化工学报 ›› 2022, Vol. 73 ›› Issue (10): 4255-4267.DOI: 10.11949/0438-1157.20220599
收稿日期:2022-04-28
修回日期:2022-07-19
出版日期:2022-10-05
发布日期:2022-11-02
通讯作者:
周欣
作者简介:张博(2000—),男,硕士研究生,201830380205@mail.scut.edu.cn
基金资助:
Bo ZHANG( ), Xiaofei CHEN, Siyao ZHAO, Xin ZHOU(
), Xiaofei CHEN, Siyao ZHAO, Xin ZHOU( )
)
Received:2022-04-28
Revised:2022-07-19
Online:2022-10-05
Published:2022-11-02
Contact:
Xin ZHOU
摘要:
乙烯是石油化学工业的基础原材料,聚合级乙烯工业纯化的关键挑战是去除其中的乙烷杂质,这一步骤难度大、能耗高。近年来,以乙烷选择性吸附剂为核心的吸附分离纯化技术快速发展,并得到学术界和工业界的关注。该技术可在温和工况下高选择性分离出乙烯中的乙烷杂质,显现出巨大潜力。本文总结了近年来乙烷选择性吸附剂(特别是乙烷选择性MOFs)的研究进展,并归纳阐释其选择性吸附机理。同时,在前人研究成果的基础上,总结可行的乙烷选择性吸附剂的设计策略,指出当前开发高效乙烷选择性吸附剂面临的挑战和未来的研究方向。
中图分类号:
张博, 陈晓霏, 赵思尧, 周欣. 高效分离乙烷/乙烯的烷烃选择性吸附剂研究进展[J]. 化工学报, 2022, 73(10): 4255-4267.
Bo ZHANG, Xiaofei CHEN, Siyao ZHAO, Xin ZHOU. Progress of ethane-selective adsorbents for efficient purification of ethylene[J]. CIESC Journal, 2022, 73(10): 4255-4267.
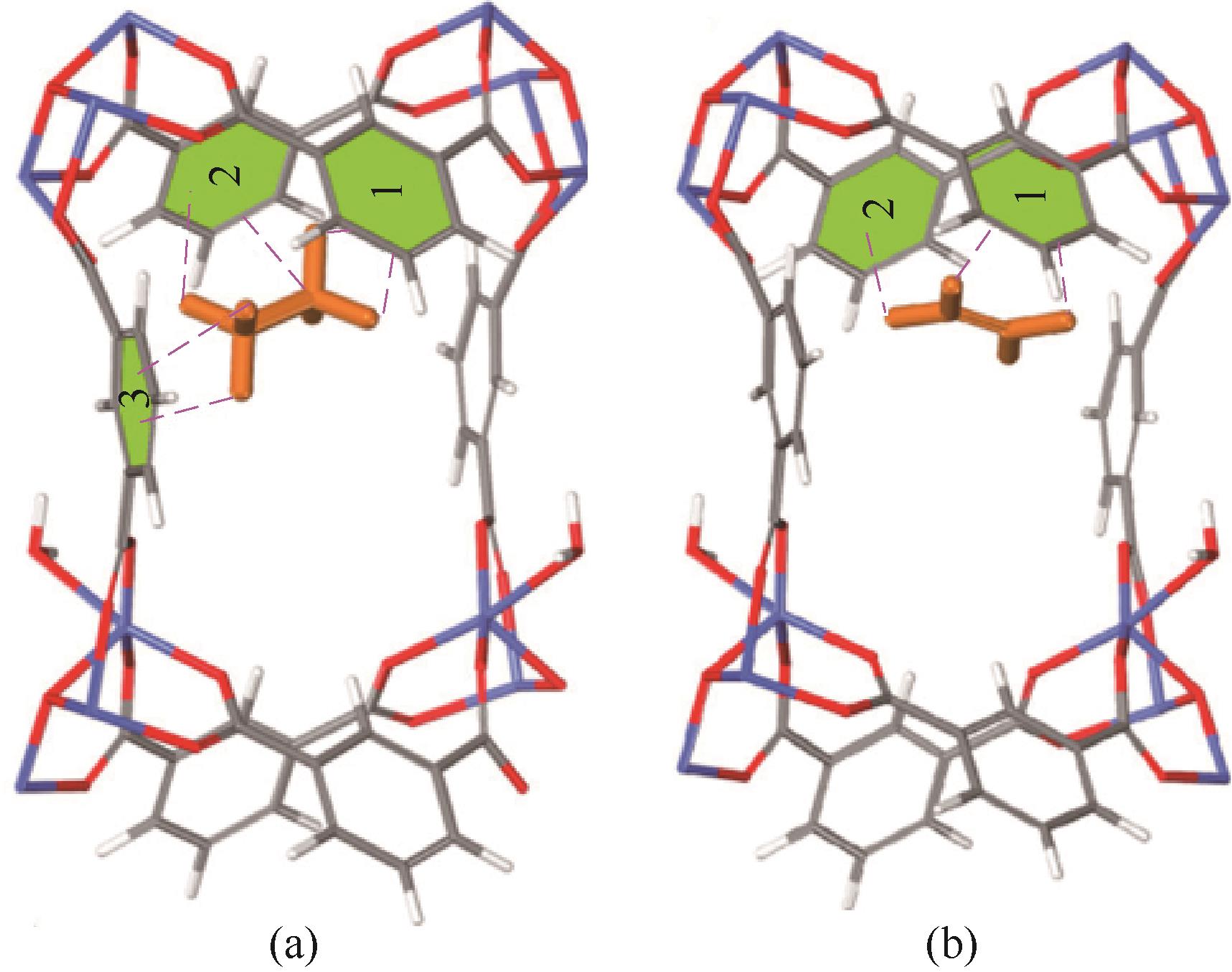
图1 DFT-D3计算比较C2H6(a)和C2H4(b)在MUF-15中的吸附位点[45]
Fig.1 Comparison of the preferential C2H6 (a) and C2H4 (b) adsorption sites observed by DFT-D3 calculations[45]

图2 DMOF、DMOF-DM和DMOF-TM配体(a);DMOF、DMOF-DM和DMOF-TM的IAST选择性(C2H6∶C2H4 = 1∶1)(b)[50]
Fig.2 Ligands for DMOF, DMOF-DM and DMOF-TM (a); C2H6/C2H4 IAST selectivity for equimolar mixtures of DMOF, DMOF-DM and DMOF-TM (b)[50]
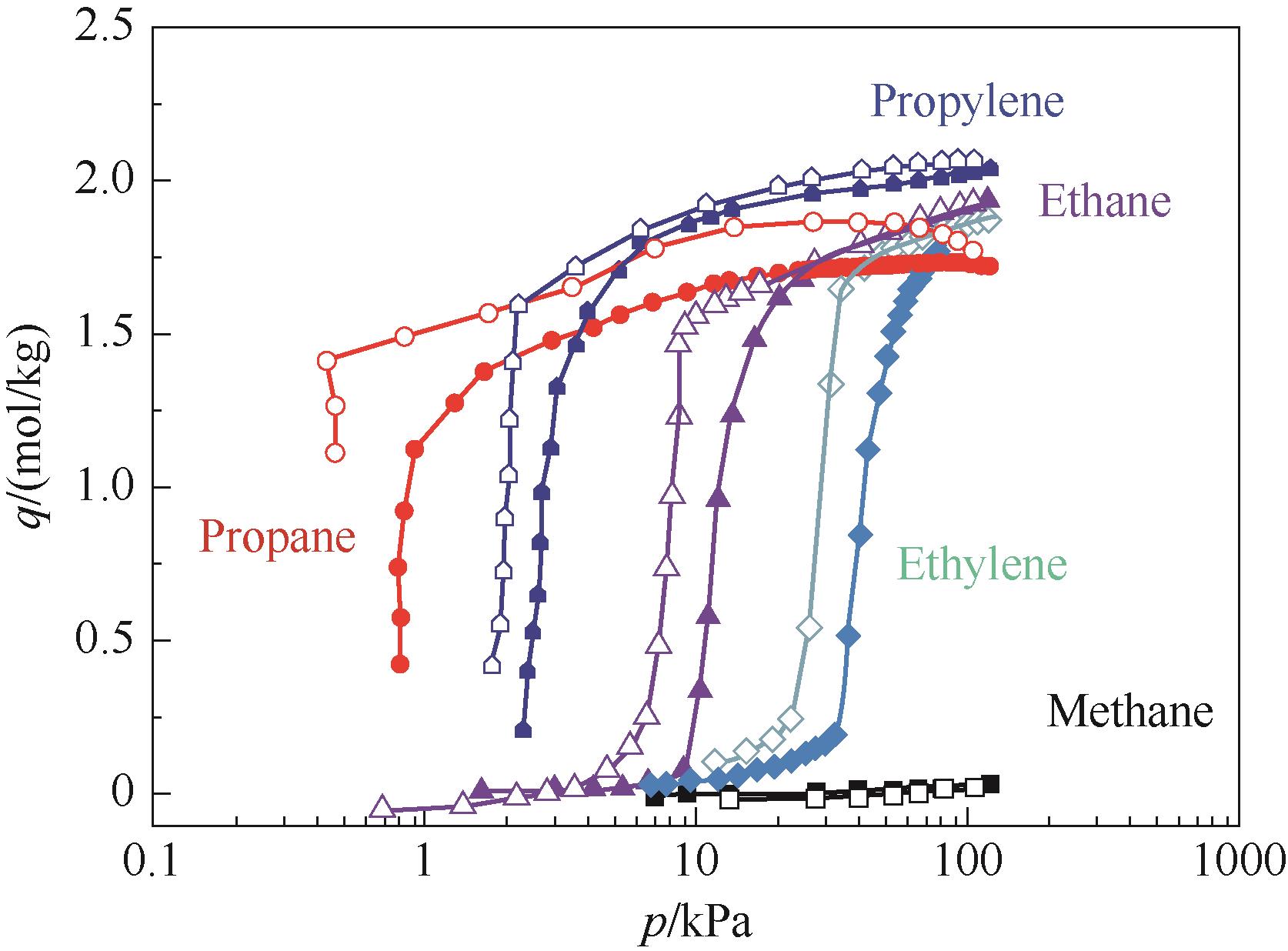
图3 298 K下ZIF-7对几种轻烃的吸附-脱附曲线[51]
Fig.3 Adsorption (closed symbols)-desorption (open symbols) isotherms of several hydrocarbons on ZIF-7 powder at 298 K[51]
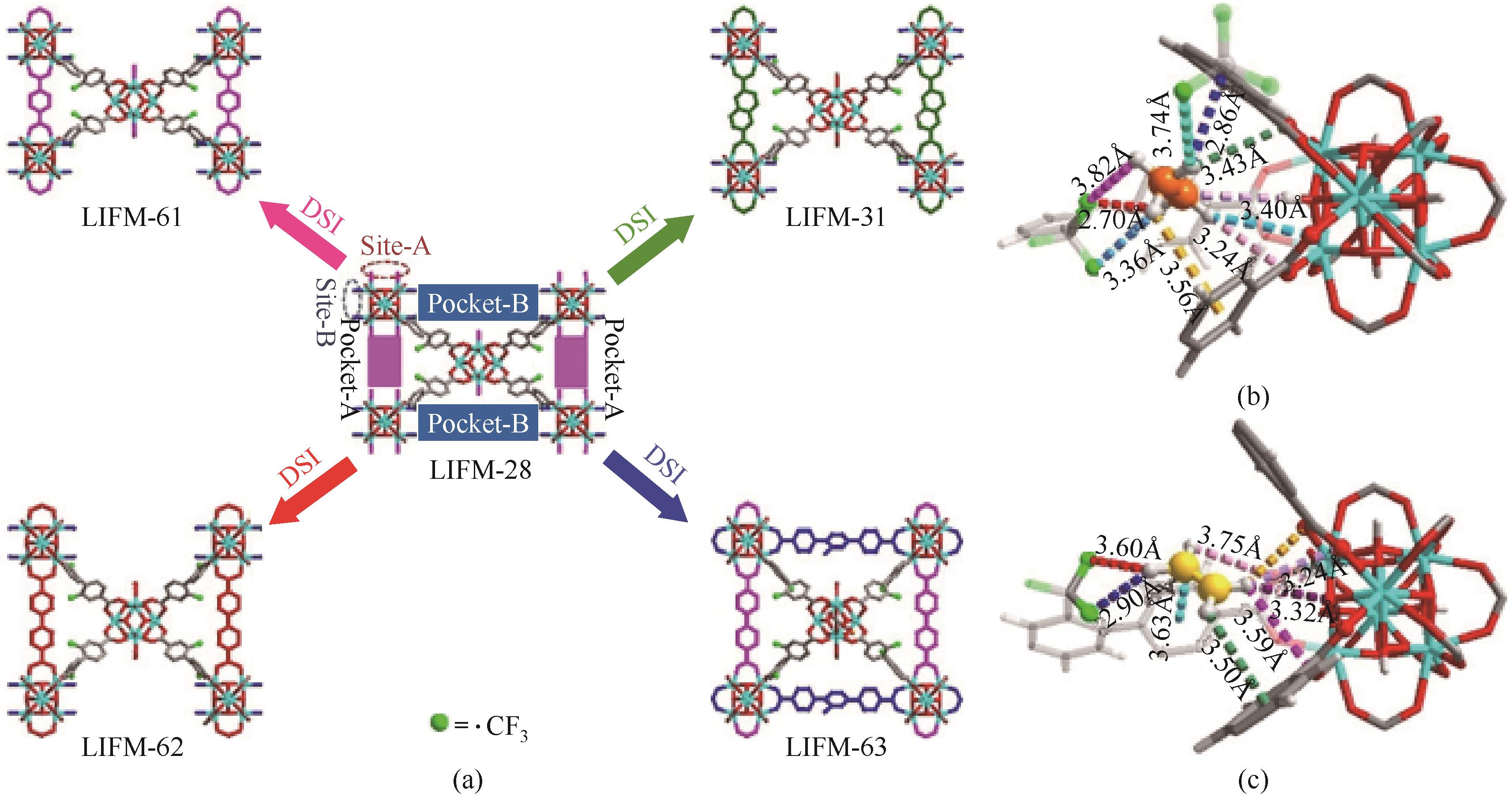
图4 LIFM-28、LIFM-61、LIFM-31、LIFM-62和LIFM-63的结构(a);乙烷(b)和乙烯(c)在LIFM-63中的优先结合位点[8]
Fig.4 Structures of LIFM-28, LIFM-61, LIFM-31, LIFM-62 and LIFM-63(a); Preferential C2H6 (b) and C2H4 (c) binding sites of LIFM-63[8]
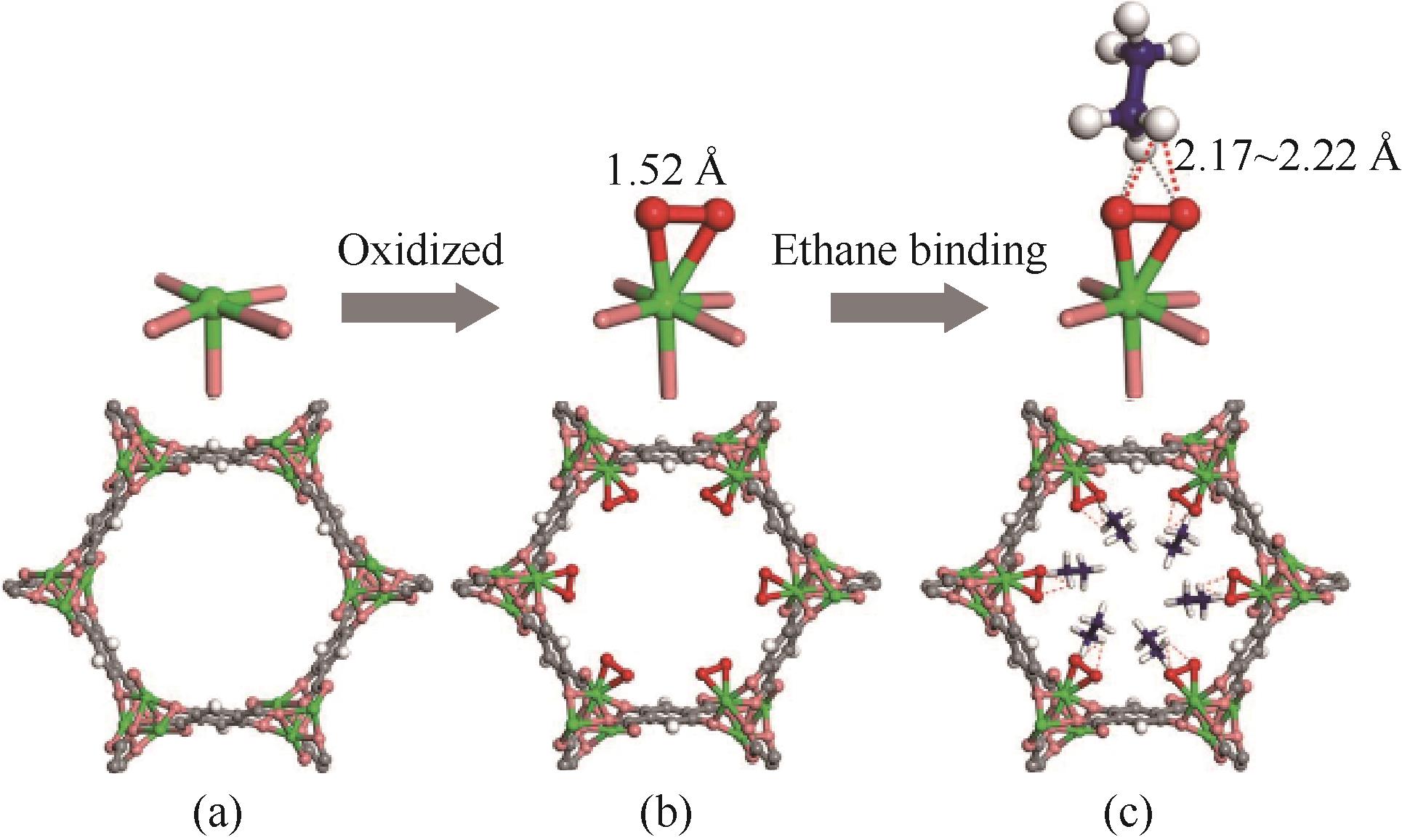
图5 中子粉末衍射确定的Fe2(dobdc)(a)、Fe2(O2)(dobdc)(b)、Fe2(O2)(dobdc)·C2D6(c)结构[55]
Fig.5 Structures of Fe2(dobdc) (a), Fe2(O2)(dobdc) (b), and Fe2(O2)(dobdc)·C2D6 (c) determined from NPD studies[55]
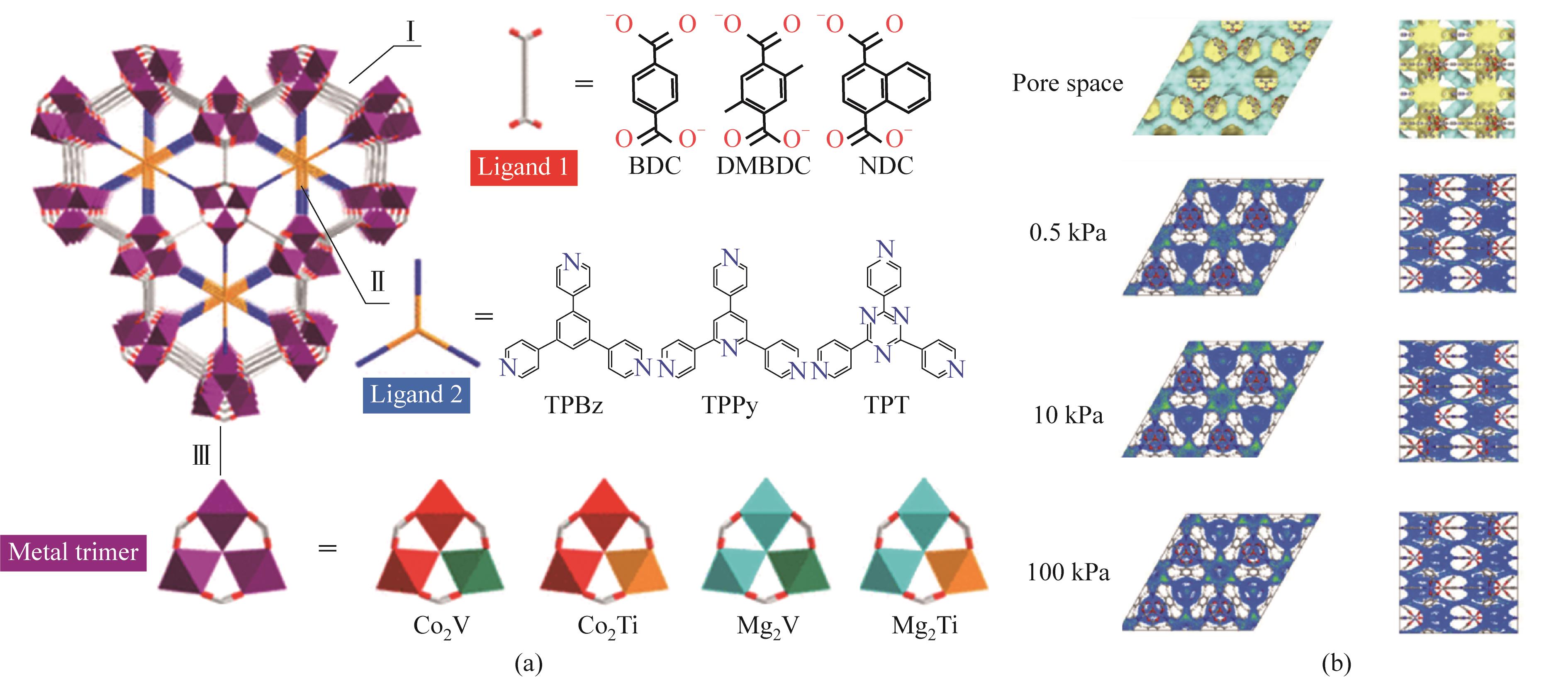
图7 pacs结构MOFs的三种模块:三种双羧基配体1、三种孔道空间分隔剂2和四种金属三聚体(a);GCMC模拟不同压力下Co2V-bdc-tpt中的乙烷密度分布(b)[62]
Fig.7 Three modules of pacs MOFs including three kinds of dicarboxylate ligand, three kinds of pore-partitioning agent and four kinds of metal trimers (a); Density distribution of C2H6 molecules mass center within Co2V-bdc-tpt under different pressures from GCMC simulations (b)[62]
| 乙烷选择性吸附剂 | 条件 | 乙烷吸附量/(mmol/g) | 乙烯吸附量/(mmol/g) | IAST选择性 | 文献 |
|---|---|---|---|---|---|
| UiO-67-(NH2)2 | 296 K,1 bar | 5.32 | 4.32 | 1.7 | [ |
| CAU-3-NDCA | 298 K,1 bar | 2.42 | 1.61 | 1.56 | [ |
| MOF-841 | 298 K,1 bar | 4.70 | 3.40 | 1.6 | [ |
| Ni(BODC)(TED) | 298 K,1 bar | 3.36 | 2.61 | 1.83① | [ |
| LIFM-63 | 298 K,1 bar | 3.0 | 2.1 | 1.6 | [ |
| MIL-53-BDC | 298 K,1 bar | 2.93 | 2.78 | 1.70 | [ |
| MIL-53-NDCA | 298 K,1 bar | 4.24 | 3.12 | 1.53 | [ |
| MIL-53-BPDC | 298 K,1 bar | 2.97 | 2.07 | 1.47 | [ |
| ZJNU-115 | 298 K,1 atm | 4.20 | 3.75 | 1.56 | [ |
| BUT-10 | 298 K,1 bar | 4.76 | 3.56 | 1.72 | [ |
| ScBPDC | 298 K,1 bar | 3.42 | 2.41 | 1.7② | [ |
| ZJNU-7 | 298 K,1 bar | 4.13 | 3.80 | 1.56 | [ |
| Co(AIN)2 | 298 K,1 bar | 3.16 | 3.14 | 2.96 | [ |
| FMOF-2 | 303 K,5 kPa | 0.90 | 0.60 | 3.3③ | [ |
| CPM-80-Zn | 298 K,1 bar | 4.77 | 4.24 | 1.8 | [ |
| CPM-81-Co | 298 K,1 bar | 5.51 | 5.07 | 1.8 | [ |
| SNNU-40 | 298 K,1 bar | 7.54 | 4.91 | 1.57 | [ |
| MIL-125 | 298 K,1 bar | 4.83 | 3.98 | 1.43 | [ |
| MUV-11 | 298 K,1 bar | 1.83 | 1.72 | 1.53 | [ |
| ZSTU-2 | 298 K,1 bar | 2.73 | 2.35 | 1.62 | [ |
| DMOF | 298 K,1 bar | 2.79④ | 2.08④ | 1.51 | [ |
| DMOF-DM | 298 K,1 bar | 4.03④ | 3.11④ | 1.70 | [ |
| DMOF-TM | 298 K,1 bar | 5.31④ | 4.99④ | 1.88 | [ |
| MOF-525 | 298 K,1 bar | 2.77 | 2.10 | 1.24 | [ |
| MOF-525(Co) | 298 K,1 bar | 2.24⑤ | 1.91⑤ | 1.11 | [ |
| UPC-612 | 298 K,1 bar | 3.57 | 2.69 | 1.4 | [ |
| UPC-613 | 298 K,1 bar | 2.56 | 2.25 | 1.47 | [ |
| JXNU-9 | 298 K,1 atm | 3.61 | 2.45 | 1.53 | [ |
| PCN-250(Fe2Zn) | 298 K,1 bar | 5.95 | 5.42 | 1.7① | [ |
| PCN-250(Fe) | 298 K,1 bar | 6.00 | 5.00 | 1.6① | [ |
| NUM-9 | 298 K,1 bar | 2.48 | 2.30 | 1.61 | [ |
| MOF-545 | 298 K,1 bar | 3.12 | 2.57 | 1.31 | [ |
| ZJU-HOF-10(sc) | 296 K,1 bar | 2.19 | 1.88 | 1.9 | [ |
| ZJU-HOF-10(v) | 296 K,1 bar | 0.64 | 0.58 | 1.54 | [ |
| ZJU-HOF-1 | 298 K,1 bar | 4.87 | 3.87 | 2.25 | [ |
| CAC-900-3 | 298 K,1 bar | 6.02 | 4.86 | 2.6 | [ |
| AAC-800-3 | 298 K,1 bar | 5.98 | 4.68 | 2.2 | [ |
| WAC-800-3 | 298 K,1 bar | 6.09 | 4.96 | 1.8 | [ |
| SIFSIX-CAC-800-3 | 298 K,1 bar | 5.02 | 4.14 | 1.4 | [ |
| DUT-8(Ni) | 303 K,1 bar | 3.96 | 2.27 | 1.65 | [ |
| RT-Cu(Qc)2 | 298 K,1 bar | 2.04 | 0.56 | 4.1 | [ |
| Ca(H2tcpb) | 298 K,1 bar | 2.78 | 2.67 | 1.75 | [ |
| NIIC-20-Bu | 298 K,1 bar | 2.50 | 1.40 | 15.4 | [ |
| ZJU-120a | 298 K,1 bar | 4.91 | 3.93 | 2.74 | [ |
| NUM-7a | 298 K,1 bar | 3.04 | 2.68 | 1.76 | [ |
表1 近年来乙烷选择性吸附剂的吸附性能比较
Table 1 Comparison of the adsorption performances of ethane selective adsorbents in recent years
| 乙烷选择性吸附剂 | 条件 | 乙烷吸附量/(mmol/g) | 乙烯吸附量/(mmol/g) | IAST选择性 | 文献 |
|---|---|---|---|---|---|
| UiO-67-(NH2)2 | 296 K,1 bar | 5.32 | 4.32 | 1.7 | [ |
| CAU-3-NDCA | 298 K,1 bar | 2.42 | 1.61 | 1.56 | [ |
| MOF-841 | 298 K,1 bar | 4.70 | 3.40 | 1.6 | [ |
| Ni(BODC)(TED) | 298 K,1 bar | 3.36 | 2.61 | 1.83① | [ |
| LIFM-63 | 298 K,1 bar | 3.0 | 2.1 | 1.6 | [ |
| MIL-53-BDC | 298 K,1 bar | 2.93 | 2.78 | 1.70 | [ |
| MIL-53-NDCA | 298 K,1 bar | 4.24 | 3.12 | 1.53 | [ |
| MIL-53-BPDC | 298 K,1 bar | 2.97 | 2.07 | 1.47 | [ |
| ZJNU-115 | 298 K,1 atm | 4.20 | 3.75 | 1.56 | [ |
| BUT-10 | 298 K,1 bar | 4.76 | 3.56 | 1.72 | [ |
| ScBPDC | 298 K,1 bar | 3.42 | 2.41 | 1.7② | [ |
| ZJNU-7 | 298 K,1 bar | 4.13 | 3.80 | 1.56 | [ |
| Co(AIN)2 | 298 K,1 bar | 3.16 | 3.14 | 2.96 | [ |
| FMOF-2 | 303 K,5 kPa | 0.90 | 0.60 | 3.3③ | [ |
| CPM-80-Zn | 298 K,1 bar | 4.77 | 4.24 | 1.8 | [ |
| CPM-81-Co | 298 K,1 bar | 5.51 | 5.07 | 1.8 | [ |
| SNNU-40 | 298 K,1 bar | 7.54 | 4.91 | 1.57 | [ |
| MIL-125 | 298 K,1 bar | 4.83 | 3.98 | 1.43 | [ |
| MUV-11 | 298 K,1 bar | 1.83 | 1.72 | 1.53 | [ |
| ZSTU-2 | 298 K,1 bar | 2.73 | 2.35 | 1.62 | [ |
| DMOF | 298 K,1 bar | 2.79④ | 2.08④ | 1.51 | [ |
| DMOF-DM | 298 K,1 bar | 4.03④ | 3.11④ | 1.70 | [ |
| DMOF-TM | 298 K,1 bar | 5.31④ | 4.99④ | 1.88 | [ |
| MOF-525 | 298 K,1 bar | 2.77 | 2.10 | 1.24 | [ |
| MOF-525(Co) | 298 K,1 bar | 2.24⑤ | 1.91⑤ | 1.11 | [ |
| UPC-612 | 298 K,1 bar | 3.57 | 2.69 | 1.4 | [ |
| UPC-613 | 298 K,1 bar | 2.56 | 2.25 | 1.47 | [ |
| JXNU-9 | 298 K,1 atm | 3.61 | 2.45 | 1.53 | [ |
| PCN-250(Fe2Zn) | 298 K,1 bar | 5.95 | 5.42 | 1.7① | [ |
| PCN-250(Fe) | 298 K,1 bar | 6.00 | 5.00 | 1.6① | [ |
| NUM-9 | 298 K,1 bar | 2.48 | 2.30 | 1.61 | [ |
| MOF-545 | 298 K,1 bar | 3.12 | 2.57 | 1.31 | [ |
| ZJU-HOF-10(sc) | 296 K,1 bar | 2.19 | 1.88 | 1.9 | [ |
| ZJU-HOF-10(v) | 296 K,1 bar | 0.64 | 0.58 | 1.54 | [ |
| ZJU-HOF-1 | 298 K,1 bar | 4.87 | 3.87 | 2.25 | [ |
| CAC-900-3 | 298 K,1 bar | 6.02 | 4.86 | 2.6 | [ |
| AAC-800-3 | 298 K,1 bar | 5.98 | 4.68 | 2.2 | [ |
| WAC-800-3 | 298 K,1 bar | 6.09 | 4.96 | 1.8 | [ |
| SIFSIX-CAC-800-3 | 298 K,1 bar | 5.02 | 4.14 | 1.4 | [ |
| DUT-8(Ni) | 303 K,1 bar | 3.96 | 2.27 | 1.65 | [ |
| RT-Cu(Qc)2 | 298 K,1 bar | 2.04 | 0.56 | 4.1 | [ |
| Ca(H2tcpb) | 298 K,1 bar | 2.78 | 2.67 | 1.75 | [ |
| NIIC-20-Bu | 298 K,1 bar | 2.50 | 1.40 | 15.4 | [ |
| ZJU-120a | 298 K,1 bar | 4.91 | 3.93 | 2.74 | [ |
| NUM-7a | 298 K,1 bar | 3.04 | 2.68 | 1.76 | [ |
| 1 | Chen D L, Wang N W, Xu C H, et al. A combined theoretical and experimental analysis on transient breakthroughs of C2H6/C2H4 in fixed beds packed with ZIF-7[J]. Microporous and Mesoporous Materials, 2015, 208: 55-65. |
| 2 | Haghighi S S, Rahimpour M R, Raeissi S, et al. Investigation of ethylene production in naphtha thermal cracking plant in presence of steam and carbon dioxide[J]. Chemical Engineering Journal, 2013, 228: 1158-1167. |
| 3 | Amghizar I, Vandewalle L A, Geem K M V, et al. New trends in olefin production[J]. Engineering, 2017, 3(2): 171-178. |
| 4 | Liao P Q, Zhang W X, Zhang J P, et al. Efficient purification of ethene by an ethane-trapping metal-organic framework[J]. Nature Communications, 2015, 6: 8697. |
| 5 | Yang S Q, Sun F Z, Liu P X, et al. Efficient purification of ethylene from C2 hydrocarbons with an C2H6/C2H2-selective metal-organic framework[J]. ACS Applied Materials & Interfaces, 2021, 13(1): 962-969. |
| 6 | Wu H X, Chen Y W, Yang W Y, et al. Ethane-selective behavior achieved on a nickel-based metal-organic framework: impact of pore effect and hydrogen bonds[J]. Industrial & Engineering Chemistry Research, 2019, 58(24): 10516-10523. |
| 7 | Cho K H, Yoon J W, Lee J H, et al. Separation of ethane/ethylene gas mixture by ethane-selective CAU-3-NDCA adsorbent[J]. Microporous and Mesoporous Materials, 2022, 330: 111572. |
| 8 | Chen C X, Wei Z W, Pham T, et al. Nanospace engineering of metal-organic frameworks through dynamic spacer installation of multifunctionalities for efficient separation of ethane from ethane/ethylene mixtures[J]. Angewandte Chemie International Edition, 2021, 60(17): 9680-9685. |
| 9 | Liang W W, Wu Y, Xiao H Y, et al. Ethane-selective carbon composites CPDA@A-ACs with high uptake and its enhanced ethane/ethylene adsorption selectivity[J]. AIChE Journal, 2018, 64(9): 3390-3399. |
| 10 | 朱登磊, 尚书勇, 谭超, 等. 基于分壁精馏塔的乙烯装置顺序分离新工艺及其模拟研究[J]. 石油学报(石油加工), 2014, 30(4): 682-686. |
| Zhu D L, Shang S Y, Tan C, et al. A new sequential separation process and its simulation for ethylene plant based on a dividing wall column[J]. Acta Petrolei Sinica(Petroleum Processing Section), 2014, 30(4): 682-686. | |
| 11 | 谭明松, 朱炜玄, 邹雄, 等. 脱乙烷塔侧采流程模拟与优化[J]. 石油化工, 2020, 49(8): 791-796. |
| Tan M S, Zhu W X, Zou X, et al. Simulation and optimization of deethanizer with side draw process[J]. Petrochemical Technology, 2020, 49(8): 791-796. | |
| 12 | González-Garay A, Mac D N, Shah N. A carbon neutral chemical industry powered by the sun[J]. Discover Chemical Engineering, 2021, 1(1): 2. |
| 13 | Sholl D S, Lively R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 14 | Wang S M, Wang F, Dong Y L, et al. Reversed C2H6/C2H4 separation in interpenetrated diamondoid coordination networks with enhanced host-guest interaction[J]. Separation and Purification Technology, 2021, 276: 119385. |
| 15 | Lu C Y, Chen Y, Wang Y, et al. Energy efficient ethylene purification in a commercially viable ethane-selective MOF[J]. Separation and Purification Technology, 2022, 282: 120126. |
| 16 | Peng J J, Sun Y W, Wu Y, et al. Selectively trapping ethane from ethylene on metal-organic framework MIL-53(Al)-FA[J]. Industrial & Engineering Chemistry Research, 2019, 58(19): 8290-8295. |
| 17 | Bereciartua P J, Cantín Á, Corma A, et al. Control of zeolite framework flexibility and pore topology for separation of ethane and ethylene[J]. Science, 2017, 358(6366): 1068-1071. |
| 18 | Ma X C, Chen R F, Zhou K, et al. Activated porous carbon with an ultrahigh surface area derived from waste biomass for acetone adsorption, CO2 capture, and light hydrocarbon separation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(31): 11721-11728. |
| 19 | Liu Y Z, Wu Y, Liang W W, et al. Bimetallic ions regulate pore size and chemistry of zeolites for selective adsorption of ethylene from ethane[J]. Chemical Engineering Science, 2020, 220: 115636. |
| 20 | Anson A, Wang Y, Lin C C H, et al. Adsorption of ethane and ethylene on modified ETS-10[J]. Chemical Engineering Science, 2008, 63(16): 4171-4175. |
| 21 | Li B Y, Zhang Y M, Krishna R, et al. Introduction of π-complexation into porous aromatic framework for highly selective adsorption of ethylene over ethane[J]. Journal of the American Chemical Society, 2014, 136(24): 8654-8660. |
| 22 | Bao Z B, Alnemrat S, Yu L, et al. Adsorption of ethane, ethylene, propane, and propylene on a magnesium-based metal-organic framework[J]. Langmuir: the ACS Journal of Surfaces and Colloids, 2011, 27(22): 13554-13562. |
| 23 | Bloch E D, Queen W L, Krishna R, et al. Hydrocarbon separations in a metal-organic framework with open iron(Ⅱ) coordination sites[J]. Science, 2012, 335(6076): 1606-1610. |
| 24 | Geier S J, Mason J A, Bloch E D, et al. Selective adsorption of ethylene over ethane and propylene over propane in the metal-organic frameworks M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Zn)[J]. Chemical Science, 2013, 4(5): 2054-2061. |
| 25 | Lin R B, Li L B, Zhou H L, et al. Molecular sieving of ethylene from ethane using a rigid metal-organic framework[J]. Nature Materials, 2018, 17(12): 1128-1133. |
| 26 | Yang Y S, Li L B, Lin R B, et al. Ethylene/ethane separation in a stable hydrogen-bonded organic framework through a gating mechanism[J]. Nature Chemistry, 2021, 13(10): 933-939. |
| 27 | Gu C, Hosono N, Zheng J J, et al. Design and control of gas diffusion process in a nanoporous soft crystal[J]. Science, 2019, 363(6425): 387-391. |
| 28 | Zhang L, Li L B, Hu E L, et al. Boosting ethylene/ethane separation within copper(Ⅰ)-chelated metal-organic frameworks through tailor-made aperture and specific π-complexation[J]. Advanced Science, 2020, 7(2): 1901918. |
| 29 | Chen Y W, Qiao Z W, Wu H X, et al. An ethane-trapping MOF PCN-250 for highly selective adsorption of ethane over ethylene[J]. Chemical Engineering Science, 2018, 175: 110-117. |
| 30 | Mukherjee S, Desai A V, Ghosh S K. Potential of metal-organic frameworks for adsorptive separation of industrially and environmentally relevant liquid mixtures[J]. Coordination Chemistry Reviews, 2018, 367: 82-126. |
| 31 | Furukawa H, Cordova K E, O'Keeffe M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149): 1230444. |
| 32 | Wang C, Liu D M, Lin W B. Metal-organic frameworks as a tunable platform for designing functional molecular materials[J]. Journal of the American Chemical Society, 2013, 135(36): 13222-13234. |
| 33 | Adil K, Belmabkhout Y, Pillai R S, et al. Gas/vapour separation using ultra-microporous metal-organic frameworks: insights into the structure/separation relationship[J]. Chemical Society Reviews, 2017, 46(11): 3402-3430. |
| 34 | Lin R B, Xiang S C, Xing H B, et al. Exploration of porous metal-organic frameworks for gas separation and purification[J]. Coordination Chemistry Reviews, 2019, 378: 87-103. |
| 35 | Cadiau A, Adil K, Bhatt P M, et al. A metal-organic framework-based splitter for separating propylene from propane[J]. Science, 2016, 353(6295): 137-140. |
| 36 | Peng Y L, Pham T, Li P F, et al. Robust ultramicroporous metal-organic frameworks with benchmark affinity for acetylene[J]. Angewandte Chemie International Edition, 2018, 57(34): 10971-10975. |
| 37 | Li L Y, Guo L D, Pu S Y, et al. A calcium-based microporous metal-organic framework for efficient adsorption separation of light hydrocarbons[J]. Chemical Engineering Journal, 2019, 358: 446-455. |
| 38 | Zhang X, Wang J X, Li L B, et al. A rod-packing hydrogen-bonded organic framework with suitable pore confinement for benchmark ethane/ethylene separation[J]. Angewandte Chemie International Edition, 2021, 60(18): 10304-10310. |
| 39 | Zhang X, Li L B, Wang J X, et al. Selective ethane/ethylene separation in a robust microporous hydrogen-bonded organic framework[J]. Journal of the American Chemical Society, 2020, 142(1): 633-640. |
| 40 | Wang J X, Gu X W, Lin Y X, et al. A novel hydrogen-bonded organic framework with highly permanent porosity for boosting ethane/ethylene separation[J]. ACS Materials Letters, 2021, 3(5): 497-503. |
| 41 | Chen S X, Huang Y F, Han X X, et al. Simultaneous and efficient removal of Cr(Ⅵ) and methyl orange on LDHs decorated porous carbons[J]. Chemical Engineering Journal, 2018, 352: 306-315. |
| 42 | Zhang P X, Zhong Y, Ding J, et al. A new choice of polymer precursor for solvent-free method: preparation of N-enriched porous carbons for highly selective CO2 capture[J]. Chemical Engineering Journal, 2019, 355: 963-973. |
| 43 | Liang W W, Zhang Y F, Wang X J, et al. Asphalt-derived high surface area activated porous carbons for the effective adsorption separation of ethane and ethylene[J]. Chemical Engineering Science, 2017, 162: 192-202. |
| 44 | Lin R B, Wu H, Li L B, et al. Boosting ethane/ethylene separation within isoreticular ultramicroporous metal-organic frameworks[J]. Journal of the American Chemical Society, 2018, 140(40): 12940-12946. |
| 45 | Qazvini O T, Babarao R, Shi Z L, et al. A robust ethane-trapping metal-organic framework with a high capacity for ethylene purification[J]. Journal of the American Chemical Society, 2019, 141(12): 5014-5020. |
| 46 | Pires J, Fernandes J, Dedecker K, et al. Enhancement of ethane selectivity in ethane-ethylene mixtures by perfluoro groups in Zr-based metal-organic frameworks[J]. ACS Applied Materials & Interfaces, 2019, 11(30): 27410-27421. |
| 47 | Lee S K, Lee Y J, Cho K, et al. A fluorinated metal-organic framework, FMOF-2, for preferential adsorption of ethane over ethylene[J]. Bulletin of the Korean Chemical Society, 2021, 42(2): 286-289. |
| 48 | Zhang Y P, Lv D F, Chen J Y, et al. Preferential adsorption of ethane over ethylene on a Zr-based metal-organic framework: impacts of C—H⋯N hydrogen bonding[J]. New Journal of Chemistry, 2021, 45(18): 8045-8053. |
| 49 | Wang X, Niu Z, Al-Enizi A M, et al. Pore environment engineering in metal-organic frameworks for efficient ethane/ethylene separation[J]. Journal of Materials Chemistry A, 2019, 7(22): 13585-13590. |
| 50 | Schneemann A, Jing Y, Evans J D, et al. Alkyl decorated metal-organic frameworks for selective trapping of ethane from ethylene above ambient pressures[J]. Dalton Transactions (Cambridge, England: 2003), 2021, 50 30: 10423-10435. |
| 51 | Gücüyener C, van den Bergh J, Gascon J, et al. Ethane/ethene separation turned on its head: selective ethane adsorption on the metal-organic framework ZIF-7 through a gate-opening mechanism[J]. Journal of the American Chemical Society, 2010, 132(50): 17704-17706. |
| 52 | Li Y P, Zhao Y N, Li S N, et al. Ultrahigh-uptake capacity-enabled gas separation and fruit preservation by a new single-walled nickel-organic framework[J]. Advanced Science, 2021, 8(12): 2003141. |
| 53 | Ma C, Wang X J, Wang X, et al. Novel glucose-based adsorbents (Glc-As) with preferential adsorption of ethane over ethylene and high capacity[J]. Chemical Engineering Science, 2017, 172: 612-621. |
| 54 | Wang X J, Wu Y, Peng J J, et al. Novel glucosamine-based carbon adsorbents with high capacity and its enhanced mechanism of preferential adsorption of C2H6 over C2H4 [J]. Chemical Engineering Journal, 2019, 358: 1114-1125. |
| 55 | Li L B, Lin R B, Krishna R, et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science, 2018, 362(6413): 443-446. |
| 56 | Lysova A A, Samsonenko D G, Kovalenko K A, et al. A series of mesoporous metal-organic frameworks with tunable windows sizes and exceptionally high ethane over ethylene adsorption selectivity[J]. Angewandte Chemie International Edition, 2020, 59(46): 20561-20567. |
| 57 | Yang L, Zhou W, Li H, et al. Reversed ethane/ethylene adsorption in a metal-organic framework via introduction of oxygen[J]. Chinese Journal of Chemical Engineering, 2020, 28(2): 593-597. |
| 58 | Pei J Y, Wang J X, Shao K, et al. Engineering microporous ethane-trapping metal-organic frameworks for boosting ethane/ethylene separation[J]. Journal of Materials Chemistry A, 2020, 8(7): 3613-3620. |
| 59 | Zheng S T, Bu J T, Li Y F, et al. Pore space partition and charge separation in cage-within-cage indium-organic frameworks with high CO2 uptake[J]. Journal of the American Chemical Society, 2010, 132(48): 17062-17064. |
| 60 | Zhang Q L, Chen J, Zhu X C, et al. 7-Connected F e 3 Ⅲ -based bio-MOF: pore space partition and gas separations[J]. Inorganic Chemistry, 2020, 59(23): 16829-16832. |
| 61 | Serre C, Mellot-Draznieks C, Surblé S, et al. Role of solvent-host interactions that lead to very large swelling of hybrid frameworks[J]. Science, 2007, 315(5820): 1828-1831. |
| 62 | Yang H J, Wang Y X, Krishna R, et al. Pore-space-partition-enabled exceptional ethane uptake and ethane-selective ethane-ethylene separation[J]. Journal of the American Chemical Society, 2020, 142(5): 2222-2227. |
| 63 | Liang W W, Xu F, Zhou X, et al. Ethane selective adsorbent Ni(bdc)(ted)0.5 with high uptake and its significance in adsorption separation of ethane and ethylene[J]. Chemical Engineering Science, 2016, 148: 275-281. |
| 64 | Wu H X, Chen Y W, Lv D F, et al. An indium-based ethane-trapping MOF for efficient selective separation of C2H6/C2H4 mixture[J]. Separation and Purification Technology, 2019, 212: 51-56. |
| 65 | Sun F Z, Yang S Q, Krishna R, et al. Microporous metal-organic framework with a completely reversed adsorption relationship for C2 hydrocarbons at room temperature[J]. ACS Applied Materials & Interfaces, 2020, 12(5): 6105-6111. |
| 66 | Wu H X, Chen Y W, Yuan Y N, et al. The modulation of ethane-selective adsorption performance in series of bimetal PCN-250 metal-organic frameworks: impact of metal composition[J]. AIChE Journal, 2022, 68(1): e17385. |
| 67 | Lee S K, Park H, Yoon J W, et al. Microporous 3D graphene-like zeolite-templated carbons for preferential adsorption of ethane[J]. ACS Applied Materials & Interfaces, 2020, 12(25): 28484-28495. |
| 68 | Xiao H Y, Wu Y, Wang X J, et al. A novel fructose-based adsorbent with high capacity and its ethane-selective adsorption property[J]. Journal of Solid State Chemistry, 2018, 268: 190-197. |
| 69 | Zhang P X, Wen X, Wang L, et al. Algae-derived N-doped porous carbons with ultrahigh specific surface area for highly selective separation of light hydrocarbons[J]. Chemical Engineering Journal, 2020, 381: 122731. |
| 70 | Wang Z H, Yang L F, Zhang P X, et al. Highly microporous activated carbons with industrial potential for selective adsorption of ethane over ethylene[J]. Industrial & Engineering Chemistry Research, 2021, 60(36): 13301-13308. |
| 71 | Gu X W, Wang J X, Wu E Y, et al. Immobilization of lewis basic sites into a stable ethane-selective MOF enabling one-step separation of ethylene from a ternary mixture[J]. Journal of American Chemical Society, 2022, 144(6): 2614-2623. |
| 72 | Jiang S S, Guo L D, Chen L H, et al. A strongly hydrophobic ethane-selective metal-organic framework for efficient ethane/ethylene separation[J]. Chemical Engineering Journal, 2022, 442: 136152. |
| 73 | Zhang J Y, Liu Z W, Liu H B, et al. Preferential adsorption performance of ethane in a robust nickel-based metal-organic framework for separating ethane from ethylene[J]. ACS Omega, 2022, 7(9): 7648-7654. |
| 74 | Cho K H, Yoon J W, Lee J H, et al. Pore control of Al-based MIL-53 isomorphs for the preferential capture of ethane in an ethane/ethylene mixture[J]. Journal of Materials Chemistry A, 2021, 9(25): 14593-14600. |
| 75 | Fan L H, Zhou P, Wang X X, et al. Rational construction and performance regulation of an In(Ⅲ)-tetraisophthalate framework for one-step adsorption-phase purification of C2H4 from C2 hydrocarbons[J]. Inorganic Chemistry, 2021, 60(14): 10819-10829. |
| 76 | He C H, Wang Y, Chen Y, et al. An ethane-favored metal-organic framework with tailored pore environment used for efficient ethylene separation[J]. Microporous and Mesoporous Materials, 2021, 320: 111096. |
| 77 | Jiang S S, Li L Y, Guo L D, et al. A robust ethane-trapping metal-organic framework for efficient purification of ethylene[J]. Science China Chemistry, 2021, 64(4): 666-672. |
| 78 | Jiang Z Z, Fan L H, Zhou P, et al. An aromatic-rich cage-based MOF with inorganic chloride ions decorating the pore surface displaying the preferential adsorption of C2H2 and C2H6 over C2H4 [J]. Inorganic Chemistry Frontiers, 2021, 8(5): 1243-1252. |
| 79 | Kang M, Kang D W, Choe J H, et al. A robust hydrogen-bonded metal-organic framework with enhanced ethane uptake and selectivity[J]. Chemistry of Materials, 2021, 33(15): 6193-6199. |
| 80 | Lei X W, Yang H J, Wang Y X, et al. Tunable metal-organic frameworks based on 8-connected metal trimers for high ethane uptake[J]. Small, 2021, 17(22): 2003167. |
| 81 | Liu P X, Wang Y, Chen Y, et al. Construction of saturated coordination titanium-based metal-organic framework for one-step C2H2/C2H6/C2H4 separation[J]. Separation and Purification Technology, 2021, 276: 119284. |
| 82 | Wang Y T, Hao C L, Fan W D, et al. One-step ethylene purification from an acetylene/ethylene/ethane ternary mixture by cyclopentadiene cobalt-functionalized metal-organic frameworks[J]. Angewandte Chemie International Edition, 2021, 60(20): 11350-11358. |
| 83 | Wang Z Q, Luo H Q, Wang Y L, et al. Octanuclear cobalt(Ⅱ) cluster-based metal-organic framework with caged structure exhibiting the selective adsorption of ethane over ethylene[J]. Inorganic Chemistry, 2021, 60(14): 10596-10602. |
| 84 | Cho K H, Yoon J W, Lee J H, et al. Effect of framework rigidity in metal-organic frameworks for adsorptive separation of ethane/ethylene[J]. Microporous and Mesoporous Materials, 2020, 307: 110473. |
| 85 | Tang Y N, Wang S, Zhou X, et al. Room temperature synthesis of Cu(Qc)2 and its application for ethane capture from light hydrocarbons[J]. Chemical Engineering Science, 2020, 213: 115355. |
| 86 | Lin Y H, Li Y Z, Wang H, et al. Separation of ethane and ethylene by a robust ethane-selective calcium-based metal-organic framework[J]. New Journal of Chemistry, 2020, 44(28): 11933-11936. |
| 87 | Xu Z Z, Xiong X H, Xiong J B, et al. A robust Th-azole framework for highly efficient purification of C2H4 from a C2H4/C2H2/C2H6 mixture[J]. Nature Communications, 2020, 11: 3163. |
| 88 | 刘普旭, 贺朝辉, 李立博, 等. 高稳定双金属MOF材料用于低浓度乙烷的高效分离[J]. 化工学报, 2020, 71(9): 4211-4218. |
| Liu P X, He C H, Li L B, et al. Stable mixed metal-organic framework for efficient C2H6/C2H4 separation[J]. CIESC Journal, 2020, 71(9): 4211-4218. | |
| 89 | Yang L, Wang Y, Chen Y, et al. Microporous metal-organic framework with specific functional sites for efficient removal of ethane from ethane/ethylene mixtures[J]. Chemical Engineering Journal, 2020, 387: 124137. |
| 90 | Chen K J, Madden D G, Mukherjee S, et al. Synergistic sorbent separation for one-step ethylene purification from a four-component mixture[J]. Science, 2019, 366(6462): 241-246. |
| 91 | Wang Y X, Yuan S, Hu Z G, et al. Pore size reduction in zirconium metal-organic frameworks for ethylene/ethane separation[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(7): 7118-7126. |
| 92 | Chen Y W, Wu H X, Lv D F, et al. Highly adsorptive separation of ethane/ethylene by an ethane-selective MOF MIL-142A[J]. Industrial & Engineering Chemistry Research, 2018, 57(11): 4063-4069. |
| 93 | Hao H G, Zhao Y F, Chen D M, et al. Simultaneous trapping of C2H2 and C2H6 from a ternary mixture of C2H2/C2H4/C2H6 in a robust metal-organic framework for the purification of C2H4 [J]. Angewandte Chemie International Edition, 2018, 57(49): 16067-16071. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 王浩, 王振雷. 基于自适应谱方法的裂解炉烧焦模型化简策略[J]. 化工学报, 2023, 74(9): 3855-3864. |
| [5] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [6] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [7] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [8] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [9] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [10] | 诸程瑛, 王振雷. 基于改进深度强化学习的乙烯裂解炉操作优化[J]. 化工学报, 2023, 74(8): 3429-3437. |
| [11] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [12] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [13] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [14] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [15] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号