化工学报 ›› 2023, Vol. 74 ›› Issue (2): 511-524.DOI: 10.11949/0438-1157.20221021
收稿日期:2022-07-25
修回日期:2022-09-26
出版日期:2023-02-05
发布日期:2023-03-21
通讯作者:
张吉松
作者简介:章承浩(1998—),男,博士研究生,zhangch20@mails.tsinghua.edu.cn
基金资助:
Chenghao ZHANG( ), Jing LUO, Jisong ZHANG(
), Jing LUO, Jisong ZHANG( )
)
Received:2022-07-25
Revised:2022-09-26
Online:2023-02-05
Published:2023-03-21
Contact:
Jisong ZHANG
摘要:
连续液相氧气氧化技术代替传统氧化技术已经成为氧化反应发展的一大趋势。但是,分子氧通常需要被合适的催化剂进行活化后才能进行高选择性氧化。近年来,氮氧自由基催化剂因其能够在温和条件下高效地催化氧气氧化反应而取得了快速发展。此外,可持续的绿色氧化工艺不仅依赖于高效环保的催化体系,还需要依托能够强化传质和反应性能的反应器技术。本文介绍了连续微反应氧化技术中常用的微反应器,归纳总结了以氮氧自由基及其衍生物为催化剂的空气/氧气氧化反应在连续有机合成中的研究进展。最后,针对现阶段氮氧自由基催化的连续液相氧化技术的潜在挑战,对该技术在精细化工领域中的应用进行了展望。
中图分类号:
章承浩, 罗京, 张吉松. 微反应器内基于氮氧自由基催化剂连续氧气/空气氧化反应的研究进展[J]. 化工学报, 2023, 74(2): 511-524.
Chenghao ZHANG, Jing LUO, Jisong ZHANG. Advances in continuous aerobic oxidation based on nitroxyl radical catalyst in microreactors[J]. CIESC Journal, 2023, 74(2): 511-524.
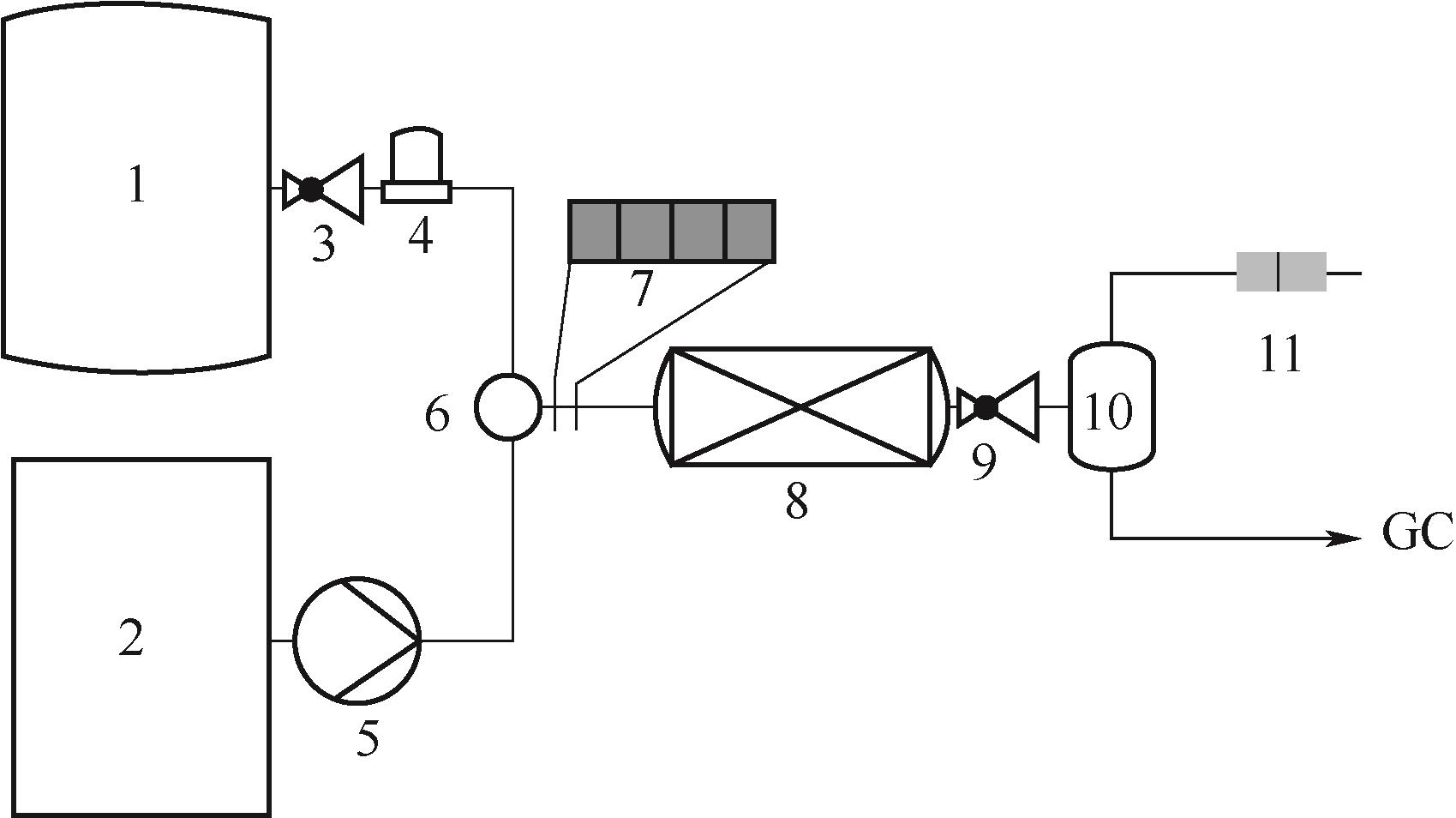
图7 连续流动三相反应器流程示意图[49]1—氧气罐;2—液体罐;3—减压阀;4—质量流量计;5—HPLC泵;6—气液混合器;7—柱塞流;8—固定床反应器;9—压力调节阀;10—气液分离器;11—液相流动通过红外检测
Fig.7 Schematic diagram of the continuous flow three-phase reactor[49]1—oxygen reservoir; 2—liquid reservoir; 3—pressure reducing valve; 4—mass flow controller; 5—HPLC pump; 6—gas-liquid mixer; 7—segmented flow; 8—fixed bed reactor; 9—back-pressure regular; 10—gas-liquid separator; 11—liquid-flow passing through transmission IR cell
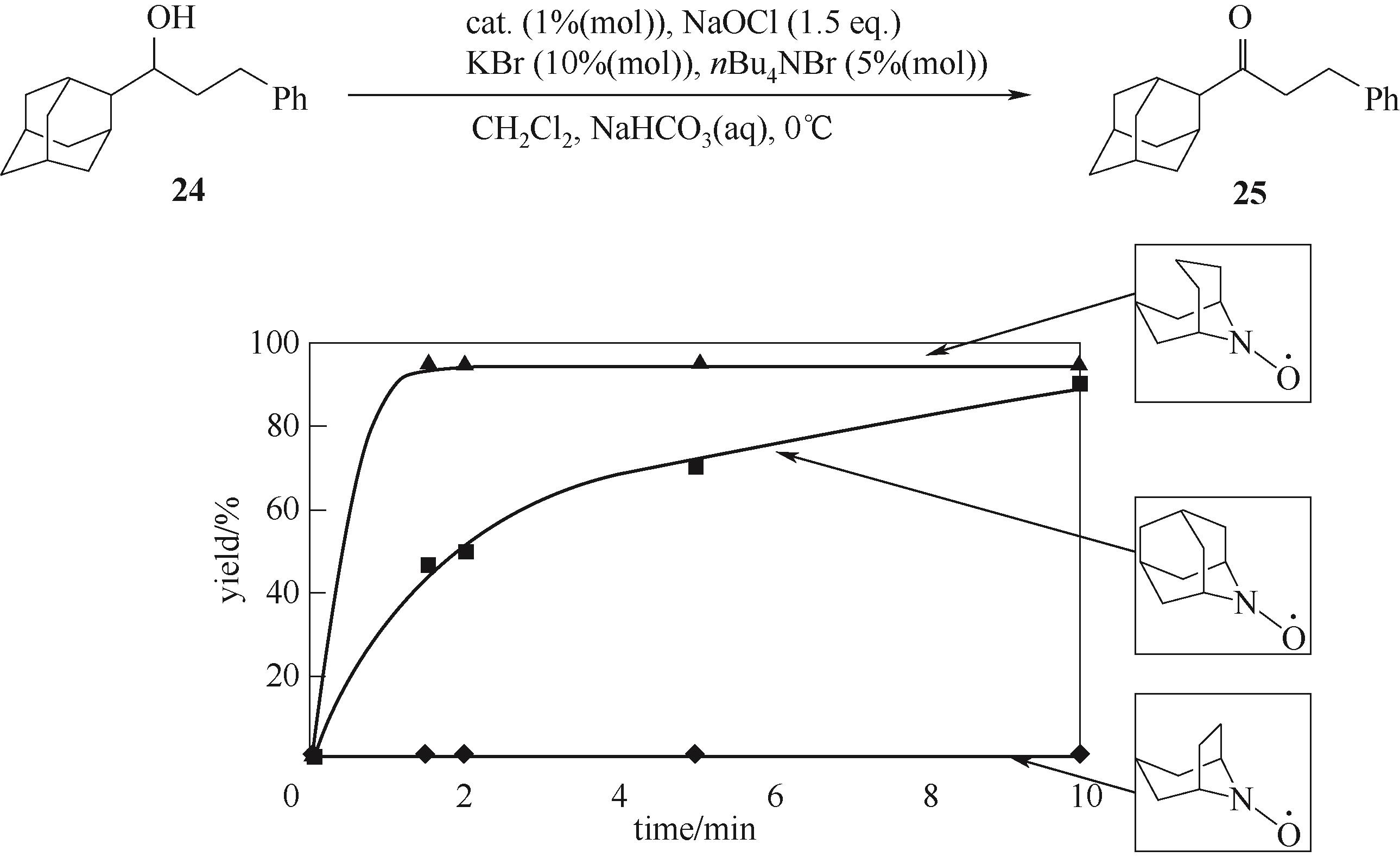
图9 Anelli条件下氮氧自由基催化剂的空间位阻效应对醇氧化的影响[57]
Fig.9 Effect of steric hindrance of nitroxide radical catalyst on alcohol oxidation using the Anelli conditions[57]
| 1 | González-Núñez M E, Mello R, Olmos A, et al. Oxidation of alcohols to carbonyl compounds with CrO3·SiO2 in supercritical carbon dioxide[J]. The Journal of Organic Chemistry, 2006, 71(3): 1039-1042. |
| 2 | Taylor R J K, Reid M, Foot J, et al. Tandem oxidation processes using manganese dioxide: discovery, applications, and current studies[J]. Accounts of Chemical Research, 2005, 38(11): 851-869. |
| 3 | Taber D F, Amedio J C, Jung K Y. Phosphorus pentoxide/dimethyl sulfoxide/triethylamine(PDT): a convenient procedure for oxidation of alcohols to ketones and aldehydes[J]. The Journal of Organic Chemistry, 1987, 52(25): 5621-5622. |
| 4 | Bryan M C, Dunn P J, Entwistle D, et al. Key green chemistry research areas from a pharmaceutical manufacturers' perspective revisited[J]. Green Chemistry, 2018, 20(22): 5082-5103. |
| 5 | Cavani F, Teles J H. Sustainability in catalytic oxidation: an alternative approach or a structural evolution? [J]. ChemSusChem, 2009, 2(6): 508-534. |
| 6 | Galvanin F, Sankar M, Cattaneo S, et al. On the development of kinetic models for solvent-free benzyl alcohol oxidation over a gold-palladium catalyst[J]. Chemical Engineering Journal, 2018, 342: 196-210. |
| 7 | Constantinou A, Wu G W, Venezia B, et al. Aerobic oxidation of benzyl alcohol in a continuous catalytic membrane reactor[J]. Topics in Catalysis, 2019, 62(17/18/19/20): 1126-1131. |
| 8 | Battino R, Rettich T R, Tominaga T. The solubility of oxygen and ozone in liquids[J]. Journal of Physical and Chemical Reference Data, 1983, 12(2): 163-178. |
| 9 | Hone C A, Kappe C O. The use of molecular oxygen for liquid phase aerobic oxidations in continuous flow[J]. Topics in Current Chemistry (Cham), 2018, 377(1): 2. |
| 10 | Dijksman A, Marino-González A, Payeras A M I, et al. Efficient and selective aerobic oxidation of alcohols into aldehydes and ketones using ruthenium/TEMPO as the catalytic system[J]. Journal of the American Chemical Society, 2001, 123(28): 6826-6833. |
| 11 | Gemoets H P L, Su Y H, Shang M J, et al. Liquid phase oxidation chemistry in continuous-flow microreactors[J]. Chemical Society Reviews, 2016, 45(1): 83-117. |
| 12 | Pasha M, Liu S E, Zhang J, et al. Recent advancements on hydrodynamics and mass transfer characteristics for CO2 absorption in microreactors[J]. Industrial & Engineering Chemistry Research, 2022, 61(34): 12249-12268. |
| 13 | Tanimu A, Jaenicke S, Alhooshani K. Heterogeneous catalysis in continuous flow microreactors: a review of methods and applications[J]. Chemical Engineering Journal, 2017, 327: 792-821. |
| 14 | Yang C X, Teixeira A R, Shi Y X, et al. Catalytic hydrogenation of N-4-nitrophenyl nicotinamide in a micro-packed bed reactor[J]. Green Chemistry, 2018, 20(4): 886-893. |
| 15 | Mohamed D K B, Yu X J, Li J S, et al. Reaction screening in continuous flow reactors[J]. Tetrahedron Letters, 2016, 57(36): 3965-3977. |
| 16 | Leclerc A, Alamé M, Schweich D, et al. Gas-liquid selective oxidations with oxygen under explosive conditions in a micro-structured reactor[J]. Lab on a Chip, 2008, 8(5): 814-817. |
| 17 | Zhang J S, Teixeira A R, Kögl L T, et al. Hydrodynamics of gas-liquid flow in micropacked beds: pressure drop, liquid holdup, and two-phase model[J]. AIChE Journal, 2017, 63(10): 4694-4704. |
| 18 | Sang L, Feng X D, Tu J C, et al. Investigation of external mass transfer in micropacked bed reactors[J]. Chemical Engineering Journal, 2020, 393: 124793. |
| 19 | Elvira K S, Solvas X C i, Wootton R C R, et al. The past, present and potential for microfluidic reactor technology in chemical synthesis[J]. Nature Chemistry, 2013, 5(11): 905-915. |
| 20 | Brooks M R, Crowl D A. Flammability envelopes for methanol, ethanol, acetonitrile and toluene[J]. Journal of Loss Prevention in the Process Industries, 2007, 20(2): 144-150. |
| 21 | Veser G. Experimental and theoretical investigation of H2 oxidation in a high-temperature catalytic microreactor[J]. Chemical Engineering Science, 2001, 56(4): 1265-1273. |
| 22 | Aellig C, Scholz D, Hermans I. Metal-free aerobic alcohol oxidation: intensification under three-phase flow conditions[J]. ChemSusChem, 2012, 5(9): 1732-1736. |
| 23 | Bennett J A, Campbell Z S, Abolhasani M. Role of continuous flow processes in green manufacturing of pharmaceuticals and specialty chemicals[J]. Current Opinion in Chemical Engineering, 2019, 26: 9-19. |
| 24 | Hartman R L. Flow chemistry remains an opportunity for chemists and chemical engineers[J]. Current Opinion in Chemical Engineering, 2020, 29: 42-50. |
| 25 | Akwi F M, Watts P. Continuous flow chemistry: where are we now? Recent applications, challenges and limitations[J]. Chemical Communications, 2018, 54(99): 13894-13928. |
| 26 | Yue J, Luo L G, Gonthier Y, et al. An experimental study of air-water Taylor flow and mass transfer inside square microchannels[J]. Chemical Engineering Science, 2009, 64(16): 3697-3708. |
| 27 | Yue J, Chen G W, Yuan Q, et al. Hydrodynamics and mass transfer characteristics in gas-liquid flow through a rectangular microchannel[J]. Chemical Engineering Science, 2007, 62(7): 2096-2108. |
| 28 | Zhang J S, Teixeira A R, Jensen K F. Automated measurements of gas-liquid mass transfer in micropacked bed reactors[J]. AIChE Journal, 2018, 64(2): 564-570. |
| 29 | Zhang C H, Duan X N, Yin J B, et al. Copper/TEMPO-catalyzed continuous aerobic alcohol oxidation in a micro-packed bed reactor[J]. Reaction Chemistry & Engineering, 2022, 7(6): 1289-1296. |
| 30 | Yue J. Multiphase flow processing in microreactors combined with heterogeneous catalysis for efficient and sustainable chemical synthesis[J]. Catalysis Today, 2018, 308: 3-19. |
| 31 | Wang N W, Matsumoto T, Ueno M, et al. A gold-immobilized microchannel flow reactor for oxidation of alcohols with molecular oxygen[J]. Angewandte Chemie International Edition, 2009, 48(26): 4744-4746. |
| 32 | Hernandez-Alvarado F, Kalaga D V, Turney D, et al. Void fraction, bubble size and interfacial area measurements in co-current downflow bubble column reactor with microbubble dispersion[J]. Chemical Engineering Science, 2017, 168: 403-413. |
| 33 | Mase N, Mizumori T, Tatemoto Y. Aerobic copper/TEMPO-catalyzed oxidation of primary alcohols to aldehydes using a microbubble strategy to increase gas concentration in liquid phase reactions[J]. Chemical Communications, 2011, 47(7): 2086. |
| 34 | Osterberg P M, Niemeier J K, Welch C J, et al. Experimental limiting oxygen concentrations for nine organic solvents at temperatures and pressures relevant to aerobic oxidations in the pharmaceutical industry[J]. Organic Process Research & Development, 2015, 19(11): 1537-1543. |
| 35 | O'Brien M, Taylor N, Polyzos A, et al. Hydrogenation in flow: homogeneous and heterogeneous catalysis using Teflon AF-2400 to effect gas-liquid contact at elevated pressure[J]. Chemical Science, 2011, 2(7): 1250. |
| 36 | Wu G W, Constantinou A, Cao E H, et al. Continuous heterogeneously catalyzed oxidation of benzyl alcohol using a tube-in-tube membrane microreactor[J]. Industrial & Engineering Chemistry Research, 2015, 54(16): 4183-4189. |
| 37 | Greene J F, Preger Y, Stahl S S, et al. PTFE-membrane flow reactor for aerobic oxidation reactions and its application to alcohol oxidation[J]. Organic Process Research & Development, 2015, 19(7): 858-864. |
| 38 | Mo Y M, Imbrogno J, Zhang H M, et al. Scalable thin-layer membrane reactor for heterogeneous and homogeneous catalytic gas-liquid reactions[J]. Green Chemistry, 2018, 20(16): 3867-3874. |
| 39 | Wu G W, Cao E H, Ellis P, et al. Development of a flat membrane microchannel packed-bed reactor for scalable aerobic oxidation of benzyl alcohol in flow[J]. Chemical Engineering Journal, 2019, 377: 120086. |
| 40 | Beejapur H A, Zhang Q, Hu K C, et al. TEMPO in chemical transformations: from homogeneous to heterogeneous[J]. ACS Catalysis, 2019, 9(4): 2777-2830. |
| 41 | 黄斌, 张宁, 洪三国, 等. 氮氧自由基催化有机物的分子氧氧化研究进展[J]. 分子催化, 2009, 23(4): 377-385. |
| Huang B, Zhang N, Hong S G, et al. Advances in molecular oxygen oxidation of organic compounds catalyzed by nitroxide free radicals[J]. Journal of Molecular Catalysis, 2009, 23(4): 377-385. | |
| 42 | Semmelhack M F, Schmid C R, Cortes D A, et al. Oxidation of alcohols to aldehydes with oxygen and cupric ion, mediated by nitrosonium ion[J]. Journal of the American Chemical Society, 1984, 106(11): 3374-3376. |
| 43 | Hoover J M, Ryland B L, Stahl S S. Mechanism of copper(Ⅰ)/TEMPO-catalyzed aerobic alcohol oxidation[J]. Journal of the American Chemical Society, 2013, 135(6): 2357-2367. |
| 44 | Hoover J M, Stahl S S. Highly practical copper ( Ⅰ ) /TEMPO catalyst system for chemoselective aerobic oxidation of primary alcohols[J]. Journal of the American Chemical Society, 2011, 133(42): 16901-16910. |
| 45 | Kim J, Stahl S S. Cu/nitroxyl catalyzed aerobic oxidation of primary amines into nitriles at room temperature[J]. ACS Catalysis, 2013, 3(7): 1652-1656. |
| 46 | Obermayer D, Balu A M, Romero A A, et al. Nanocatalysis in continuous flow: supported iron oxide nanoparticles for the heterogeneous aerobic oxidation of benzyl alcohol[J]. Green Chemistry, 2013, 15(6): 1530. |
| 47 | 阮万民, 王建黎. 负载型TEMPO催化剂研究进展[J]. 工业催化, 2015, 23(12): 961-965. |
| Ruan W M, Wang J L. Progress in supported TEMPO catalysts[J]. Industrial Catalysis, 2015, 23(12): 961-965. | |
| 48 | Megiel E. Surface modification using TEMPO and its derivatives[J]. Advances in Colloid and Interface Science, 2017, 250: 158-184. |
| 49 | Aellig C, Scholz D, Conrad S, et al. Intensification of TEMPO-mediated aerobic alcohol oxidations under three-phase flow conditions[J]. Green Chemistry, 2013, 15(7): 1975. |
| 50 | Vernet G, Salehi M S, Lopatka P, et al. Cu-catalyzed aerobic oxidation of diphenyl sulfide to diphenyl sulfoxide within a segmented flow regime: modeling of a consecutive reaction network and reactor characterization[J]. Chemical Engineering Journal, 2021, 416: 129045. |
| 51 | Kashiwagi Y, Chiba S, Anzai J I. Oxidation of alcohols with nitroxyl radical under polymer-supported two-phase conditions[J]. New Journal of Chemistry, 2003, 27(11): 1545. |
| 52 | Hu Z Z, Kerton F M. Simple copper/TEMPO catalyzed aerobic dehydrogenation of benzylic amines and anilines[J]. Organic & Biomolecular Chemistry, 2012, 10(8): 1618-1624. |
| 53 | Tian H W, Yu X C, Li Q, et al. General, green, and scalable synthesis of imines from alcohols and amines by a mild and efficient copper-catalyzed aerobic oxidative reaction in open air at room temperature[J]. Advanced Synthesis & Catalysis, 2012, 354(14/15): 2671-2677. |
| 54 | Lauber M B, Stahl S S. Efficient aerobic oxidation of secondary alcohols at ambient temperature with an ABNO/NO x catalyst system[J]. ACS Catalysis, 2013, 3(11): 2612-2616. |
| 55 | Shibuya M, Tomizawa M, Suzuki I, et al. 2-Azaadamantane N-oxyl (AZADO) and 1-me-AZADO: highly efficient organocatalysts for oxidation of alcohols[J]. ChemInform, 2006, 37(45): no. |
| 56 | Anelli P L, Banfi S, Montanari F, et al. Oxidation of diols with alkali hypochlorites catalyzed by oxammonium salts under two-phase conditions[J]. The Journal of Organic Chemistry, 1989, 54(12): 2970-2972. |
| 57 | Shibuya M, Tomizawa M, Sasano Y, et al. An expeditious entry to 9-azabicyclo[3.3.1]nonane N-oxyl (ABNO): another highly active organocatalyst for oxidation of alcohols[J]. The Journal of Organic Chemistry, 2009, 74(12): 4619-4622. |
| 58 | Steves J E, Preger Y, Martinelli J R, et al. Process development of CuⅠ/ABNO/NMI-catalyzed aerobic alcohol oxidation[J]. Organic Process Research & Development, 2015, 19(11): 1548-1553. |
| 59 | Zultanski S L, Zhao J Y, Stahl S S. Practical synthesis of amides via copper/ABNO-catalyzed aerobic oxidative coupling of alcohols and amines[J]. Journal of the American Chemical Society, 2016, 138(20): 6416-6419. |
| 60 | Sonobe T, Oisaki K, Kanai M. Catalytic aerobic production of imines en route to mild, green, and concise derivatizations of amines[J]. Chemical Science, 2012, 3(11): 3249. |
| 61 | Miles K C, Abrams M L, Landis C R, et al. KetoABNO/NO x cocatalytic aerobic oxidation of aldehydes to carboxylic acids and access to α‑chiral carboxylic acids via sequential asymmetric hydroformylation/oxidation[J]. Organic Letters, 2016, 18(15): 3590-3593. |
| 62 | Iwabuchi Y. Discovery and exploitation of AZADO: the highly active catalyst for alcohol oxidation[J]. Chemical & Pharmaceutical Bulletin, 2013, 61(12): 1197-1213. |
| 63 | Hayashi M, Sasano Y, Nagasawa S, et al. 9-Azanoradamantane N-oxyl (Nor-AZADO): a highly active organocatalyst for alcohol oxidation[J]. Chemical & Pharmaceutical Bulletin, 2011, 59(12): 1570-1573. |
| 64 | Nakai S, Yatabe T, Suzuki K, et al. Methyl-selective α-oxygenation of tertiary amines to formamides by employing copper/moderately hindered nitroxyl radical (DMN-AZADO or 1-me-AZADO)[J]. Angewandte Chemie International Edition, 2019, 58(46): 16651-16659. |
| 65 | Recupero F, Punta C. Free radical functionalization of organic compounds catalyzed by N-hydroxyphthalimide[J]. ChemInform, 2007, 38(51): 3800-3842. |
| 66 | Ishii Y, Sakaguchi S. Recent progress in aerobic oxidation of hydrocarbons by N-hydroxyimides[J]. Catalysis Today, 2006, 117(1/2/3): 105-113. |
| 67 | Jafarpour M, Rezaeifard A, Yasinzadeh V, et al. Starch-coated maghemite nanoparticles functionalized by a novel cobalt Schiff base complex catalyzes selective aerobic benzylic C—H oxidation[J]. RSC Advances, 2015, 5(48): 38460-38469. |
| 68 | Serra A C, Rocha Gonsalves A M D. Mild oxygen activation with isobutyraldehyde promoted by simple salts[J]. Tetrahedron Letters, 2011, 52(27): 3489-3491. |
| 69 | Nagy B S, Kappe C O, Ötvös S B. N-hydroxyphthalimide catalyzed aerobic oxidation of aldehydes under continuous flow conditions[J]. Advanced Synthesis and Catalysis, 2022, 364(12): 1998-2008. |
| 70 | Minisci F, Punta C, Recupero F, et al. Aerobic oxidation of N-alkylamides catalyzed by N-hydroxyphthalimide under mild conditions. Polar and enthalpic effects[J]. The Journal of Organic Chemistry, 2002, 67(8): 2671-2676. |
| 71 | Cecchetto A, Minisci F, Recupero F, et al. A new selective free radical synthesis of aromatic aldehydes by aerobic oxidation of tertiary benzylamines catalysed by N-hydroxyimides and C o ( Ⅱ ) under mild conditions. Polar and enthalpic effects[J]. Tetrahedron Letters, 2002, 43(19): 3605-3607. |
| 72 | Fukuda O, Sakaguchi S, Ishii Y. A new strategy for catalytic Baeyer-Villiger oxidation of KA-oil with molecular oxygen using N-hydroxyphthalimide[J]. Tetrahedron Letters, 2001, 42(20): 3479-3481. |
| 73 | Dai P F, Qu J P, Kang Y B. Organocatalyzed aerobic oxidation of aldehydes to acids[J]. Organic Letters, 2019, 21(5): 1393-1396. |
| 74 | Yun L, Zhao J N, Tang X F, et al. Selective oxidation of benzylic sp3 C—H bonds using molecular oxygen in a continuous-flow microreactor[J]. Organic Process Research & Development, 2021, 25(7): 1612-1618. |
| 75 | Zhu Y G, Wang Q, Cornwall R G, et al. Organocatalytic asymmetric epoxidation and aziridination of olefins and their synthetic applications[J]. Chemical Reviews, 2014, 114(16): 8199-8256. |
| 76 | Minisci F, Gambarotti C, Pierini M, et al. Molecule-induced homolysis of N-hydroxyphthalimide(NHPI) by peracids and dioxirane. A new, simple, selective aerobic radical epoxidation of alkenes[J]. Tetrahedron Letters, 2006, 47(9): 1421-1424. |
| 77 | Spaccini R, Liguori L, Punta C, et al. Organocatalyzed epoxidation of alkenes in continuous flow using a multi-jet oscillating disk reactor[J]. ChemSusChem, 2012, 5(2): 261-265. |
| 78 | Sawatari N, Yokota T, Sakaguchi S, et al. Alkane oxidation with air catalyzed by lipophilic N-hydroxyphthalimides without any solvent[J]. The Journal of Organic Chemistry, 2001, 66(23): 7889-7891. |
| 79 | Ueda C, Noyama M, Ohmori H, et al. Reactivity of phthalimide-N-oxyl: a kinetic study[J]. Chemical and Pharmaceutical Bulletin, 1987, 35(4): 1372-1377. |
| 80 | Jarrahpour A, Fadavi A, Zarei M. Synthesis of structurally diverse 2-azetidinones via staudinger reaction on a solid support[J]. Bulletin of the Chemical Society of Japan, 2011, 84(3): 320-327. |
| 81 | Shi G J, Xu S H, Bao Y, et al. Selective aerobic oxidation of toluene to benzaldehyde on immobilized CoO x on SiO2 catalyst in the presence of N-hydroxyphthalimide and hexafluoropropan-2-ol[J]. Catalysis Communications, 2019, 123: 73-78. |
| 82 | Kasperczyk K, Orlinska B, Witek E, et al. Polymer-supported N-hydroxyphthalimide as catalyst for toluene and p-methoxytoluene aerobic oxidation[J]. Catalysis Letters, 2015, 145(10): 1856-1867. |
| 83 | Melone L, Prosperini S, Gambarotti C, et al. Selective catalytic aerobic oxidation of substituted ethylbenzenes under mild conditions[J]. Journal of Molecular Catalysis A: Chemical, 2012, 355: 155-160. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [6] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [7] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [8] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [9] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [10] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [11] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [12] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [13] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [14] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [15] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号