化工学报 ›› 2022, Vol. 73 ›› Issue (10): 4498-4506.DOI: 10.11949/0438-1157.20220826
尤红运( ), 林景骏, 黄凯越, 舒日洋(
), 林景骏, 黄凯越, 舒日洋( ), 田志鹏, 王超, 陈颖
), 田志鹏, 王超, 陈颖
收稿日期:2022-06-13
修回日期:2022-08-31
出版日期:2022-10-05
发布日期:2022-11-02
通讯作者:
舒日洋
作者简介:尤红运(1996—),男,硕士研究生,2112002273@mail2.gdut.edu.cn
基金资助:
Hongyun YOU( ), Jingjun LIN, Kaiyue HUANG, Riyang SHU(
), Jingjun LIN, Kaiyue HUANG, Riyang SHU( ), Zhipeng TIAN, Chao WANG, Ying CHEN
), Zhipeng TIAN, Chao WANG, Ying CHEN
Received:2022-06-13
Revised:2022-08-31
Online:2022-10-05
Published:2022-11-02
Contact:
Riyang SHU
摘要:
系统研究了木质素酚类化合物在Ru/C和Al2O3催化体系下溶剂效应对加氢反应的影响。在乙醇溶剂中,苯酚在35℃下即可完全转化为环己醇,具有最佳的加氢效果。研究表明极性溶剂比非极性溶剂的加氢效果更好,是因为催化剂分散均匀,强化了催化剂和反应物间的传质与扩散。在醇类极性溶剂中,乙醇的加氢反应效率最高,对比研究显示溶剂的极性越强,苯酚加氢的转化效果越好。还建立了Kamlet-Taft表达式参数与苯酚转化率间的关系关联式,分析了各参数的影响效果,详细阐述了Ru/C和Al2O3催化体系下详细的苯酚加氢反应路径和机理,并将该催化体系应用于其他木质素酚类化合物的加氢反应,也取得了很好的反应效果,大部分木质素酚类化合物均加氢饱和转化成稳定的环状醇类化合物。
中图分类号:
尤红运, 林景骏, 黄凯越, 舒日洋, 田志鹏, 王超, 陈颖. 溶剂效应对木质素酚类化合物加氢反应的影响机理[J]. 化工学报, 2022, 73(10): 4498-4506.
Hongyun YOU, Jingjun LIN, Kaiyue HUANG, Riyang SHU, Zhipeng TIAN, Chao WANG, Ying CHEN. Mechanism of solvent effect on hydrogenation of lignin-derived phenolic compounds[J]. CIESC Journal, 2022, 73(10): 4498-4506.
| 序号 | 催化剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | Ru/C | 14.6 | 95.9 | 4.1 |
| 2 | Ru/C + WO3 | 6.2 | 93.6 | 6.4 |
| 3 | Ru/C + Nb2O5 | 9.2 | 94.1 | 5.9 |
| 4 | Ru/C + ZrO2 | 13.7 | 96.1 | 3.9 |
| 5 | Ru/C + TiO2 | 23.2 | 97.5 | 2.5 |
| 6 | Ru/C + HPW | 2.1 | 84.7 | 15.3 |
| 7 | Ru/C + HSiW | 2.2 | 86.5 | 13.5 |
| 8 | Ru/C + Al2O3 | 100 | 100 | 0 |
| 9 | Ru/C + Al2O3① | 69.8 | 99.1 | 0.9 |
| 10 | Ru/C + Al2O3② | 30.4 | 98.0 | 2.0 |
| 11 | Al2O3 | 0 | — | — |
表1 不同催化剂对苯酚加氢反应的影响
Table 1 The effect of different catalysts on the phenol hydrogenation
| 序号 | 催化剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | Ru/C | 14.6 | 95.9 | 4.1 |
| 2 | Ru/C + WO3 | 6.2 | 93.6 | 6.4 |
| 3 | Ru/C + Nb2O5 | 9.2 | 94.1 | 5.9 |
| 4 | Ru/C + ZrO2 | 13.7 | 96.1 | 3.9 |
| 5 | Ru/C + TiO2 | 23.2 | 97.5 | 2.5 |
| 6 | Ru/C + HPW | 2.1 | 84.7 | 15.3 |
| 7 | Ru/C + HSiW | 2.2 | 86.5 | 13.5 |
| 8 | Ru/C + Al2O3 | 100 | 100 | 0 |
| 9 | Ru/C + Al2O3① | 69.8 | 99.1 | 0.9 |
| 10 | Ru/C + Al2O3② | 30.4 | 98.0 | 2.0 |
| 11 | Al2O3 | 0 | — | — |
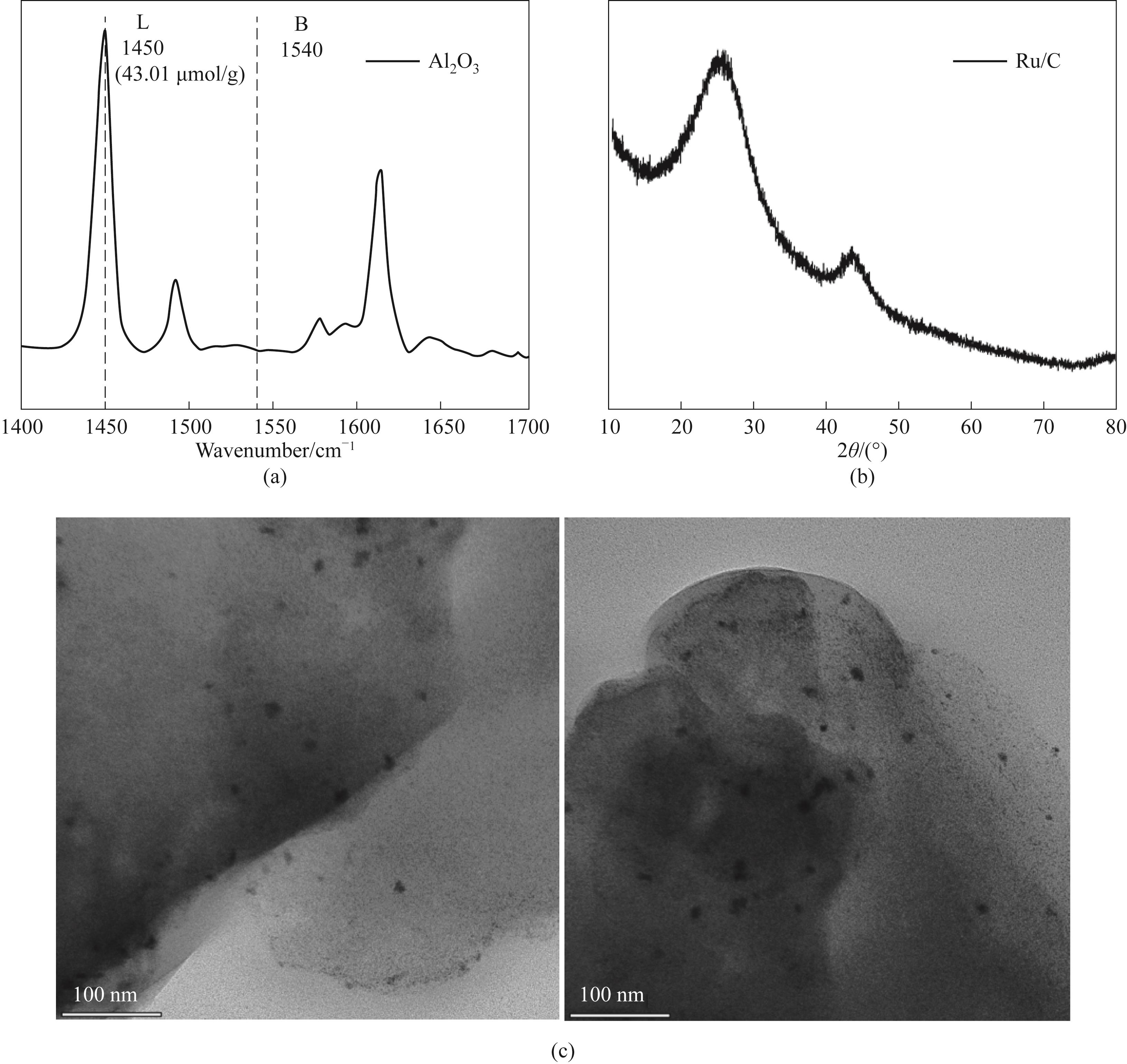
图1 (a)Al2O3的Py-IR光谱(解吸温度150℃);(b)Ru/C催化剂的XRD谱图;(c)Ru/C催化剂的TEM图
Fig.1 (a)Py-IR spectra of Al2O3 (desorption temperature 150℃);(b)XRD pattern of the Ru/C catalyst;(c)TEM images of the Ru/C catalyst
| 序号 | 溶剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | 正己烷 | 17.6 | 81.2 | 18.8 |
| 2 | 正辛烷 | 25.7 | 84.7 | 15.3 |
| 3 | 正十二烷 | 20.2 | 86.3 | 13.7 |
| 4 | 甲醇 | 24.6 | 97.9 | 2.1 |
| 5 | 乙醇 | 100 | 100 | 0 |
| 6 | 正丙醇 | 57.1 | 100 | 0 |
| 7 | 正丁醇 | 52.6 | 95.4 | 4.6 |
| 8 | 正己醇 | 31.0 | 97.2 | 2.8 |
| 9 | 正辛醇 | 14.4 | 91.3 | 8.7 |
表2 不同溶剂对苯酚加氢的影响
Table 2 The phenol hydrogenation results of different solvents
| 序号 | 溶剂 | 转化率/% | 选择性/% | |
|---|---|---|---|---|
| 环己醇 | 环己酮 | |||
| 1 | 正己烷 | 17.6 | 81.2 | 18.8 |
| 2 | 正辛烷 | 25.7 | 84.7 | 15.3 |
| 3 | 正十二烷 | 20.2 | 86.3 | 13.7 |
| 4 | 甲醇 | 24.6 | 97.9 | 2.1 |
| 5 | 乙醇 | 100 | 100 | 0 |
| 6 | 正丙醇 | 57.1 | 100 | 0 |
| 7 | 正丁醇 | 52.6 | 95.4 | 4.6 |
| 8 | 正己醇 | 31.0 | 97.2 | 2.8 |
| 9 | 正辛醇 | 14.4 | 91.3 | 8.7 |
| 溶剂 | π* | α | β | 转化率/% | ||
|---|---|---|---|---|---|---|
| 甲醇 | 55.4 | 0.7647 | 0.60 | 0.98 | 0.66 | 24.6 |
| 乙醇 | 51.9 | 0.6563 | 0.54 | 0.86 | 0.75 | 100 |
| 正丙醇 | 50.7 | 0.6192 | 0.52 | 0.84 | 0.90 | 57.1 |
| 正丁醇 | 50.2 | 0.6037 | 0.47 | 0.84 | 0.84 | 52.6 |
| 正己醇 | 48.8 | 0.5604 | 0.40 | 0.80 | 0.84 | 31.0 |
| 正辛醇 | 48.3 | 0.5449 | 0.40 | 0.77 | 0.81 | 14.4 |
表3 几种有机溶剂性质参数及苯酚加氢反应转化率
Table 3 The property parameters of some organic solvents and conversion of phenol hydrogenation
| 溶剂 | π* | α | β | 转化率/% | ||
|---|---|---|---|---|---|---|
| 甲醇 | 55.4 | 0.7647 | 0.60 | 0.98 | 0.66 | 24.6 |
| 乙醇 | 51.9 | 0.6563 | 0.54 | 0.86 | 0.75 | 100 |
| 正丙醇 | 50.7 | 0.6192 | 0.52 | 0.84 | 0.90 | 57.1 |
| 正丁醇 | 50.2 | 0.6037 | 0.47 | 0.84 | 0.84 | 52.6 |
| 正己醇 | 48.8 | 0.5604 | 0.40 | 0.80 | 0.84 | 31.0 |
| 正辛醇 | 48.3 | 0.5449 | 0.40 | 0.77 | 0.81 | 14.4 |
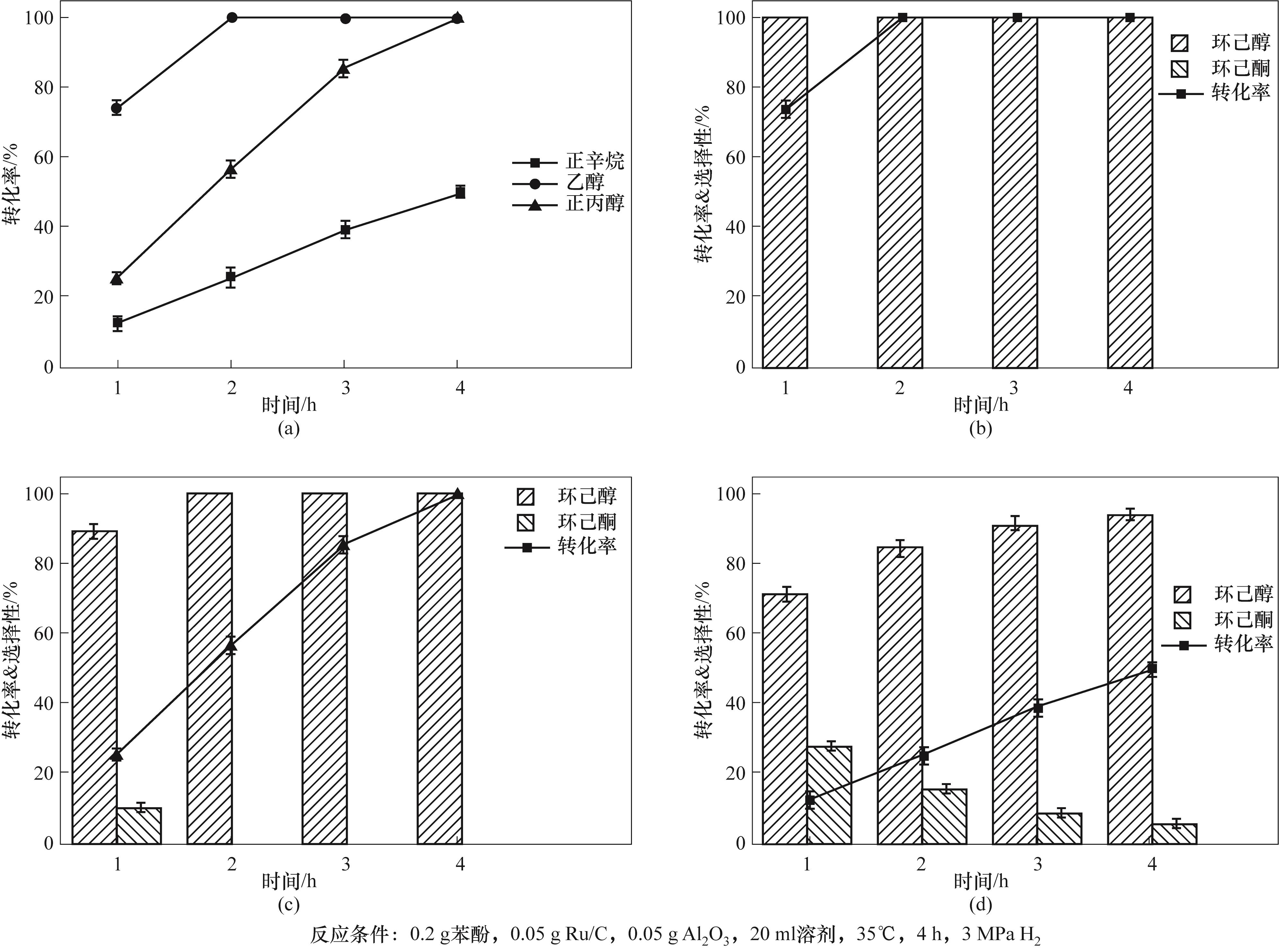
图3 (a)不同溶剂中苯酚转化率随时间的变化情况;(b)乙醇溶剂中苯酚加氢反应的产物分布情况;(c)正丙醇溶剂中苯酚加氢反应的产物分布情况;(d)正辛烷溶剂中苯酚加氢反应的产物分布情况
Fig.3 (a)Variation of phenol conversion with time in different solvents;(b)Product distribution of phenol hydrogenation in ethanol solvent;(c)Product distribution of phenol hydrogenation in n-propanol solvent;(d)Product distribution of phenol hydrogenation in octane solvent
| 底物 | 转化率/% | 选择性/% | ||
|---|---|---|---|---|
 | 100 |
7.7 |
92.3 | |
 | 100 |
7.8 |
92.2 | |
 | 100 |
2.7 |
94.9 |
2.4 |
 | 100 |
9.8 |
90.2 | |
 | 100 |
10.3 |
89.7 | |
 | 65.5 |
100 | ||
 | 39.4 |
100 | ||
表4 其他酚类模型化合物的催化加氢反应
Table 4 Catalytic hydrogenation of other phenolic model compounds
| 底物 | 转化率/% | 选择性/% | ||
|---|---|---|---|---|
 | 100 |
7.7 |
92.3 | |
 | 100 |
7.8 |
92.2 | |
 | 100 |
2.7 |
94.9 |
2.4 |
 | 100 |
9.8 |
90.2 | |
 | 100 |
10.3 |
89.7 | |
 | 65.5 |
100 | ||
 | 39.4 |
100 | ||
| 1 | Huber G W, Chheda J N, Barrett C J, et al. Production of liquid alkanes by aqueous-phase processing of biomass-derived carbohydrates[J]. Science, 2005, 308(5727): 1446-1450. |
| 2 | Yang Z, Luo B W, Shu R Y, et al. Efficient hydrodeoxygenation of phenolic compounds and raw lignin-oil under a temperature-controlled phase-transfer catalysis[J]. Fuel, 2021, 291: 120091. |
| 3 | Dou X M, Jiang X, Li W Z, et al. Highly efficient conversion of Kraft lignin into liquid fuels with a Co-Zn-beta zeolite catalyst[J]. Applied Catalysis B: Environmental, 2020, 268: 118429. |
| 4 | 王江丽, 薛敏, 赵承科, 等. 木质素分级对其应用性能的影响[J]. 化工学报, 2022, 73(5): 1894-1907. |
| Wang J L, Xue M, Zhao C K, et al. Influences of lignin fractionation on its utilization[J]. CIESC Journal, 2022, 73(5): 1894-1907. | |
| 5 | 王东玲, 王文锦, 彭梓芳, 等. 醇溶剂提取松木木质素及其结构表征[J]. 化工学报, 2020, 71(8): 3761-3769. |
| Wang D L, Wang W J, Peng Z F, et al. Structure characterization of pine lignin extracted by different alcohol solvents[J]. CIESC Journal, 2020, 71(8): 3761-3769. | |
| 6 | Li C Z, Zhao X C, Wang A Q, et al. Catalytic transformation of lignin for the production of chemicals and fuels[J]. Chemical Reviews, 2015, 115(21): 11559-11624. |
| 7 | Long J X, Xu Y, Wang T J, et al. Efficient base-catalyzed decomposition and in situ hydrogenolysis process for lignin depolymerization and char elimination[J]. Applied Energy, 2015, 141: 70-79. |
| 8 | Zhang X, Wang K G, Chen J H, et al. Mild hydrogenation of bio-oil and its derived phenolic monomers over Pt-Ni bimetal-based catalysts[J]. Applied Energy, 2020, 275: 115154. |
| 9 | 李雁斌, 徐莹, 马隆龙, 等. 邻甲酚液相原位加氢反应[J]. 高等学校化学学报, 2014, 35(12): 2654-2661. |
| Li Y B, Xu Y, Ma L L, et al. Research on in situ hydrogenation of o-cresol over Ni/CMK-3 catalysts[J]. Chemical Journal of Chinese Universities, 2014, 35(12): 2654-2661. | |
| 10 | Wen J L, Sun S L, Yuan T Q, et al. Understanding the chemical and structural transformations of lignin macromolecule during torrefaction[J]. Applied Energy, 2014, 121: 1-9. |
| 11 | Shu R Y, Zhong Z J, You H Y, et al. Hydrodeoxygenation of lignin-derived phenolic compounds over Ru/TiO2-CeO2 catalyst prepared by photochemical reduction method[J]. Journal of the Energy Institute, 2021, 99: 1-8. |
| 12 | Niu Z, Zhang S, Sun Y, et al. Controllable synthesis of N i / S i O ₂ hollow spheres and their excellent catalytic performance in 4-nitrophenol reduction[J]. Dalton Transactions, 2014, 43(44): 16911-16918. |
| 13 | Shu R Y, Zhang Q, Xu Y, et al. Hydrogenation of lignin-derived phenolic compounds over step by step precipitated Ni/SiO2 [J]. RSC Advances, 2016, 6(7): 5214-5222. |
| 14 | Mao C, Zheng J W, Matsagar B M, et al. Highly-efficient Ru/Al-SBA-15 catalysts with strong Lewis acid sites for the water-assisted hydrogenation of p-phthalic acid[J]. Catalysis Science & Technology, 2020, 10(8): 2443-2451. |
| 15 | Rao D M, Xue X G, Cui G Q, et al. Metal-acid site synergistic catalysis in Ru–ZrO2 toward selective hydrogenation of benzene to cyclohexene[J]. Catalysis Science & Technology, 2018, 8(1): 236-243. |
| 16 | You H Y, Yang Z, Lin J J, et al. Hydrogenation of lignin-derived phenolic compounds over Ru/C enhanced by Al2O3 catalyst at room temperature[J]. ChemistrySelect, 2022, 7(19): e202200709. |
| 17 | Li A Q, Shen K, Chen J Y, et al. Highly selective hydrogenation of phenol to cyclohexanol over MOF-derived non-noble Co-Ni@NC catalysts[J]. Chemical Engineering Science, 2017, 166: 66-76. |
| 18 | Li F, Cao B, Zhu W X, et al. Hydrogenation of phenol over Pt/CNTs: the effects of Pt loading and reaction solvents[J]. Catalysts, 2017, 7(5): 145. |
| 19 | Takagi H, Isoda T, Kusakabe K, et al. Effects of solvents on the hydrogenation of mono-aromatic compounds using noble-metal catalysts[J]. Energy & Fuels, 1999, 13(6): 1191-1196. |
| 20 | Shi Y K, Qu G F, Ning P, et al. Advances of application of ionic liquids in catalytic oxidation reactions[J]. Advanced Materials Research, 2011, 233/234/235: 499-506. |
| 21 | Neri G, Visco A M, Donato A, et al. Hydrogenation of phenol to cyclohexanone over palladium and alkali-doped palladium catalysts[J]. Applied Catalysis A: General, 1994, 110(1): 49-59. |
| 22 | Chen Y Z, Liaw C W, Lee L I. Selective hydrogenation of phenol to cyclohexanone over palladium supported on calcined Mg/Al hydrotalcite[J]. Applied Catalysis A: General, 1999, 177(1): 1-8. |
| 23 | Kimura Y, Haraguchi K. Clay-alcohol-water dispersions: anomalous viscosity changes due to network formation of clay nanosheets induced by alcohol clustering[J]. Langmuir, 2017, 33(19): 4758-4768. |
| 24 | 章胜男, 韩东梅, 任山, 等. 有机电极材料固定化策略[J]. 化学进展, 2020, 32(1): 103-118. |
| Zhang S N, Han D M, Ren S, et al. Immobilization strategies of organic electrode materials[J]. Progress in Chemistry, 2020, 32(1): 103-118. | |
| 25 | Wang X Y, Rinaldi R. Solvent effects on the hydrogenolysis of diphenyl ether with raney nickel and their implications for the conversion of lignin[J]. ChemSusChem, 2012, 5(8): 1455-1466. |
| 26 | Mazzieri V A, L'Argentiére P C, Fígoli N S. Influence of methanol addition during selective hydrogenation of benzene to cyclohexene[J]. Reaction Kinetics and Catalysis Letters, 2004, 81(1): 107-112. |
| 27 | Kim G J, Kim M S, Byun J Y, et al. Effects of Ru addition to Pd/Al2O3 catalysts on methanol steam reforming reaction: a mechanistic study[J]. Applied Catalysis A: General, 2019, 572: 115-123. |
| 28 | Strobel A B, Egert T, Langguth P. Predicting leachables solubilization in polysorbate 80 solutions by a linear solvation energy relationship (LSER)[J]. Pharmaceutical Research, 2021, 38(9): 1549-1561. |
| 29 | Marcus Y. The properties of organic liquids that are relevant to their use as solvating solvents[J]. ChemInform, 1994, 25(12): 409-460. |
| 30 | Liu H Z, Jiang T, Han B X, et al. Selective phenol hydrogenation to cyclohexanone over a dual supported Pd-Lewis acid catalyst[J]. Science, 2009, 326(5957): 1250-1252. |
| 31 | Laurent E, Delmon B. Influence of oxygen-, nitrogen-, and sulfur-containing compounds on the hydrodeoxygenation of phenols over sulfided cobalt-molybdenum/.gamma.-alumina and nickel-molybdenum/.gamma.-alumina catalysts[J]. Industrial & Engineering Chemistry Research, 1993, 32(11): 2516-2524. |
| 32 | Zhuang L, Li H X, Dai W L, et al. Liquid phase hydrogenation of phenol to cyclohexenone over a Pd-La-B amorphous catalyst[J]. Chemistry Letters, 2003, 32(11): 1072-1073. |
| 33 | Velu S, Kapoor M P, Inagaki S, et al. Vapor phase hydrogenation of phenol over palladium supported on mesoporous CeO2 and ZrO2 [J]. Applied Catalysis A: General, 2003, 245(2): 317-331. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [4] | 曹跃, 余冲, 李智, 杨明磊. 工业数据驱动的加氢裂化装置多工况切换过渡状态检测[J]. 化工学报, 2023, 74(9): 3841-3854. |
| [5] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [8] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [9] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [10] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [11] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [12] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [13] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [14] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| [15] | 张谭, 刘光, 李晋平, 孙予罕. Ru基氮还原电催化剂性能调控策略[J]. 化工学报, 2023, 74(6): 2264-2280. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号