |
| ChCl/urea(1∶2) | 86.58 | 313.20~333.20 | 0.1~3.0 | 0.017~0.241[ | 1.206[ | 182.2~184.5[ | 644.4[ | 49.54[ |
| ChCl/gly(1∶2) | 107.94 | 313.15~333.15 | 0.1~5.7 | 0.030~0.556[ | 1.192[ | 239.1~243.5[ | 680.7[ | 33.46[ |
| ChCl/EG(1∶2) | 87.92 | 313.15~333.15 | 0.1~5.5 | 0.035~0.511[ | 1.117[ | 192.2~196.4[ | 602.0[ | 40.99[ |
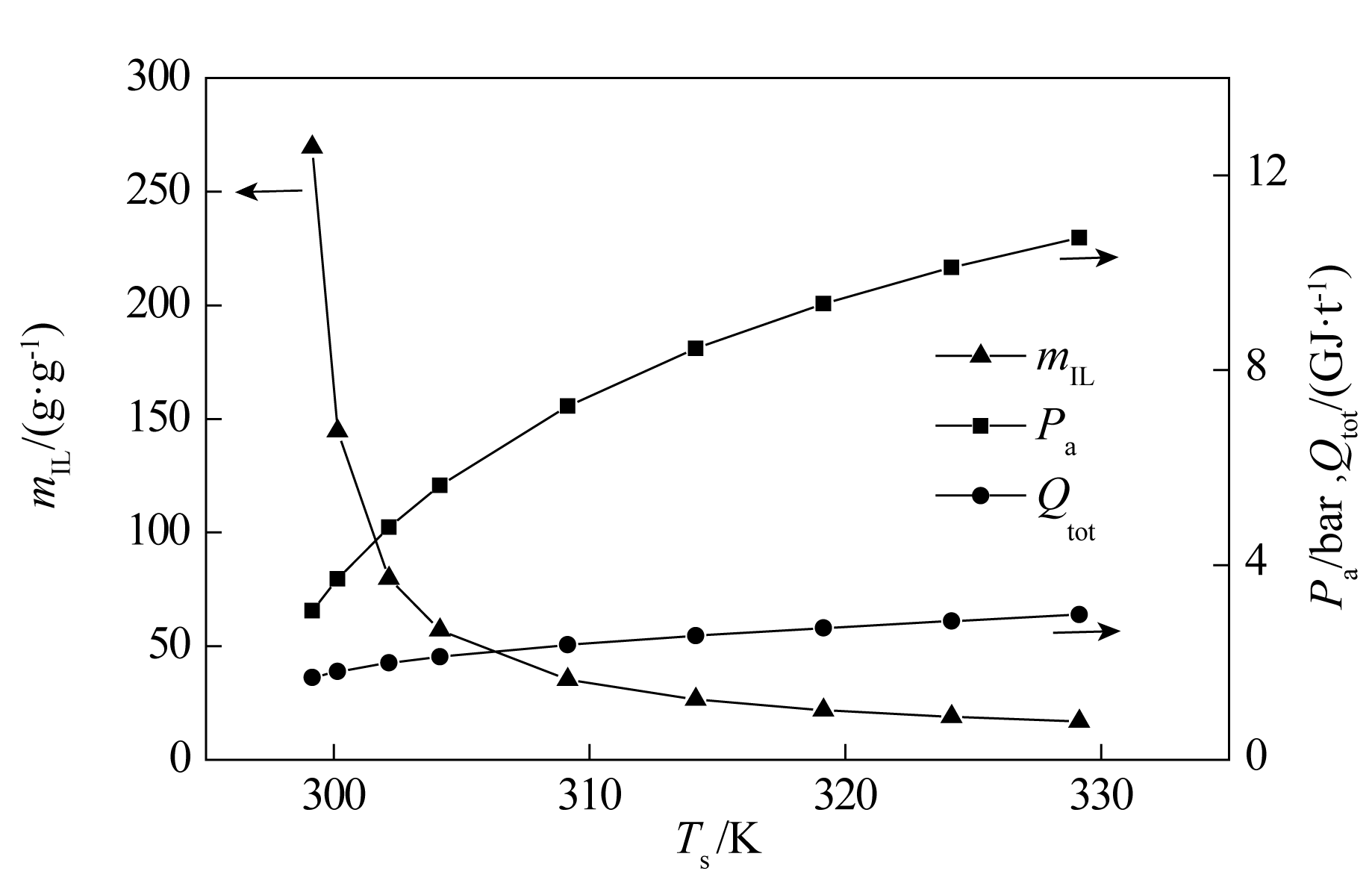
图4 [Bmim][BF4]的吸收压力、离子液体用量、总能耗与解吸温度的关系
Fig.4 The relationship between absorption pressures, the amounts of ILs needed, the energy uses and desorption temperature of [Bmim][BF4]
| 离子液体/低共熔溶剂 | Ts/K | Pa/bar | mIL/DES/(g?g-1) | Qtot/(GJ?t-1) |
|---|---|---|---|---|
| [Bmim][BF4] | 299.15~314.85 | 3.06~8.58 | 25.75~269.40 | 1.54~2.29 |
| [Hmim][BF4] | 299.15~319.15 | 3.29~11.31 | 21.05~264.70 | 1.39~1.85 |
| [Omim][BF4] | 299.15~319.15 | 3.31~11.06 | 20.12~257.05 | 1.05~1.91 |
| ChCl/EG(1∶2) | 314.85~319.15 | 2.34~2.38 | 15.18~17.72 | 2.37~2.40 |
表3 筛选出的离子液体/低共熔溶剂的解吸温度、吸收压力、吸收剂用量和总能耗
Table 3 The desorption temperature, absorption pressure, amounts of absorbents needed and total energy uses of the screened ILs/DESs
| 离子液体/低共熔溶剂 | Ts/K | Pa/bar | mIL/DES/(g?g-1) | Qtot/(GJ?t-1) |
|---|---|---|---|---|
| [Bmim][BF4] | 299.15~314.85 | 3.06~8.58 | 25.75~269.40 | 1.54~2.29 |
| [Hmim][BF4] | 299.15~319.15 | 3.29~11.31 | 21.05~264.70 | 1.39~1.85 |
| [Omim][BF4] | 299.15~319.15 | 3.31~11.06 | 20.12~257.05 | 1.05~1.91 |
| ChCl/EG(1∶2) | 314.85~319.15 | 2.34~2.38 | 15.18~17.72 | 2.37~2.40 |
| 参数 | 数值 |
|---|---|
| 分子量MIL/(g?mol-1) | 18 |
| 溶解度 | 0.253~0.290[ |
| 密度ρ/(g?cm-3) | 0.997[ |
| 比热容cp/(J?mol-1?K-1) | 75.3~75.4[ |
表4 水的摩尔质量、密度、NH3溶解度和比定压热容
Table 4 The molecular weight, density, NH3 solubility and isobaric heat capacity of water
| 参数 | 数值 |
|---|---|
| 分子量MIL/(g?mol-1) | 18 |
| 溶解度 | 0.253~0.290[ |
| 密度ρ/(g?cm-3) | 0.997[ |
| 比热容cp/(J?mol-1?K-1) | 75.3~75.4[ |

图8 [Omim][BF4]和水的吸收剂用量、总能耗与解吸温度的关系
Fig.8 The relationship between the amounts of absorbents needed, the total energy uses and desorption temperature of [Omim][BF4] and water
| 体系 | Pa | mIL/DES | Qtot | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | AARD/% | c×10-5 | d | e | AARD/% | f | g | h×103 | AARD/% | |
| [Emim][BF4] | 191.99 | -78.94 | 4.73 | 25.84 | 0.27 | -6.96 | 0.61 | -17.07 | 921.53 | -12.44 | 1.38 |
| [Bmim][BF4] | 272.41 | -155.94 | 5.05 | 9.02 | 0.36 | -9.30 | 0.03 | -7.70 | 527.65 | -9.03 | 3.20 |
| [Hmim][BF4] | 309.51 | -224.34 | 5.48 | -1.76 | 0.43 | -10.50 | 0.09 | -3.60 | 310.54 | -6.69 | 6.96 |
| [Omim][BF4] | 299.36 | -262.65 | 5.77 | -7.78 | 0.39 | -11.70 | 0.11 | -1.55 | 163.35 | -4.31 | 8.03 |
| [Bmim][PF6] | 340.73 | -215.43 | 5.51 | -7.09 | 0.48 | -11.11 | 0.08 | -2.48 | 232.74 | -5.47 | 5.60 |
| ChCl/urea(1∶2) | 31.58 | -0.29 | 0.14 | 0.83 | -0.01 | 23.59 | 7.47 | -9.35 | 253.40 | -1.71 | 2.47 |
| ChCl/gly(1∶2) | 6.82 | -298.99 | 3.28 | 2.02 | |||||||
| ChCl/EG(1∶2) | 11.76 | -365.08 | 2.84 | 2.10 | |||||||
表5 规律的拟合系数与平均相对偏差
Table 5 The fitting coefficients and average absolute relative deviation of the law
| 体系 | Pa | mIL/DES | Qtot | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a | b | AARD/% | c×10-5 | d | e | AARD/% | f | g | h×103 | AARD/% | |
| [Emim][BF4] | 191.99 | -78.94 | 4.73 | 25.84 | 0.27 | -6.96 | 0.61 | -17.07 | 921.53 | -12.44 | 1.38 |
| [Bmim][BF4] | 272.41 | -155.94 | 5.05 | 9.02 | 0.36 | -9.30 | 0.03 | -7.70 | 527.65 | -9.03 | 3.20 |
| [Hmim][BF4] | 309.51 | -224.34 | 5.48 | -1.76 | 0.43 | -10.50 | 0.09 | -3.60 | 310.54 | -6.69 | 6.96 |
| [Omim][BF4] | 299.36 | -262.65 | 5.77 | -7.78 | 0.39 | -11.70 | 0.11 | -1.55 | 163.35 | -4.31 | 8.03 |
| [Bmim][PF6] | 340.73 | -215.43 | 5.51 | -7.09 | 0.48 | -11.11 | 0.08 | -2.48 | 232.74 | -5.47 | 5.60 |
| ChCl/urea(1∶2) | 31.58 | -0.29 | 0.14 | 0.83 | -0.01 | 23.59 | 7.47 | -9.35 | 253.40 | -1.71 | 2.47 |
| ChCl/gly(1∶2) | 6.82 | -298.99 | 3.28 | 2.02 | |||||||
| ChCl/EG(1∶2) | 11.76 | -365.08 | 2.84 | 2.10 | |||||||
| 25 | Duan X Z, Gao B, Zhang C, et al. Solubility and thermodynamic properties of NH3 in choline chloride-based deep eutectic solvents[J]. J. Chem. Thermodyn., 2019, 133: 79-84. |
| 26 | Xu W G, Li L, Ma X X, et al. Density, surface tension, and refractive index of ionic liquids homologue of 1-alkyl-3-methylimidazolium tetrafluoroborate [Cnmim][BF4] (n = 2, 3, 4, 5, 6)[J]. J. Chem. Eng. Data, 2012, 57(8): 2177-2184. |
| 27 | Matkowska D, Hofman T. High-pressure volumetric properties of ionic liquids: 1-butyl-3-methylimidazolium tetrafluoroborate, [C4mim][BF4], 1-butyl-3-methylimidazolium methylsulfate [C4mim][MeSO4] and 1-ethyl-3-methylimidazolium ethylsulfate, [C2mim][EtSO4][J]. J. Mol. Liq., 2012, 165: 161-167. |
| 28 | Sanmamed Y A, González-Salgado D, Troncoso J, et al. Experimental methodology for precise determination of density of RTILs as a function of temperature and pressure using vibrating tube densimeters[J]. J. Chem. Thermodyn., 2010, 42(4): 553-563. |
| 29 | Sanmamed Y A, González-Salgado D, Troncoso J, et al. Viscosity-induced errors in the density determination of room temperature ionic liquids using vibrating tube densitometry[J]. Fluid Phase Equilibr., 2007, 252(1/2): 96-102. |
| 30 | Salgado J, Regueira T, Lugo L, et al. Density and viscosity of three (2, 2, 2-trifluoroethanol + 1-butyl-3-methylimidazolium) ionic liquid binary systems[J]. J. Chem. Thermodyn., 2014, 70: 101-110. |
| 31 | Yadav A, Pandey S. Densities and viscosities of (choline chloride + urea) deep eutectic solvent and its aqueous mixtures in the temperature range 293.15 K to 363.15 K[J]. J. Chem. Eng. Data, 2014, 59(7): 2221-2229. |
| 32 | Shahbaz K, Bagh F S G, Mjalli F S, et al. Prediction of refractive index and density of deep eutectic solvents using atomic contributions[J]. Fluid Phase Equilibr., 2013, 354: 304-311. |
| 33 | Leron R B, Li M H. High-pressure volumetric properties of choline chloride–ethylene glycol based deep eutectic solvent and its mixtures with water[J]. Thermochim. Acta, 2012, 546: 54-60. |
| 34 | Waliszewski D, Stepniak I, Piekarski H, et al. Heat capacities of ionic liquids and their heats of solution in molecular liquids[J]. Thermochim. Acta, 2005, 433: 149-152. |
| 35 | Paulechka Y U, Blokhin A V, Kabo G J. Evaluation of thermodynamic properties for non-crystallizable ionic liquids[J]. Thermochim. Acta, 2015, 604: 122-128. |
| 36 | Waliszewski D. Heat capacities of the mixtures of ionic liquids with methanol at temperatures from 283.15 K to 323.15 K[J]. J. Chem. Thermodyn., 2008, 40(2): 203-207. |
| 1 | Brautbar N, Wu M P, Richter E D. Chronic ammonia inhalation and interstitial pulmonary fibrosis: a case report and review of the literature[J]. Arch. Environ. Health, 2003, 58(9): 592-596. |
| 2 | Zhang Y S, Luan S J, Chen L L, et al. Estimating the volatilization of ammonia from synthetic nitrogenous fertilizers used in China[J]. J. Environ. Manage., 2011, 92(3): 480-493. |
| 3 | Matthews E. Nitrogenous fertilizers: global distribution of consumption and associated emissions of nitrous oxide and ammonia[J]. Global Biogeochem. Cy., 1994, 8(4): 411-439. |
| 4 | Jung S Y, Lee S J, Park J J, et al. The simultaneous removal of hydrogen sulfide and ammonia over zinc-based dry sorbent supported on alumina[J]. Sep. Purif. Technol., 2008, 63(2): 297-302. |
| 5 | Amblard M, Burch R, Southward B W L. A study of the mechanism of selective conversion of ammonia to nitrogen on Ni/γ-Al2O3 under strongly oxidising conditions[J]. Catal. Today, 2000, 59(3/4): 365-371. |
| 37 | Zhang Z H, Cui T, Zhang J L, et al. Thermodynamic investigation of room temperature ionic liquid: the heat capacity and thermodynamic functions of BMIPF6[J]. J. Therm. Anal. Calorim., 2010, 101: 1143-1148. |
| 38 | Leron R B, Li M H. Molar heat capacities of choline chloride-based deep eutectic solvents and their binary mixtures with water[J]. Thermochim. Acta, 2012, 530: 52-57. |
| 39 | Valderrama J O, Robles P A. Critical properties, normal boiling temperatures, and acentric factors of fifty ionic liquids[J]. Ind. Eng. Chem. Res., 2007, 46(4): 1338-1344. |
| 40 | Peyrovedin H, Haghbakhsh R, Duarte A R C, et al. A global model for the estimation of speeds of sound in deep eutectic solvents[J]. Molecules, 2020, 25(7): 1626. |
| 41 | 陈五平. 无机化工工艺学 (一): 合成氨[M]. 北京: 化学工业出版社, 1997: 2. |
| Chen W P. Inorganic Chemical Technology (Ⅰ): Synthetic Ammonia [M]. Beijing: Chemical Industry Press, 1997: 2. | |
| 6 | Rumburg B, Neger M, Mount G H, et al. Liquid and atmospheric ammonia concentrations from a dairy lagoon during an aeration experiment[J]. Atmospheric Environ., 2004, 38(10): 1523-1533. |
| 7 | Dasgupta P K, Dong S. Solubility of ammonia in liquid water and generation of trace levels of standard gaseous ammonia[J]. Atmospheric Environ., 1986, 20(3): 565-570. |
| 8 | Rumpf B, Maurer G. Solubility of ammonia in aqueous solutions of phosphoric acid: model development and application[J]. J. Solution Chem., 1994, 23(1): 37-51. |
| 9 | Yokozeki A, Shiflett M B. Ammonia solubilities in room-temperature ionic liquids[J]. Ind. Eng. Chem. Res., 2007, 46(5): 1605-1610. |
| 10 | Gonzalez-Miquel M, Palomar J, Omar S, et al. CO2/N2 selectivity prediction in supported ionic liquid membranes (SILMs) by COSMO-RS[J]. Ind. Eng. Chem. Res., 2011, 50(9): 5739-5748. |
| 11 | Zhao Y S, Gani R, Afzal R M, et al. Ionic liquids for absorption and separation of gases: an extensive database and a systematic screening method[J]. AIChE J., 2017, 63(4): 1353-1367. |
| 12 | Zhang J Y, Huang K. Densities and viscosities of, and NH3 solubilities in deep eutectic solvents composed of ethylamine hydrochloride and acetamide[J]. J. Chem. Thermodyn., 2019, 139: 105883. |
| 13 | Oliveira F S, Rebelo L P N, Marrucho I M. Influence of different inorganic salts on the ionicity and thermophysical properties of 1-ethyl-3-methylimidazolium acetate ionic liquid[J]. J. Chem. Eng. Data, 2015, 60(3): 781-789. |
| 14 | Ning H, Hou M Q, Mei Q Q, et al. The physicochemical properties of some imidazolium-based ionic liquids and their binary mixtures[J]. Sci. China Chem., 2012, 55(8): 1509-1518. |
| 15 | 冯伟珍. 合成氨系统弛放气、吹出气中的氨回收[J]. 磷肥与复肥, 2018, 33(7): 32-34. |
| Feng W Z. Recycling of ammonia in purge gas and blow out gas of synthesis ammonia system[J]. Phosphate & Compound Fertilizer, 2018, 33(7): 32-34. | |
| 16 | 梁鹏. 合成氨生产中的废气利用及节能效益[J]. 中国石油和化工标准与质量, 2019, 39(14): 16-17. |
| Liang P. Waste gas utilization and energy saving benefits in ammonia production[J]. China Petroleum and Chemical Standard and Quality, 2019, 39(14): 16-17. | |
| 17 | Redlich O, Kwong J N S. On the thermodynamics of solutions (Ⅴ): An equation of state. Fugacities of gaseous solutions[J]. Chem. Rev., 1949, 44(1): 233-244. |
| 18 | Yan Y, Chen C C. Thermodynamic modeling of CO2 solubility in aqueous solutions of NaCl and Na2SO4[J]. J. Supercrit. Fluid, 2010, 55(2): 623-634. |
| 19 | Oexmann J, Kather A. Minimising the regeneration heat duty of post-combustion CO2 capture by wet chemical absorption: the misguided focus on low heat of absorption solvents[J]. Int. J. Greenh. Gas Control., 2010, 4(1): 36-43. |
| 20 | Zhang Y Y, Ji X Y, Xie Y J, et al. Thermodynamic analysis of CO2 separation from biogas with conventional ionic liquids[J]. Appl. Energ., 2018, 217: 75-87. |
| 21 | Glasser L. Lattice and phase transition thermodynamics of ionic liquids[J]. Thermochim. Acta, 2004, 421(1/2): 87-93. |
| 22 | Fang D W, Yan Q, Li D, et al. Estimation of physicochemical properties of 1-alkyl-3-methylimidazolium glutamate[J]. J. Chem. Thermodyn., 2014, 79: 12-18. |
| 23 | Li G H, Zhou Q, Zhang X P, et al. Solubilities of ammonia in basic imidazolium ionic liquids[J]. Fluid Phase Equilibr., 2010, 297(1): 34-39. |
| 24 | Zhong F Y, Huang K, Peng H L. Solubilities of ammonia in choline chloride plus urea at (298.2—353.2) K and (0—300) kPa[J]. J. Chem. Thermodyn., 2019, 129: 5-11. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [6] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [7] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [8] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [9] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [10] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [11] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [12] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [13] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [14] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [15] | 龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号