化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4921-4930.DOI: 10.11949/0438-1157.20210041
演康1,3( ),杨颂1,3,刘守军1,2,3(
),杨颂1,3,刘守军1,2,3( ),杨超2,樊惠玲2,3,上官炬2,3
),杨超2,樊惠玲2,3,上官炬2,3
收稿日期:2021-01-08
修回日期:2021-04-23
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
刘守军
作者简介:演康(1995—),男,硕士研究生,基金资助:
Kang YAN1,3( ),Song YANG1,3,Shoujun LIU1,2,3(
),Song YANG1,3,Shoujun LIU1,2,3( ),Chao YANG2,Huiling FAN2,3,Ju SHANGGUAN2,3
),Chao YANG2,Huiling FAN2,3,Ju SHANGGUAN2,3
Received:2021-01-08
Revised:2021-04-23
Online:2021-09-05
Published:2021-09-05
Contact:
Shoujun LIU
摘要:
将金属氧化物活性组分通过浸渍负载的方式分散到多孔载体上,是制备高活性金属氧化物脱硫剂的常用方法。然而,由于活性组分的负载易使载体孔隙率下降,导致活性组分的脱硫能力不能充分发挥。本文直接以廉价的低阶煤为原料,经过预处理后在煤中加入硝酸锌,通过物理-化学活化法一步制备ZnO基活性炭常温脱硫剂,即将活性炭的制备与活性组分的负载一步完成。研究了硝酸锌加入量、活化温度和活化时间对脱硫剂脱硫性能的影响。结果表明:当硝酸锌加入量为20%(质量),活化温度为850℃,活化时间为1 h时,脱硫剂的穿透时间为210 min,其对应的穿透硫容为71.4 mg/g,其脱硫性能是同等实验条件下商业活性炭负载ZnO脱硫剂的5.3倍,较高的脱硫性能主要归因于其发达的介孔孔隙,不仅有利于传质,而且有利于硫化产物的存储。
中图分类号:
演康, 杨颂, 刘守军, 杨超, 樊惠玲, 上官炬. 低阶煤原位制备ZnO基活性炭脱硫剂[J]. 化工学报, 2021, 72(9): 4921-4930.
Kang YAN, Song YANG, Shoujun LIU, Chao YANG, Huiling FAN, Ju SHANGGUAN. In-situ preparation of ZnO-based activated carbon desulfurizer from low-rank coal[J]. CIESC Journal, 2021, 72(9): 4921-4930.
| 工业分析/% | 元素分析/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vad | Mad | Aad | FCad | Cad | Had | Oad① | Nad | Sad | |
| 31.57 | 22.16 | 7.24 | 39.03 | 47.54 | 4.61 | 16.97 | 0.61 | 0.87 | |
表1 WM的工业分析及元素分析
Table 1 Industrial analysis and elemental analysis of WM
| 工业分析/% | 元素分析/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vad | Mad | Aad | FCad | Cad | Had | Oad① | Nad | Sad | |
| 31.57 | 22.16 | 7.24 | 39.03 | 47.54 | 4.61 | 16.97 | 0.61 | 0.87 | |

图3 不同硝酸锌加入量所制备脱硫剂的穿透曲线(a)和对应的穿透硫容(b)
Fig.3 The breakthrough curves (a) and the breakthrough sulfur capacity (b) of the adsorbents prepared with different Zn(NO3)2 impregnation content
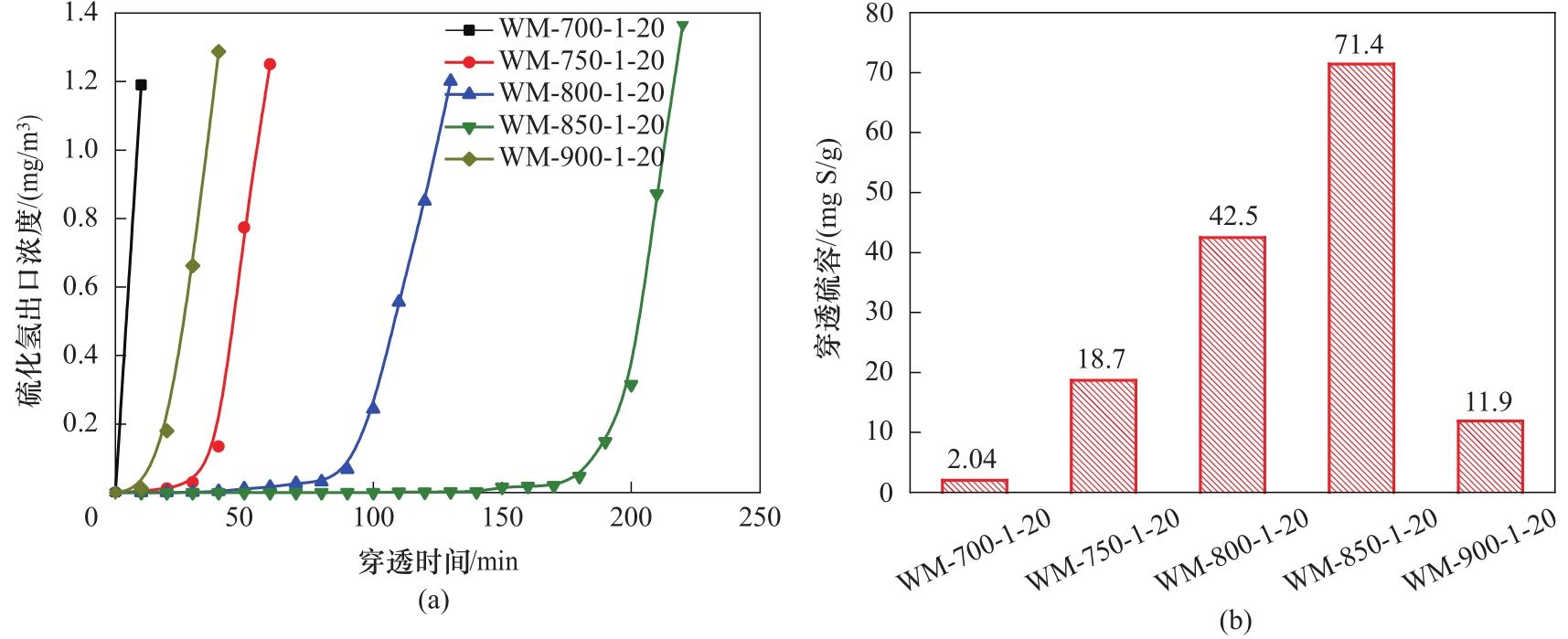
图4 不同活化温度所制备脱硫剂的穿透曲线(a)和对应的穿透硫容(b)
Fig.4 The breakthrough curves (a) and the breakthrough sulfur capacity (b) of the adsorbents prepared with different activation temperature

图5 不同活化时间所制备脱硫剂的穿透曲线(a)和对应的穿透硫容(b)
Fig.5 The breakthrough curves (a) and the breakthrough sulfur capacity (b) of the adsorbents prepared with different activation time

图6 商业活性炭负载ZnO脱硫剂和原位制备ZnO活性炭脱硫剂的穿透曲线(a)和对应的穿透硫容(b)
Fig.6 The breakthrough curves (a) and the breakthrough sulfur capacity (b) of the commercial activated carbon supported ZnO adsorbent and in-situ prepared ZnO activated carbon adsorbent

图8 AC-20和WM-850-1-20的N2吸脱附等温线(a)及孔径分布(b)
Fig. 8 The adsorption and desorption isotherms (a) and the pore size distribution (b) of AC-20 and WM-850-1-20
| 样品 | 比表面积/(m2/g) | 总孔/(cm3/g) | 微孔/(cm3/g) | 介孔/(cm3/g) | 介孔/总孔 |
|---|---|---|---|---|---|
| AC-20 | 913 | 0.47 | 0.37 | 0.10 | 0.21 |
| WM-850-1-20 | 355 | 0.25 | 0.08 | 0.17 | 0.68 |
表2 AC-20和WM-850-1-20的织构性质
Table 2 Textural properties of AC-20 and WM-850-1-20
| 样品 | 比表面积/(m2/g) | 总孔/(cm3/g) | 微孔/(cm3/g) | 介孔/(cm3/g) | 介孔/总孔 |
|---|---|---|---|---|---|
| AC-20 | 913 | 0.47 | 0.37 | 0.10 | 0.21 |
| WM-850-1-20 | 355 | 0.25 | 0.08 | 0.17 | 0.68 |
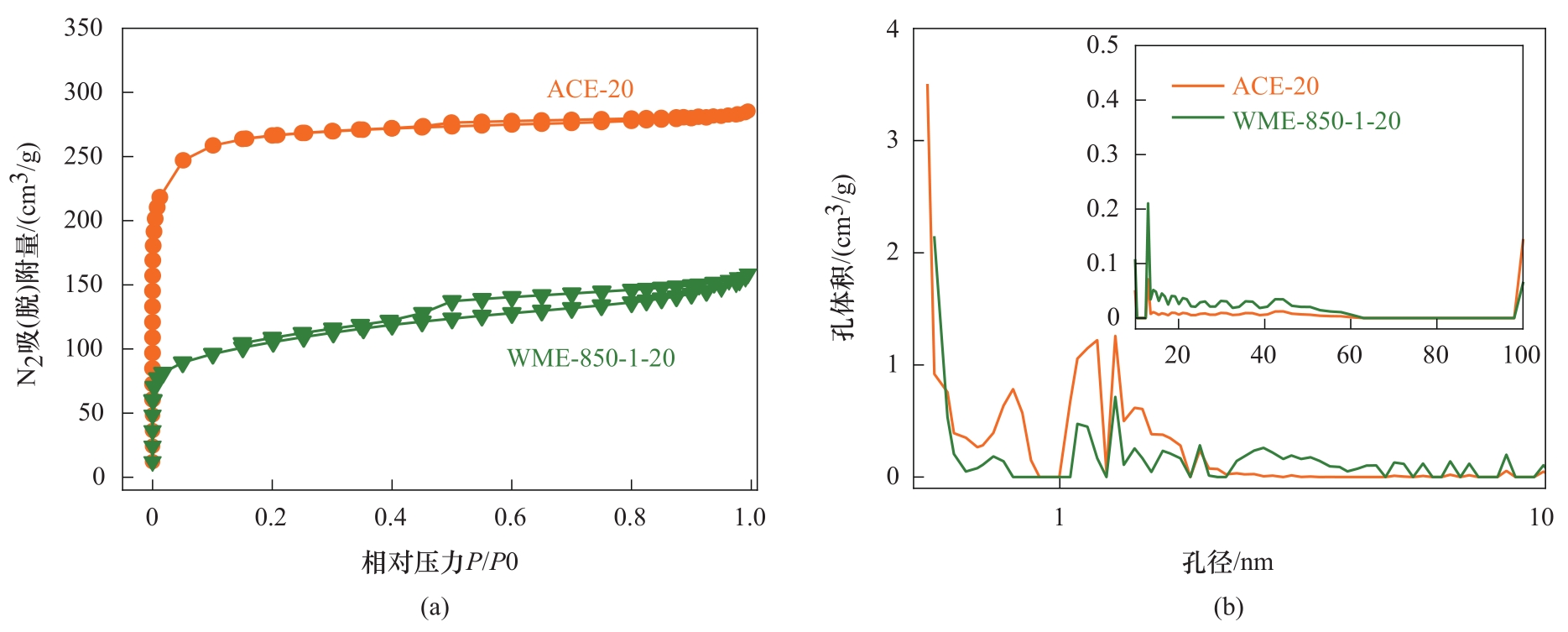
图11 ACE-20和WME-850-1-20的N2吸脱附等温线(a)及孔径分布(b)
Fig. 11 The N2 adsorption and desorption isotherms (a) and the pore size distribution (b) of ACE-20 and WME-850-1-20
| 样品 | 比表面积/(m2/g) | 总孔/(cm3/g) | 微孔/(cm3/g) | 介孔/(cm3/g) | 介孔/总孔 |
|---|---|---|---|---|---|
| ACE-20 | 854 | 0.44 | 0.35 | 0.09 | 0.20 |
| WME-850-1-20 | 302 | 0.20 | 0.05 | 0.15 | 0.75 |
表3 ACE-20和WME-850-1-20的织构性质
Table 3 Textural properties of ACE-20 and WME-850-1-20
| 样品 | 比表面积/(m2/g) | 总孔/(cm3/g) | 微孔/(cm3/g) | 介孔/(cm3/g) | 介孔/总孔 |
|---|---|---|---|---|---|
| ACE-20 | 854 | 0.44 | 0.35 | 0.09 | 0.20 |
| WME-850-1-20 | 302 | 0.20 | 0.05 | 0.15 | 0.75 |
| 1 | Rasi S, Läntelä J, Rintala J. Trace compounds affecting biogas energy utilisation—a review[J]. Energy Conversion and Management, 2011, 52(12): 3369-3375. |
| 2 | Rosso I, Galletti C, Bizzi M, et al. Zinc oxide sorbents for the removal of hydrogen sulfide from syngas[J]. Industrial & Engineering Chemistry Research, 2003, 42(8): 1688-1697. |
| 3 | Li L, Sun T H, Shu C H, et al. Low temperature H2S removal with 3-D structural mesoporous molecular sieves supported ZnO from gas stream[J]. Journal of Hazardous Materials, 2016, 311: 142-150. |
| 4 | Yang C, Wang J, Fan H L, et al. Contributions of tailored oxygen vacancies in ZnO/Al2O3 composites to the enhanced ability for H2S removal at room temperature[J]. Fuel, 2018, 215: 695-703. |
| 5 | Yang C, Wang J, Fan H L, et al. Activated carbon-assisted fabrication of cost-efficient ZnO/SiO2 desulfurizer with characteristic of high loadings and high dispersion[J]. Energy & Fuels, 2018, 32(5): 6064-6072. |
| 6 | 耿强. 熔渗法制备氧化锌基脱硫剂及其常温脱硫性能研究[D]. 太原: 太原理工大学, 2019. |
| Geng Q. Study on preparation of zinc oxide-based desulfurizer by infiltration method and its desulfurization performance at room temperature[D]. Taiyuan: Taiyuan University of Technology, 2019. | |
| 7 | 胡佩雷, 徐华龙, 沈伟. 改性Zr-Na/zeolite双功能沸石脱除水溶液中氨氮和磷性能[J]. 精细化工, 2018, 35(9): 1601-1608. |
| Hu P L, Xu H L, Shen W. Removal of ammonium and phosphate from aqueous solution by dual-functional Zr-Na modified zeolite[J]. Fine Chemicals, 2018, 35(9): 1601-1608. | |
| 8 | 李灿, 马福秋, 葛春元, 等. 改性介孔二氧化硅对硫化氢的吸附研究[J]. 中国环保产业, 2018(7): 39-42. |
| Li C, Ma F Q, Ge C Y, et al. Study on adsorption of sulfureted hydrogen by metallic oxide modification and meso-porous silicon dioxide[J]. China Environmental Protection Industry, 2018(7): 39-42. | |
| 9 | 王爱民, 白妮, 张国涛, 等. 污泥-兰炭末基成型活性炭的制备及吸附性能研究[J]. 精细化工, 2017, 34(2): 207-213. |
| Wang A M, Bai N, Zhang G T, et al. Study on preparation of pressed active carbon based on sewage sludge and fine semi-coke and properties of adsorption[J]. Fine Chemicals, 2017, 34(2): 207-213. | |
| 10 | Nguyen-Thanh D, Bandosz T J. Activated carbons with metal containing bentonite binders as adsorbents of hydrogen sulfide[J]. Carbon, 2005, 43(2): 359-367. |
| 11 | Sun F G, Liu J, Chen H C, et al. Nitrogen-rich mesoporous carbons: highly efficient, regenerable metal-free catalysts for low-temperature oxidation of H2S[J]. ACS Catalysis, 2013, 3(5): 862-870. |
| 12 | Bagreev A, Angel Menendez J, Dukhno I, et al. Bituminous coal-based activated carbons modified with nitrogen as adsorbents of hydrogen sulfide[J]. Carbon, 2004, 42(3): 469-476. |
| 13 | Li Y M, Liu X. Activated carbon/ZnO composites prepared using hydrochars as intermediate and their electrochemical performance in supercapacitor[J]. Materials Chemistry and Physics, 2014, 148(1/2): 380-386. |
| 14 | 宋华, 王璐, 张娇静, 等. 氧化铁改性活性炭的制备及其吸附脱硫性能[J]. 化工进展, 2013, 32(3): 639-644, 651. |
| Song H, Wang L, Zhang J J, et al. Adsorption of H2S by iron oxide modified activate carbon[J]. Chemical Industry and Engineering Progress, 2013, 32(3): 639-644, 651. | |
| 15 | 李芬, 张彦平, 杨莹, 等. 活性炭负载纳米ZnO的结构及常温脱除H2S的性能[J]. 硅酸盐学报, 2012, 40(6): 800-805. |
| Li F, Zhang Y P, Yang Y, et al. Structure of activated carbon supported with nano-ZnO and its removal performance of H2S at room temperature[J]. Journal of the Chinese Ceramic Society, 2012, 40(6): 800-805. | |
| 16 | Boutillara Y, Tombeur J L, De Weireld G, et al. In-situ copper impregnation by chemical activation with CuCl2 and its application to SO2 and H2S capture by activated carbons[J]. Chemical Engineering Journal, 2019, 372: 631-637. |
| 17 | 申烨华, 李文超, 陈邦, 等. 氧化锌法制备活性炭: 106115699A[P]. 2016-11-16. |
| Shen Y H, Li W C, Chen B, et al. Zinc oxide method for preparing activated carbon: 106115699A[P]. 2016-11-16. | |
| 18 | 黄文辉, 杨起, 唐修义, 等. 中国炼焦煤资源分布特点与深部资源潜力分析[J]. 中国煤炭地质, 2010, 22(5): 1-6. |
| Huang W H, Yang Q, Tang X Y, et al. Distribution features of coal for coking resource in China and deep part potential analysis[J]. Coal Geology of China, 2010, 22(5): 1-6. | |
| 19 | 邢宝林, 黄光许, 谌伦建, 等. 高品质低阶煤基活性炭的制备与表征[J]. 煤炭学报, 2013, 38(S1): 217-222. |
| Xing B L, Huang G X, Chen L J, et al. Preparation and characterization of high quality low-rank coal based activated carbon[J]. Journal of China Coal Society, 2013, 38(S1): 217-222. | |
| 20 | 王秀芳, 田勇, 张会平. 高比表面积煤质活性炭的制备与活化机理[J]. 化工学报, 2009, 60(3): 733-737. |
| Wang X F, Tian Y, Zhang H P. Preparation and activation mechanism of high specific surface area coal-based activated carbon[J]. CIESC Journal, 2009, 60(3): 733-737. | |
| 21 | Yang C, Yang S, Fan H L, et al. Tuning the ZnO-activated carbon interaction through nitrogen modification for enhancing the H2S removal capacity[J]. Journal of Colloid and Interface Science, 2019, 555: 548-557. |
| 22 | 解强, 姚鑫, 杨川, 等. 压块工艺条件下煤种对活性炭孔结构发育的影响[J]. 煤炭学报, 2015, 40(1): 196-202. |
| Xie Q, Yao X, Yang C, et al. Effect of coalification degree of coals on the porosity of coal-based granular activated carbon prepared by briquetting method[J]. Journal of China Coal Society, 2015, 40(1):196-202. | |
| 23 | 易牡丹, 丘克强. 由酚醛树脂基板CO2活化法制备高性能活性炭[J]. 应用化工, 2012, 41(7): 1127-1131. |
| Yi M D, Qiu K Q. Preparation of high-properties activated carbon from phenolic resin laminated board with CO2 activation[J]. Applied Chemical Industry, 2012, 41(7): 1127-1131. | |
| 24 | Shi R H, Zhang Z R, Fan H L, et al. Cu-based metal-organic framework/activated carbon composites for sulfur compounds removal[J]. Applied Surface Science, 2017, 394: 394-402. |
| 25 | 邵纯红, 孙曙光, 张爽, 等. 纳米CuO/ZnO去除H2S反应条件及机理研究[J]. 化学工程师, 2010, 24(2): 13-15. |
| Shao C H, Sun S G, Zhang S, et al. Reaction condition and mechanism research with nano CuO/ZnO to remove H2S[J]. Chemical Engineer, 2010, 24(2): 13-15. | |
| 26 | Zhang R P, Wang Y, Jia M Q, et al. One-pot hydrothermal synthesis of ZnS quantum dots/graphene hybrids as a dual anode for sodium ion and lithium ion batteries[J]. Applied Surface Science, 2018, 437: 375-383. |
| 27 | Hao X Q, Wang Y C, Zhou J, et al. Zinc vacancy-promoted photocatalytic activity and photostability of ZnS for efficient visible-light-driven hydrogen evolution[J]. Applied Catalysis B: Environmental, 2018, 221: 302-311. |
| 28 | Yang C, Wang Y S, Fan H L, et al. Bifunctional ZnO-MgO/activated carbon adsorbents boost H2S room temperature adsorption and catalytic oxidation[J]. Applied Catalysis B: Environmental, 2020, 266: 118674. |
| 29 | Brazhnyk D V, Zaitsev Y P, Bacherikova I V, et al. Oxidation of H2S on activated carbon KAU and influence of the surface state[J]. Applied Catalysis B: Environmental, 2007, 70(1/2/3/4): 557-566. |
| 30 | 谭小耀, 吴迪镛, 袁权. 浸渍活性炭脱硫过程中孔结构及气体湿度的影响[J]. 化工学报, 1997, 48(2): 237-240. |
| Tan X Y, Wu D Y, Yuan Q. Influence of the pore structure and gas humidity on desulfurization by impregnated activated carbon[J]. Journal of Chemical Industry and Engineering (China), 1997, 48(2): 237-240. | |
| 31 | 李芬. 纳米锌基脱硫剂室温脱硫效能及再生研究[D]. 哈尔滨: 哈尔滨工业大学, 2007. |
| Li F. Study on desulfurization performance at ambient temperature and regeneration of nanocrystalline zinc-base sorbent[D]. Harbin: Harbin Institute of Technology, 2007. | |
| 32 | Wang L J, Fan H L, Shangguan J, et al. Design of a sorbent to enhance reactive adsorption of hydrogen sulfide[J]. ACS Applied Materials & Interfaces, 2014, 6(23): 21167-21177. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [3] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [4] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [5] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [6] | 吕龙义, 及文博, 韩沐达, 李伟光, 高文芳, 刘晓阳, 孙丽, 王鹏飞, 任芝军, 张光明. 铁基导电材料强化厌氧去除卤代有机污染物:研究进展及未来展望[J]. 化工学报, 2023, 74(8): 3193-3202. |
| [7] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [8] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [9] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [10] | 王新悦, 王俊杰, 曹思贤, 王翠, 李灵坤, 吴宏宇, 韩静, 吴昊. 玻璃内包材界面修饰对机械应力诱导的单克隆抗体聚集体形成的影响[J]. 化工学报, 2023, 74(6): 2580-2588. |
| [11] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [12] | 王蕾, 王磊, 白云龙, 何柳柳. SA膜状锂离子筛的制备及其锂吸附性能[J]. 化工学报, 2023, 74(5): 2046-2056. |
| [13] | 蔺彩虹, 王丽, 吴瑜, 刘鹏, 杨江峰, 李晋平. 沸石中碱金属阳离子对CO2/N2O吸附分离性能的影响[J]. 化工学报, 2023, 74(5): 2013-2021. |
| [14] | 李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066. |
| [15] | 肖川宝, 李林洋, 刘武锋, 钟年丙, 解泉华, 钟登杰, 常海星. 光催化与离子交换吸附耦合有效去除2,4,6-三氯苯酚[J]. 化工学报, 2023, 74(4): 1587-1597. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号