化工学报 ›› 2019, Vol. 70 ›› Issue (10): 3956-3966.DOI: 10.11949/0438-1157.20190648
收稿日期:2019-06-10
修回日期:2019-09-18
出版日期:2019-10-05
发布日期:2019-10-05
通讯作者:
陈立芳
作者简介:李姜无忌(1997—),男,硕士研究生,基金资助:
Wuji LIJIANG( ),Qiaoying ZHU,Lifang CHEN(
),Qiaoying ZHU,Lifang CHEN( ),Hongye CHENG,Zhiwen QI
),Hongye CHENG,Zhiwen QI
Received:2019-06-10
Revised:2019-09-18
Online:2019-10-05
Published:2019-10-05
Contact:
Lifang CHEN
摘要:
纺织印染废水中的有机污染物如亚甲基蓝的高效吸附净化是环境领域的重要研究课题,三氧化钼独特的片层结构极具吸附应用潜力。通过微波一步法制备了不同氧空穴浓度的氧化钼,具有不同的表面电荷分布,利用氧空穴氧化钼表面所带负电荷选择性高效吸附阳离子偶氮染料亚甲基蓝。揭示了氧空穴浓度与吸附性能之间的关系,发现氧空穴浓度越高则氧化钼吸附速率越快;吸附过程符合Langmuir等温线模型和准二级动力学模型,表明该吸附过程属于单分子层吸附,并分析了其吸附机理。氧空穴氧化钼(MoO3- x )为金属氧化物在染料吸附领域的发展和应用提供一定的基础数据和理论基础。
中图分类号:
李姜无忌,朱巧影,陈立芳,成洪业,漆志文. 氧缺陷位MoO3- x 的制备及其吸附性能研究[J]. 化工学报, 2019, 70(10): 3956-3966.
Wuji LIJIANG,Qiaoying ZHU,Lifang CHEN,Hongye CHENG,Zhiwen QI. Preparation of oxygen defect vacancies MoO3- x and its adsorption properties[J]. CIESC Journal, 2019, 70(10): 3956-3966.
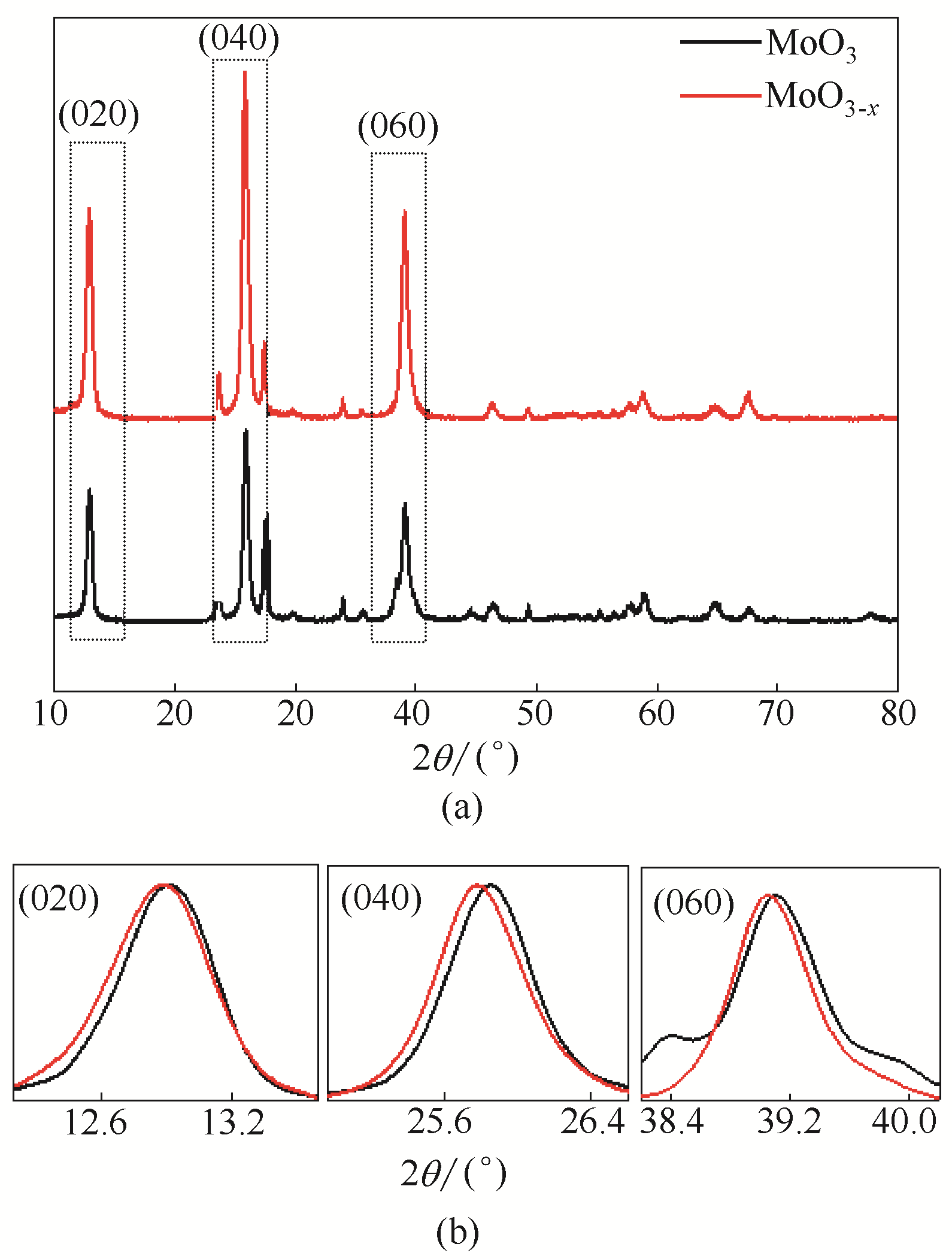
图1 MoO3- x 和MoO3的XRD谱图(a);放大的MoO3- x 和MoO3的XRD谱图中(020)、(040)和(060)晶面衍射峰(b)
Fig.1 XRD patterns (a) and selected XRD pattern of (020), (040) and (060) diffraction peaks (b) of MoO3- x and MoO3
| Sample | Mass decrease/% | Oxygen vacancy concentration |
|---|---|---|
| MoO3 | 0 | MoO3 |
| MoO3- x -120-150 | 0.314 | MoO2.97 |
| MoO3- x -150-45 | 0.634 | MoO2.94 |
| MoO3- x -180-10 | 1.293 | MoO2.88 |
| MoO3- x -180-20 | 1.266 | MoO2.89 |
表1 氧化钼样品的氧空穴浓度
Table 1 Oxygen vacancy concentration of MoO3– x characterized by thermogravimetric analysis
| Sample | Mass decrease/% | Oxygen vacancy concentration |
|---|---|---|
| MoO3 | 0 | MoO3 |
| MoO3- x -120-150 | 0.314 | MoO2.97 |
| MoO3- x -150-45 | 0.634 | MoO2.94 |
| MoO3- x -180-10 | 1.293 | MoO2.88 |
| MoO3- x -180-20 | 1.266 | MoO2.89 |
| Absorbent sample | Q max/(mg·g-1) | Ref. |
|---|---|---|
| graphene | 153.8 | [ |
| Fe-MOF | 187 | [ |
| activated carbon | 207 | [ |
| biomassed bamboo | 606 | [ |
| Fe(Ⅲ)/Cr(Ⅲ) hydroxide | 22.8 | [ |
| zeolite | 53.1 | [ |
| clay | 300 | [ |
| MoO3 | 629 | this work |
| MoO3- x | 758 | this work |
表 2 不同吸附剂对MB的吸附能力对比
Table 2 Summary of adsorption capacity of various materials for MB
| Absorbent sample | Q max/(mg·g-1) | Ref. |
|---|---|---|
| graphene | 153.8 | [ |
| Fe-MOF | 187 | [ |
| activated carbon | 207 | [ |
| biomassed bamboo | 606 | [ |
| Fe(Ⅲ)/Cr(Ⅲ) hydroxide | 22.8 | [ |
| zeolite | 53.1 | [ |
| clay | 300 | [ |
| MoO3 | 629 | this work |
| MoO3- x | 758 | this work |
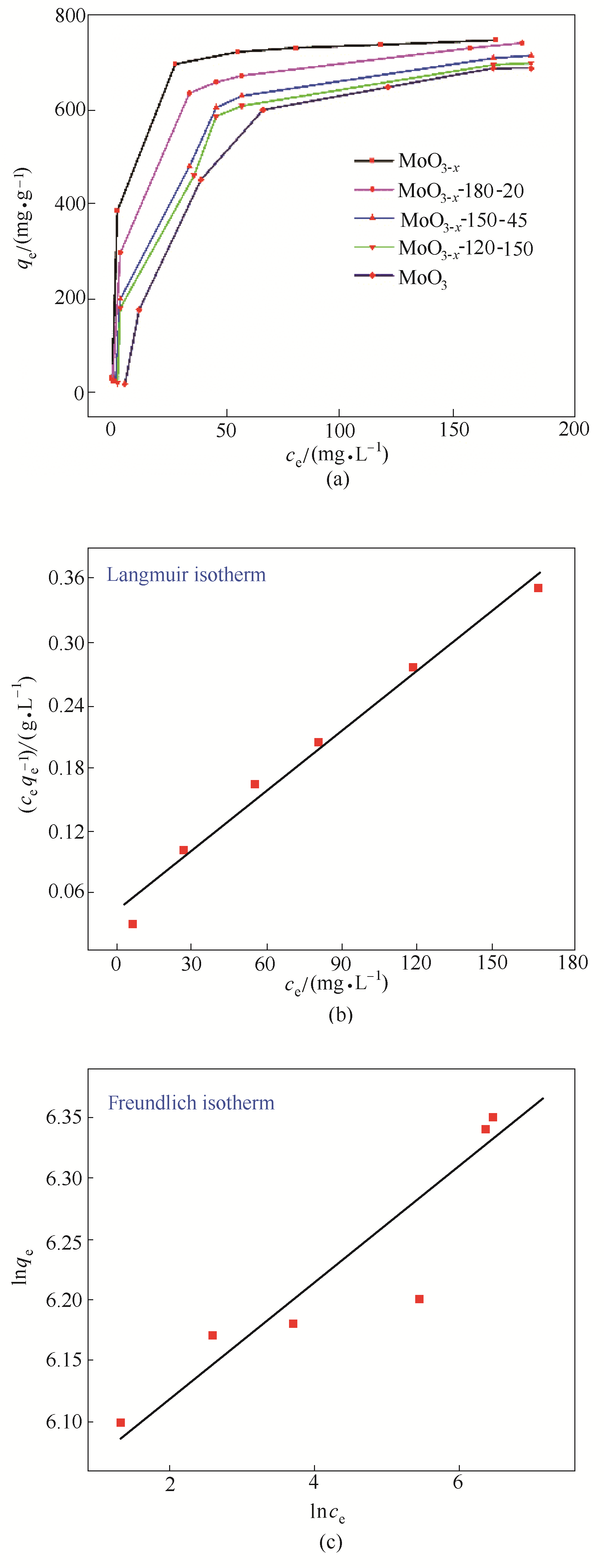
图7 (a) 25℃下不同含氧空穴的MoO3- x 对MB的吸附等温线;(b) MoO3- x 吸附MB的Langmuir吸附等温线模型拟合曲线;(c) MoO3- x 吸附MB的Freundlich吸附等温线模型拟合曲线
Fig.7 (a) Adsorption isotherm of MoO3- x with different oxygen vacancies concentration to MB under 25℃, (b) Langmuir isothermal equations of MoO3- x to MB, (c) Freundlich isothermal equations of MoO3- x to MB
| Adsorbent | Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|---|
| q m/(mg·g-1) | R L | R 2 | 1/n | K F/((mg·g-1)(L·mg-1)1/ n ) | R 2 | ||
| MoO3- x | 748 | 0.042 | 0.978 | 0.24 | 134.3 | 0.844 | |
| MoO3- x -180-20 | 739 | 0.096 | 0.993 | 2.36 | 126.8 | 0.568 | |
| MoO3- x -150-45 | 721 | 0.356 | 0.976 | 4.56 | 263.4 | 0.497 | |
| MoO3- x -120-150 | 704 | 0.569 | 0.965 | 8.21 | 85.55 | 0.368 | |
| MoO3 | 629 | 0.875 | 0.998 | 13 | 0.7658 | 0.426 | |
表3 MoO3- x 和MoO3吸附MB的Langmuir和Freundlich模型参数
Table 3 Adsorption isothermal equation parameters of MoO3- x and MoO3 to MB
| Adsorbent | Langmuir isotherm | Freundlich isotherm | |||||
|---|---|---|---|---|---|---|---|
| q m/(mg·g-1) | R L | R 2 | 1/n | K F/((mg·g-1)(L·mg-1)1/ n ) | R 2 | ||
| MoO3- x | 748 | 0.042 | 0.978 | 0.24 | 134.3 | 0.844 | |
| MoO3- x -180-20 | 739 | 0.096 | 0.993 | 2.36 | 126.8 | 0.568 | |
| MoO3- x -150-45 | 721 | 0.356 | 0.976 | 4.56 | 263.4 | 0.497 | |
| MoO3- x -120-150 | 704 | 0.569 | 0.965 | 8.21 | 85.55 | 0.368 | |
| MoO3 | 629 | 0.875 | 0.998 | 13 | 0.7658 | 0.426 | |
| Adsorbent | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| k 1/(g·mg-1·h-1) | q e,cal/(mg·g-1) | R 2 | k 2/(g·mg-1·min-1) | q e,cal/(mg·g-1) | R 2 | |
| MoO3- x | 0.536 | 6.9×1014 | 0.2882 | 0.040 | 762 | 0.99973 |
| MoO3 | 1.822×10-4 | 7.12×1022 | 0.3554 | 0.00252 | 785 | 0.99628 |
表4 25℃下动力学方程参数
Table 4 Kinetics equation parameters under 25℃
| Adsorbent | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| k 1/(g·mg-1·h-1) | q e,cal/(mg·g-1) | R 2 | k 2/(g·mg-1·min-1) | q e,cal/(mg·g-1) | R 2 | |
| MoO3- x | 0.536 | 6.9×1014 | 0.2882 | 0.040 | 762 | 0.99973 |
| MoO3 | 1.822×10-4 | 7.12×1022 | 0.3554 | 0.00252 | 785 | 0.99628 |
| 1 | Shannon M A , Bohn P W , Elimelech M , et al . Science and technology for water purification in the coming decades[J]. Nature, 2008, 452(7185): 301-310. |
| 2 | Ghasemi M , Mashhadi S , Asif M , et al . Microwave-assisted synthesis of tetraethylenepentamine functionalized activated carbon with high adsorption capacity for Malachite green dye[J]. Journal of Molecular Liquids, 2016, 213(1): 317-325. |
| 3 | 任南琪, 周显娇, 郭婉茜, 等 . 染料废水处理技术研究进展[J]. 化工学报, 2013, 64(1): 84-94. |
| Ren N Q , Zhou X J , Guo W Q , et al . A review on treatment methods of dye wastewater[J]. CIESC Journal, 2013, 64(1): 84-94. | |
| 4 | Hoffmann M R , Martin S T , Choi W , et al . Environmental applications of semiconductor photocatalysis[J]. Chemical Reviews, 1995, 95(1): 69-96. |
| 5 | Pathania D , Sharma S , Singh P . Removal of methylene blue by adsorption onto activated carbon developed from Ficus carica bast[J]. Arabian Journal of Chemistry, 2017, 10(1): S1445-S1451. |
| 6 | Yagub M T , Sen T K , Ang H M . Equilibrium, kinetics, and thermodynamics of methylene blue adsorption by pine tree leaves[J]. Water, Air, & Soil Pollution, 2012, 223(8): 5267-5282. |
| 7 | Dabrowski A . Adsorption from theory to practice[J]. Advances in Colloid and Interface Science, 2001, 93(1): 135-224. |
| 8 | Ali I , Asim M , Khan T A . Low cost adsorbents for the removal of organic pollutants from wastewater[J]. Journal of Environmental Management, 2012, 113(1): 170-183. |
| 9 | Zhu Q , Wang Z , Chen L , et al . Ionic-liquid-controlled two-dimensional monolayer Bi2MoO6 and its adsorption of azo molecules[J]. ACS Applied Nano Materials, 2018, 1(9): 5083-5091. |
| 10 | 赵亚红, 薛振华, 王喜明, 等 . 羧甲基纤维素/蒙脱土纳米复合材料对刚果红染料的吸附及解吸性能[J]. 化工学报, 2012, 63(8): 2655-2660. |
| Zhao Y H , Xue Z H , Wang X M , et al . Adsorption and desorption properties for Congo red dye of carboxymethylcellulose/montmorillonite nanocomposites[J].CIESC Journal, 2012, 63(8): 2655-2660. | |
| 11 | Allen S J , Koumanova B . Decolourisation of water/wastewater using adsorption[J]. Journal of the University of Chemical Technology and Metallurgy, 2005, 40(3): 175-192. |
| 12 | Gougoulias N , Papachatzis A , Kalorizou H . Role of acid blue 25 dye as active site for the adsorption of Cd2+, and Zn2+, using activated carbons[J]. Dyes & Pigments, 2013, 96(2): 459-466. |
| 13 | Sarkar M , Majumdar P . Application of response surface methodology for optimization of heavy metal biosorption using surfactant modified chitosan bead[J]. Chemical Engineering Journal, 2011, 175(1): 376-387. |
| 14 | Lee M Y , Hong K J , Kajiuchi T , et al . Synthesis of chitosan-based polymeric surfactants and their adsorption properties for heavy metals and fatty acids[J]. International Journal of Biological Macromolecules, 2005, 36(3): 152-158. |
| 15 | Liu S , Ding Y , Li P . Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N, N-dimethyl dehydroabietylamine oxide[J]. Chemical Engineering Journal, 2014, 248(1): 135-144. |
| 16 | Nie L H , Tan Q , Zhu W . Fast adsorption removal of Congo red on hierarchically porous γ-Al2O3 hollow microspheres prepared by microwave-assisted hydrothermal method[J]. Acta Physico-Chimica Sinica, 2015, 31(9): 1815-1822. |
| 17 | He Q , Ni Y , Ye S . Preparation of flowerlike BiOBr/Bi2MoO6 composite superstructures and the adsorption behavior to dyes[J]. Journal of Physics and Chemistry of Solids, 2017, 104(1): 286-292. |
| 18 | 汪泽华, 蔡卫权, 郭蕾, 等 . P123辅助SB粉溶胶制备大孔径介孔γ-Al2O3及其对甲基蓝的强化吸附性能[J]. 化工学报, 2012, 63(8): 2623-2628. |
| Wang Z H , Cai W Q , Guo L , et al . P123-assisted synthesis of enlarged mesoporous γ-Al2O3 from SB pseudoboehmite sol and its enhanced adsorption performance towards methyl blue [J]. CIESC Journal, 2012, 63(8): 2623-2628. | |
| 19 | 徐刚, 郜洪文 . 甜菜碱OSB-12@高岭土杂化材料对染料吸附机理研究[J]. 化学学报, 2012, 70(24): 2496-2500. |
| Xu G , Gao H W . Betaine OSB-12@kaolin hybrid material synthesized for adsorption of dyes[J]. Acta Chimica Sinica, 2012, 70(24): 2496-2500. | |
| 20 | Lou X W , Zeng H C . Hydrothermal synthesis of α-MoO3 nanorods via acidification of ammonium heptamolybdate tetrahydrate [J]. Chemistry of Materials, 2002, 14(11): 4781-4789. |
| 21 | Sheehan P E , Lieber C M . Nanotribology and nanofabrication of MoO3 structures by atomic force microscopy[J]. Science, 1996, 272(5265): 1158-1161. |
| 22 | Song J , Ni X , Gao L , et al . Synthesis of metastable h-MoO3 by simple chemical precipitation[J]. Materials Chemistry and Physics, 2007, 102(2/3): 245-248. |
| 23 | Siciliano T , Tepore A , Filippo E , et al . Characteristics of molybdenum trioxide nanobelts prepared by thermal evaporation technique[J]. Materials Chemistry and Physics, 2009, 114(2/3): 687-691. |
| 24 | Schöllhorn R , Kuhlmann R , Besenhard J O . Topotactic redox reactions and ion exchange of layered MoO3 bronzes[J]. Materials Research Bulletin, 1976, 11(1): 83-90. |
| 25 | Prasad A K , Kubinski D J , Gouma P I . Comparison of sol–gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection [J]. Sensors and Actuators B: Chemical, 2003, 93(1/2/3): 25-30. |
| 26 | Kim H S , Cook J B , Lin H , et al . Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3- x [J]. Nature Materials, 2017, 16(4): 454. |
| 27 | Dieterle M , Weinberg G , Mestl G . Raman spectroscopy of molybdenum oxides (Ⅰ): Structural characterization of oxygen defects in MoO3- x by DR UV/VIS, Raman spectroscopy and X-ray diffraction [J]. Physical Chemistry Chemical Physics, 2002, 4(5): 812-821. |
| 28 | Tao P , Xu Y , Zhou Y , et al . Nitrogen oxide (NO) gas-sensing properties of Bi2MoO6 nanosheets synthesized by a hydrothermal method[J]. Materials Research, 2017, 20(3): 786-790. |
| 29 | Vasilopoulou M , Douvas A M , Georgiadou D G , et al . The influence of hydrogenation and oxygen vacancies on molybdenum oxides work function and gap states for application in organic optoelectronics[J]. Journal of the American Chemical Society, 2012, 134(39): 16178-16187. |
| 30 | Greiner M T , Chai L , Helander M G , et al . Transition metal oxide work functions: the influence of cation oxidation state and oxygen vacancies[J]. Advanced Functional Materials, 2012, 22(21): 4557–4568. |
| 31 | Kong X Y , Ng B J , Tan K H , et al . Simultaneous generation of oxygen vacancies on ultrathin BiOBr nanosheets during visible-light-driven CO2, photoreduction evoked superior activity and long-term stability[J]. Catalysis Today, 2018, 314: 20-27. |
| 32 | Zhang L , Wang W , Jiang D , et al . Photoreduction of CO2 on BiOCl nanoplates with the assistance of photoinduced oxygen vacancies[J]. Nano Research, 2015, 8(3): 821-831. |
| 33 | Cervantes F J , Garciaespinosa A , Morenoreynosa M A , et al . Immobilized redox mediators on anion exchange resins and their role on the reductive decolorization of azo dyes[J]. Environmental Science & Technology, 2010, 44(5): 1747-1753. |
| 34 | Liu T , Li Y , Du Q , et al . Adsorption of methylene blue from aqueous solution by graphene[J]. Colloids and Surfaces B- Biointerfaces, 2012, 90: 197-203. |
| 35 | Haque E , Jun J W , Jhung S H . Adsorptive removal of methyl orange and methylene blue from aqueous solution with a metal-organic framework material, iron terephthalate (MOF-235)[J]. Journal of Hazardous Materials, 2011, 185(1): 507-511. |
| 36 | Li Y , Du Q , Liu T , et al . Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes[J]. Chemical Engineering Research & Design: Transactions of the Institution of Chemical Engineers, 2013, 91(2): 361-368. |
| 37 | Guo J Z , Li B , Liu L , et al . Removal of methylene blue from aqueous solutions by chemically modified bamboo[J]. Chemosphere, 2014, 111: 225-231. |
| 38 | Namasivayam A , Sumithra S . Removal of direct red 12B and methylene blue from water by adsorption onto Fe(Ⅲ)/Cr(Ⅲ) hydroxide, an industrial solid waste[J]. Journal of Environmental Management, 2005, 74(3): 207-215. |
| 39 | Dogan M , Alkan M , Onager Y . Adsorption of methylene blue from aqueous solution onto perlite[J]. Water Air & Soil Pollution, 2000, 120(3/4): 229-248. |
| 40 | Bagane M , Guiza S . Removal of a dye from textile effluents by adsorption[J]. Annales de Chimie-Science des Materiaux, 2000, 25(8): 615-626. |
| 41 | Tian P , Han X , Ning G , et al . Synthesis of porous hierarchical MgO and its superb adsorption properties[J]. ACS Applied Materials & Interfaces, 2013, 5(23): 12411-12418. |
| 42 | 陈自正, 沈卫华, 陈立芳, 等 . 纳米H2TiO3锂吸附剂的水热合成及其吸附性能[J]. 中国有色金属学报, 2017, 27(3): 547-554. |
| Chen Z Z , Shen W H , Chen L F , et al . Hydrothermal synthesis and adsorption properties of nano scale H2TiO3 adsorbent [J].The Chinese Journal of Nonferrous Metals, 2017, 27(3): 547-554. | |
| 43 | Atia A A , Donia A M , Al-Amrani W A . Adsorption/desorption behavior of acid orange 10 on magnetic silica modified with amine groups[J]. Chemical Engineering Journal, 2009, 150(1): 55-62. |
| 44 | Senturk H B , Ozdes D , Gundogdu A , et al . Removal of phenol from aqueous solutions by adsorption onto organomodified Tirebolu bentonite: equilibrium, kinetic and thermodynamic study[J]. Journal of Hazardous Materials, 2009, 172(1): 353-362. |
| 45 | Zhang J , Xiao H , Yang Y . Preparation of hemicellulose-containing latex and its application as absorbent toward dyes[J]. Journal of Materials Science, 2015, 50(4): 1673-1678. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [3] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [4] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [5] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [6] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [9] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [10] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [11] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [12] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [13] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [14] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [15] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号