化工学报 ›› 2021, Vol. 72 ›› Issue (11): 5582-5589.DOI: 10.11949/0438-1157.20210935
收稿日期:2021-07-08
修回日期:2021-08-20
出版日期:2021-11-05
发布日期:2021-11-12
通讯作者:
兰文杰
作者简介:玄雪梅(1995—),女,博士研究生,
Xuemei XUAN( ),Miao WANG,Dizong CAI,Rui ZHANG,Wenjie LAN(
),Miao WANG,Dizong CAI,Rui ZHANG,Wenjie LAN( )
)
Received:2021-07-08
Revised:2021-08-20
Online:2021-11-05
Published:2021-11-12
Contact:
Wenjie LAN
摘要:
离子液体催化剂可以显著提高烷基化反应速率,开发适用于该工艺的高效反应器具有重要意义。基于微反应器在过程强化方面的优势,设计了适用于烷基化反应的微反应器,考察了烷基化模拟体系的流动规律,在较大操作范围内实现了催化剂与产物的连续分相。以上述研究为基础,实现了C4烷基化反应的微型化,分别考察了停留时间、反应温度、分散尺寸对反应性能的影响。研究结果表明,微反应器内的烷基化反应在2 s内完成。C8选择性和TMPs(三甲基戊烷)/DMHs(二甲基己烷)分别最高可达70.90%与13.4,均高于相同反应条件下搅拌釜反应器内的反应结果,证明微反应器在优化烷基化反应性能方面拥有巨大潜力。
中图分类号:
玄雪梅, 王苗, 蔡迪宗, 张睿, 兰文杰. 离子液体催化烷基化体系在微反应器内的流动和反应基础研究[J]. 化工学报, 2021, 72(11): 5582-5589.
Xuemei XUAN, Miao WANG, Dizong CAI, Rui ZHANG, Wenjie LAN. Fundamental study on flow and reaction performance of isobutane alkylation catalyzed by ionic liquid in microreactor[J]. CIESC Journal, 2021, 72(11): 5582-5589.
| 混合烃组分 | 摩尔分数/% |
|---|---|
| 异丁烷 | 97.7540 |
| 正丁烷 | 0.0675 |
| 反-2-丁烯 | 0.0192 |
| 正-1-丁烯 | 0.0254 |
| 异丁烯 | 0.0201 |
| 顺-2-丁烯 | 2.1138 |
表1 混合烃原料组成
Table 1 Components of the feedstock
| 混合烃组分 | 摩尔分数/% |
|---|---|
| 异丁烷 | 97.7540 |
| 正丁烷 | 0.0675 |
| 反-2-丁烯 | 0.0192 |
| 正-1-丁烯 | 0.0254 |
| 异丁烯 | 0.0201 |
| 顺-2-丁烯 | 2.1138 |
| 试剂 | 分子式 | 密度/ (g/cm3) | 黏度/ (mPa·s) |
|---|---|---|---|
| 正辛烷 | C8H18 | 0.70 | 0.55 |
| 基础离子液体 | Et3NHCl-1.8AlCl3 | 1.24 | 34.15 |
| 复合离子液体 | Et3NHCl-1.8AlCl3-xCuCl | 1.29 | 75.57 |
表2 两相流体的密度和黏度
Table 2 Density and viscosity of the fluid
| 试剂 | 分子式 | 密度/ (g/cm3) | 黏度/ (mPa·s) |
|---|---|---|---|
| 正辛烷 | C8H18 | 0.70 | 0.55 |
| 基础离子液体 | Et3NHCl-1.8AlCl3 | 1.24 | 34.15 |
| 复合离子液体 | Et3NHCl-1.8AlCl3-xCuCl | 1.29 | 75.57 |
| 序号 | 体系 | 界面张力/(mN/m) | |
|---|---|---|---|
| 分散相 | 连续相 | ||
| (W1) | 正辛烷 | 基础离子液体 | 7.75 |
| (W2) | 正辛烷 | 复合离子液体 | 10.21 |
表3 两相流体界面张力
Table 3 Interfacial tension of two-phase fluid
| 序号 | 体系 | 界面张力/(mN/m) | |
|---|---|---|---|
| 分散相 | 连续相 | ||
| (W1) | 正辛烷 | 基础离子液体 | 7.75 |
| (W2) | 正辛烷 | 复合离子液体 | 10.21 |
| 产物 | 产物分布/%(质量) | 平均值 | 标准偏差/%(质量) | 相对标准偏差/% | ||
|---|---|---|---|---|---|---|
| 1# | 2# | 3# | ||||
| C5 | 6.98 | 7.66 | 7.84 | 7.49 | 0.40 | 6.06 |
| C6 | 9.15 | 9.7 | 10.72 | 9.86 | 0.53 | 8.08 |
| C7 | 7.38 | 6.79 | 7.75 | 7.31 | 0.52 | 6.62 |
| C8 | 41.05 | 38.39 | 38.78 | 39.41 | 1.54 | 3.64 |
| 35.44 | 37.46 | 34.92 | 35.94 | 1.56 | 3.73 | |
| C8组分 | ||||||
| TMPs | 35.16 | 33.2 | 33.34 | 33.9 | 1.13 | 3.23 |
| DMHs | 5.61 | 4.89 | 5.17 | 5.22 | 0.43 | 6.95 |
表4 平行实验结果
Table 4 Parallel experiments results
| 产物 | 产物分布/%(质量) | 平均值 | 标准偏差/%(质量) | 相对标准偏差/% | ||
|---|---|---|---|---|---|---|
| 1# | 2# | 3# | ||||
| C5 | 6.98 | 7.66 | 7.84 | 7.49 | 0.40 | 6.06 |
| C6 | 9.15 | 9.7 | 10.72 | 9.86 | 0.53 | 8.08 |
| C7 | 7.38 | 6.79 | 7.75 | 7.31 | 0.52 | 6.62 |
| C8 | 41.05 | 38.39 | 38.78 | 39.41 | 1.54 | 3.64 |
| 35.44 | 37.46 | 34.92 | 35.94 | 1.56 | 3.73 | |
| C8组分 | ||||||
| TMPs | 35.16 | 33.2 | 33.34 | 33.9 | 1.13 | 3.23 |
| DMHs | 5.61 | 4.89 | 5.17 | 5.22 | 0.43 | 6.95 |
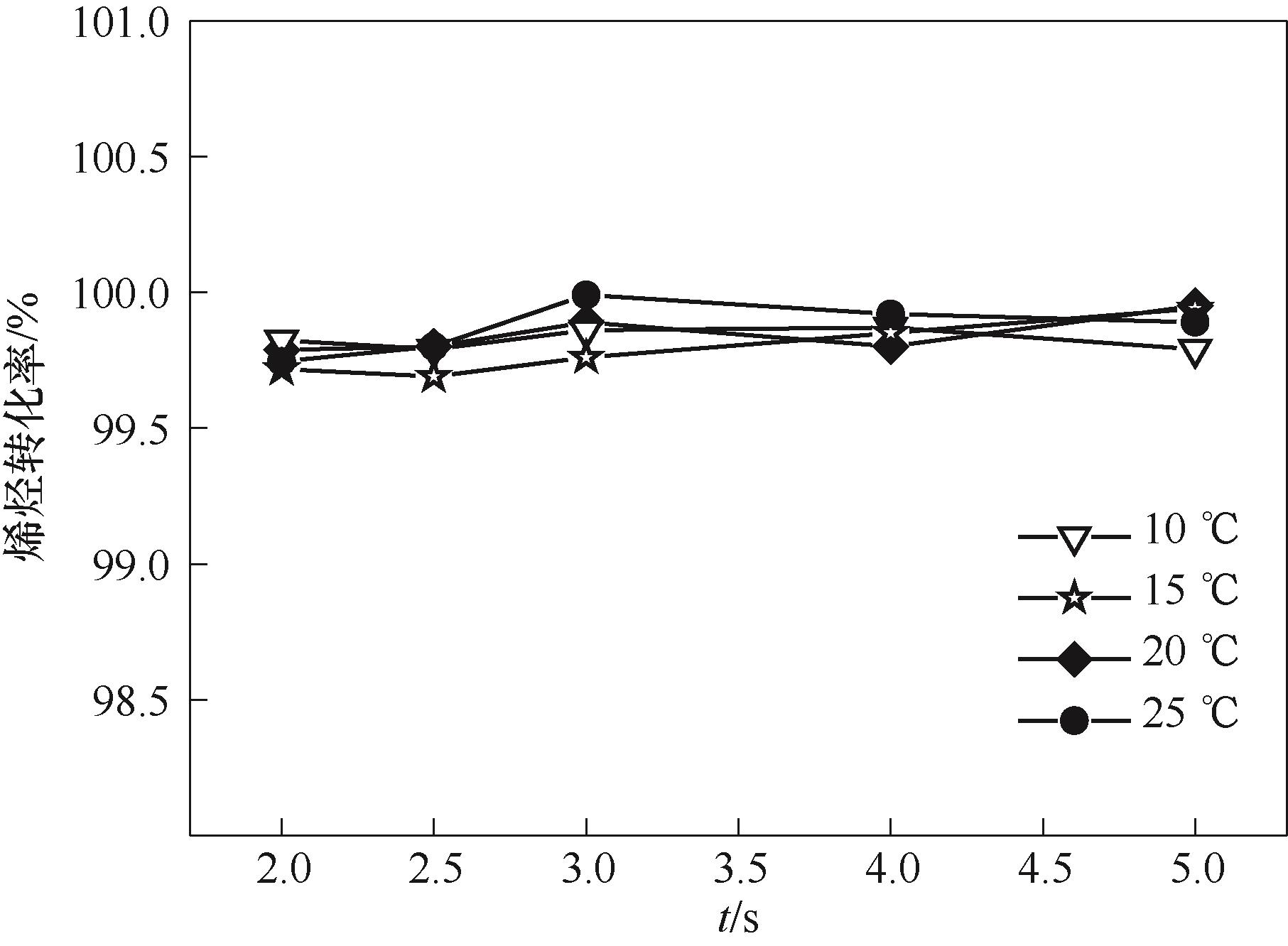
图7 烯烃转化率随停留时间的变化 (Qc = 40 μl/min,Qd = 20 μl/min, ddrop = 300 μm)
Fig.7 Variation of olefin conversion with residence time (Qc = 40 μl/min,Qd = 20 μl/min, ddrop = 300 μm)
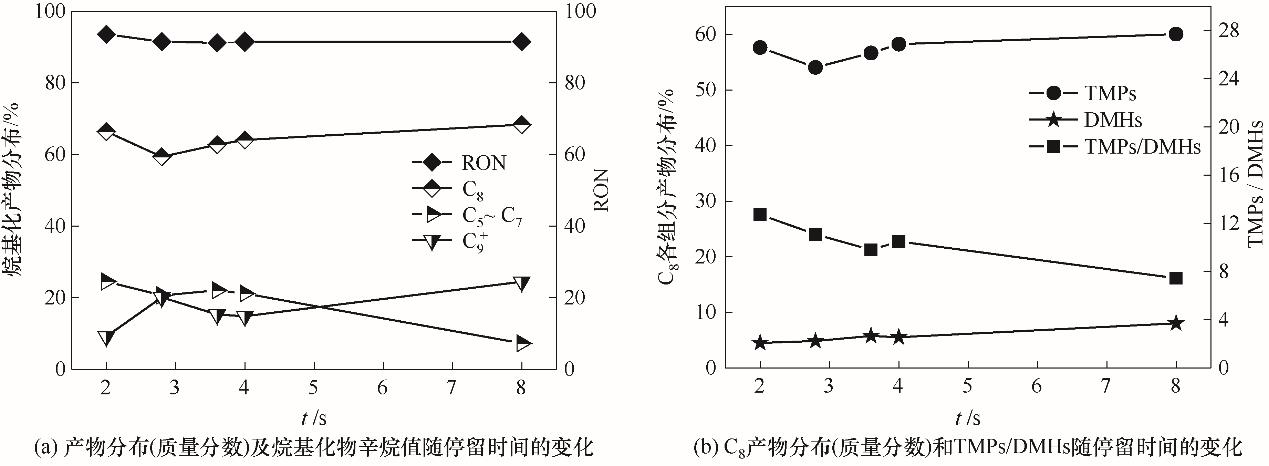
图8 停留时间对反应性能的影响(Qc = 40 μl/min,Qd = 20 μl/min, ddrop = 300 μm, T = 10℃)
Fig.8 The effect of residence time on reaction performance(Qc = 40 μl/min,Qd = 20 μl/min, ddrop = 300 μm, T = 10℃)
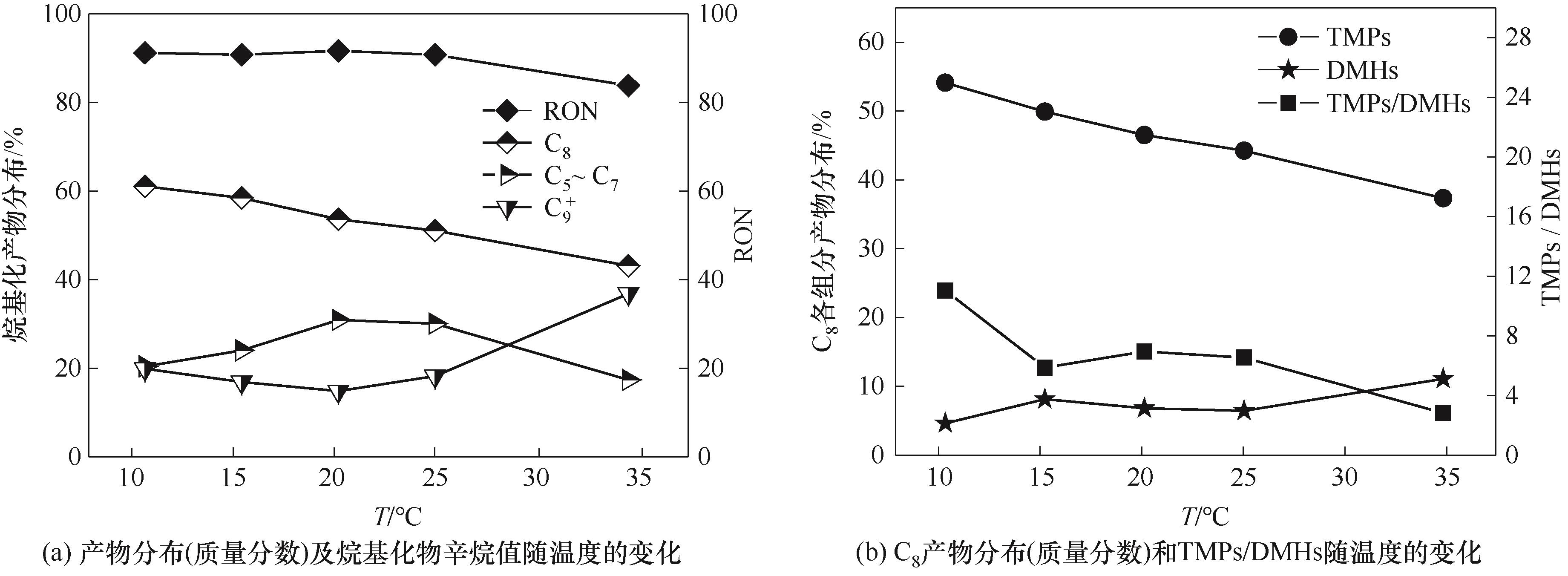
图9 温度对反应性能的影响(Qc = 40 μl/min,Qd = 20 μl/min, ddrop = 300 μm, t = 2 s)
Fig.9 The effect of temperature on reaction performance(Qc = 40 μl/min,Qd = 20 μl/min, ddrop = 300 μm, t = 2 s)
| 产物 | 产物分布(质量分数)/% | |||
|---|---|---|---|---|
| 100 μm① | 200 μm② | 500 μm② | 86 μm③[ | |
| C5 | 9.65 | 7.37 | 10.88 | 4.36 |
| C6 | 6.53 | 6.43 | 5.65 | 7.22 |
| C7 | 5.78 | 6.12 | 3.90 | 5.56 |
| C8 | 70.90 | 66.18 | 33.60 | 61.36 |
| 7.12 | 13.90 | 45.98 | 21.50 | |
| C8分布 | ||||
| TMPs | 66 | 59.89 | 29.11 | 56.63 |
| DMHs | 4.93 | 6.09 | 4.12 | 5.28 |
| TMPs/DMHs | 13.4 | 9.83 | 7.06 | 8.10 |
| RON | 95.3 | 94 | 83.7 | 92.11 |
| 烯烃转化率 | 94.96 | 99.65 | 99.34 | |
表5 分散尺寸对烷基化产物分布的影响
Table 5 Effect of droplet size on yields
| 产物 | 产物分布(质量分数)/% | |||
|---|---|---|---|---|
| 100 μm① | 200 μm② | 500 μm② | 86 μm③[ | |
| C5 | 9.65 | 7.37 | 10.88 | 4.36 |
| C6 | 6.53 | 6.43 | 5.65 | 7.22 |
| C7 | 5.78 | 6.12 | 3.90 | 5.56 |
| C8 | 70.90 | 66.18 | 33.60 | 61.36 |
| 7.12 | 13.90 | 45.98 | 21.50 | |
| C8分布 | ||||
| TMPs | 66 | 59.89 | 29.11 | 56.63 |
| DMHs | 4.93 | 6.09 | 4.12 | 5.28 |
| TMPs/DMHs | 13.4 | 9.83 | 7.06 | 8.10 |
| RON | 95.3 | 94 | 83.7 | 92.11 |
| 烯烃转化率 | 94.96 | 99.65 | 99.34 | |
| 1 | 毕建国. 烷基化油生产技术的进展[J]. 化工进展, 2007, 26(7): 934-939. |
| Bi J G. Advances in alkylate manufacture technology[J]. Chemical Industry and Engineering Progress, 2007, 26(7): 934-939. | |
| 2 | Gan P X, Tang S W. Research progress in ionic liquids catalyzed isobutane/butene alkylation[J]. Chinese Journal of Chemical Engineering, 2016, 24(11): 1497-1504. |
| 3 | 李迪, 孙伟振, 奚桢浩, 等. 混合丁烯/异丁烷硫酸烷基化反应动力学[J]. 化工学报, 2015, 66(2): 585-590. |
| Li D, Sun W Z, Xi Z H, et al. Alkylation kinetics of mixed butenes/isobutane by sulfuric acid [J]. CIESC Journal, 2015, 66(2): 585-590. | |
| 4 | Hommeltoft S I. Isobutane alkylation recent developments and future perspectives[J]. Applied Catalysis A: General, 2001, 221(1): 421-428. |
| 5 | Albright L F. Present and future alkylation processes in refineries[J]. Industrial & Engineering Chemistry Research, 2009, 48(3): 1409-1413. |
| 6 | Corma A, Martínez A. Chemistry, catalysts, and processes for isoparaffin-olefin alkylation: actual situation and future trends[J]. Catalysis Reviews, 1993, 35(4): 483-570. |
| 7 | Busca G. Acid catalysts in industrial hydrocarbon chemistry[J]. Chemical Reviews, 2007, 107(11): 5366-5410. |
| 8 | Olah G A, Mathew T, Goeppert A, et al. Ionic liquid and solid HF equivalent amine-poly(hydrogen fluoride) complexes effecting efficient environmentally friendly isobutane-isobutylene alkylation[J]. Journal of the American Chemical Society, 2005, 127(16): 5964-5969. |
| 9 | 刘鹰, 刘植昌, 徐春明. 异丁烷与2-丁烯在含有抑制剂离子液体中的烷基化反应[J]. 化工学报, 2005, 56(11): 2119-2123. |
| Liu Y, Liu Z C, Xu C M. Alkylation of isobutane and 2-butene in inhibited chloroaluminate ionic liquids[J]. Journal of Chemical Industry and Engineering (China), 2005, 56(11): 2119-2123. | |
| 10 | Li L T, Zhang J S, Du C C, et al. Intensification of the sulfuric acid alkylation process with trifluoroacetic acid[J]. AIChE Journal, 2019, 65(1): 113-119. |
| 11 | Huang C P, Liu Z C, Xu C M, et al. Effects of additives on the properties of chloroaluminate ionic liquids catalyst for alkylation of isobutane and butene[J]. Applied Catalysis A: General, 2004, 277(1/2): 41-43. |
| 12 | Liu Y, Hu R S, Xu C M, et al. Alkylation of isobutene with 2-butene using composite ionic liquid catalysts[J]. Applied Catalysis A: General, 2008, 346(1/2): 189-193. |
| 13 | Liu Z C, Meng X H, Zhang R, et al. Reaction performance of isobutane alkylation catalyzed by a composite ionic liquid at a short contact time[J]. AIChE Journal, 2014, 60(6): 2244-2253. |
| 14 | Hu P C, Wang Y D, Meng X H, et al. Isobutane alkylation with 2-butene catalyzed by amide-AlCl3-based ionic liquid analogues[J]. Fuel, 2017, 189: 203-209. |
| 15 | Wang H, Meng X Z, Zhao G Y, et al. Isobutane/butene alkylation catalyzed by ionic liquids: a more sustainable process for clean oil production[J]. Green Chemistry, 2017, 19(6): 1462-1489. |
| 16 | Hartman R L, McMullen J P, Jensen K F. Deciding whether to go with the flow: evaluating the merits of flow reactors for synthesis[J]. Angewandte Chemie International Edition, 2011, 50(33): 7502-7519. |
| 17 | Aschauer S J, Jess A. Effective and intrinsic kinetics of the two-phase alkylation of i-paraffins with olefins using chloroaluminate ionic liquids as catalyst[J]. Industrial & Engineering Chemistry Research, 2012, 51(50): 16288-16298. |
| 18 | Zhang J S, Wang K, Teixeira A R, et al. Design and scaling up of microchemical systems: a review[J]. Annual Review of Chemical and Biomolecular Engineering, 2017, 8(1): 285-305. |
| 19 | Günther A, Jensen K F. Multiphase microfluidics: from flow characteristics to chemical and materials synthesis[J]. Lab on a Chip, 2006, 6(12): 1487-1503. |
| 20 | Yoshida J I, Kim H, Nagaki A. Green and sustainable chemical synthesis using flow microreactors[J]. ChemSusChem, 2011, 4(3): 331-340. |
| 21 | Jensen K F. Flow chemistry—microreaction technology comes of age[J]. AIChE Journal, 2017, 63(3): 858-869. |
| 22 | Burns J R, Ramshaw C. Development of a microreactor for chemical production[J]. Chemical Engineering Research and Design, 1999, 77(3): 206-211. |
| 23 | Yao X J, Zhang Y, Du L Y, et al. Review of the applications of microreactors[J]. Renewable and Sustainable Energy Reviews, 2015, 47: 519-539. |
| 24 | Su Y H, Zhao Y C, Chen G W, et al. Liquid-liquid two-phase flow and mass transfer characteristics in packed microchannels[J]. Chemical Engineering Science, 2010, 65(13): 3947-3956. |
| 25 | Dummann G, Quittmann U, Gröschel L, et al. The capillary-microreactor: a new reactor concept for the intensification of heat and mass transfer in liquid-liquid reactions[J]. Catalysis Today, 2003, 79/80: 433-439. |
| 26 | Li L T, Zhang J S, Wang K, et al. Caprolactam as a new additive to enhance alkylation of isobutane and butene in H2SO4[J]. Industrial & Engineering Chemistry Research, 2016, 55(50): 12818-12824. |
| 27 | Li L T, Zhang J S, Du C C, et al. Kinetics study of sulfuric acid alkylation of isobutane and butene using a microstructured chemical system[J]. Industrial & Engineering Chemistry Research, 2019, 58(3): 1150-1158. |
| 28 | Li S W, Xu J H, Wang Y J, et al. Low-temperature bonding of poly-(methyl methacrylate) microfluidic devices under an ultrasonic field[J]. Journal of Micromechanics and Microengineering, 2009, 19(1): 015035. |
| 29 | Yao C Q, Zhao Y C, Ma H Y, et al. Two-phase flow and mass transfer in microchannels: a review from local mechanism to global models[J]. Chemical Engineering Science, 2021, 229: 116017. |
| 30 | Lan W J, Liu D, Guo X Q, et al. Study on liquid-liquid droplet flow separation in a T-shaped microseparator[J]. Industrial & Engineering Chemistry Research, 2020, 59(26): 12262-12269. |
| 31 | Qi L, Meng X H, Zhang R, et al. Droplet size distribution and droplet size correlation of chloroaluminate ionic liquid-heptane dispersion in a stirred vessel[J]. Chemical Engineering Journal, 2015, 268: 116-124. |
| [1] | 周绍华, 詹飞龙, 丁国良, 张浩, 邵艳坡, 刘艳涛, 郜哲明. 短管节流阀内流动噪声的实验研究及降噪措施[J]. 化工学报, 2023, 74(S1): 113-121. |
| [2] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [3] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [4] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [5] | 王玉兵, 李杰, 詹宏波, 朱光亚, 张大林. R134a在菱形离散肋微小通道内的流动沸腾换热实验研究[J]. 化工学报, 2023, 74(9): 3797-3806. |
| [6] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [7] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [8] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [9] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [10] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [11] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [12] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [13] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [14] | 何晓崐, 刘锐, 薛园, 左然. MOCVD生长AlN单晶薄膜的气相和表面化学反应综述[J]. 化工学报, 2023, 74(7): 2800-2813. |
| [15] | 文兆伦, 李沛睿, 张忠林, 杜晓, 侯起旺, 刘叶刚, 郝晓刚, 官国清. 基于自热再生的隔壁塔深冷空分工艺设计及优化[J]. 化工学报, 2023, 74(7): 2988-2998. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号