化工学报 ›› 2019, Vol. 70 ›› Issue (S2): 85-93.DOI: 10.11949/0438-1157.20190431
收稿日期:2019-04-26
修回日期:2019-07-10
出版日期:2019-09-06
发布日期:2019-09-06
通讯作者:
花儿
作者简介:郑勋(1995—),男,硕士研究生,基金资助:
Xun ZHENG1( ),Yu XU1,Er HUA1,2(
),Yu XU1,Er HUA1,2( )
)
Received:2019-04-26
Revised:2019-07-10
Online:2019-09-06
Published:2019-09-06
Contact:
Er HUA
摘要:
通过前期实验研究,己基乙二胺-三氟甲磺酸([HHex][TFS])型质子化离子液体(protic ionic liquid,PIL)的极性部位具有两个氨基,亲水性较强,能够与水混溶90%(质量)(H2O/ PIL)以上。因此利用密度泛函理论(density functional theory,DFT),在M06-2X/6-311G(d,p)的水平下,对 [HHex][TFS]与H2O分子间形成的氢键作用进行了研究。设计了[HHex][TFS]分别与nH2O (n = 1, 2, 6) 相结合的构型,并得到了较稳定构型共8种(S1~S8),计算了其分子间的相互作用能(ΔE 0 BSSE)、分子振动频率(Δν)、二阶微扰能、电子密度(ρ c)以及Laplace值(?2 ρ c)。分析结果显示,[HHex][TFS]与水分子间形成了较强的氢键,[HHex][TFS]与H2O结合数量增加,构型中氢键相互作用增强,即S4(n=1)<S6(n=2)<S8(n=6)。
中图分类号:
郑勋,徐宇,花儿. 己基乙二胺-TFS型质子化离子液体与水分子间氢键相互作用的研究[J]. 化工学报, 2019, 70(S2): 85-93.
Xun ZHENG,Yu XU,Er HUA. Water and hydrogen bonding study on protic ionic liquids composed of hexylethylenediamine cation and trifluoromethanesulfonate anion[J]. CIESC Journal, 2019, 70(S2): 85-93.
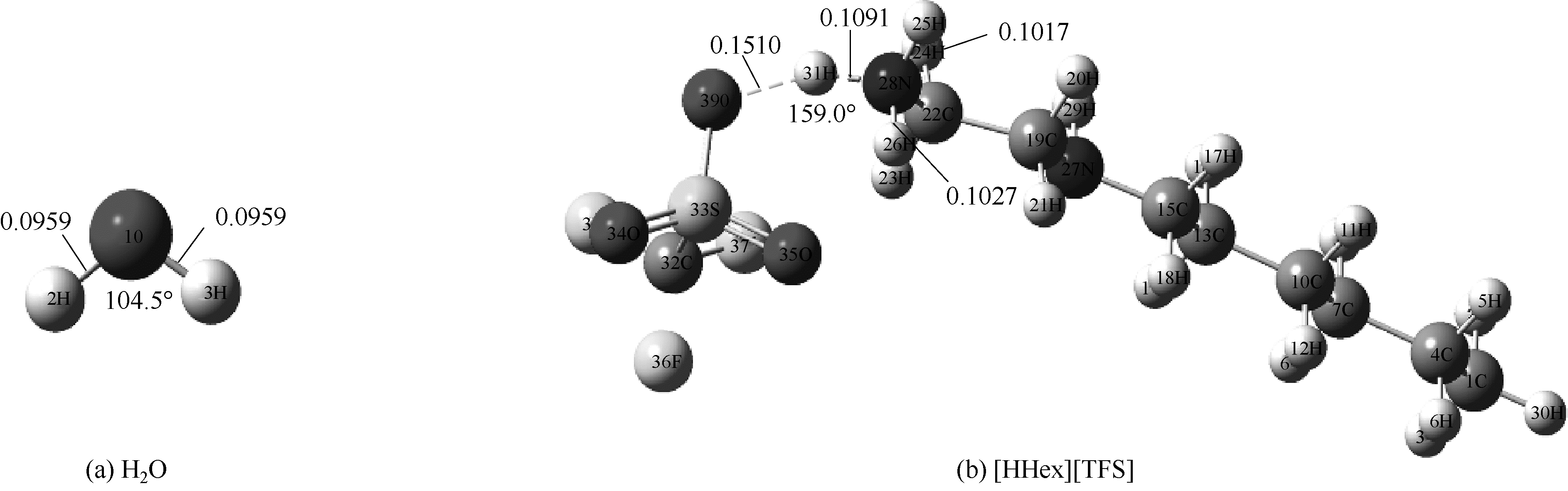
图1 在M06-2X/6-311G(d,p)水平下优化得到[HHex][TFS]分子和H2O分子构型(图中显示了主要氢键部位的键长键角)
Fig.1 Optimized structures of [HHex][TFS] and H2O at M06-2X/6-311G(d,p) level(main hydrogen bond is shown and it’s bond length and angles are in nanometer and degree, respectively)
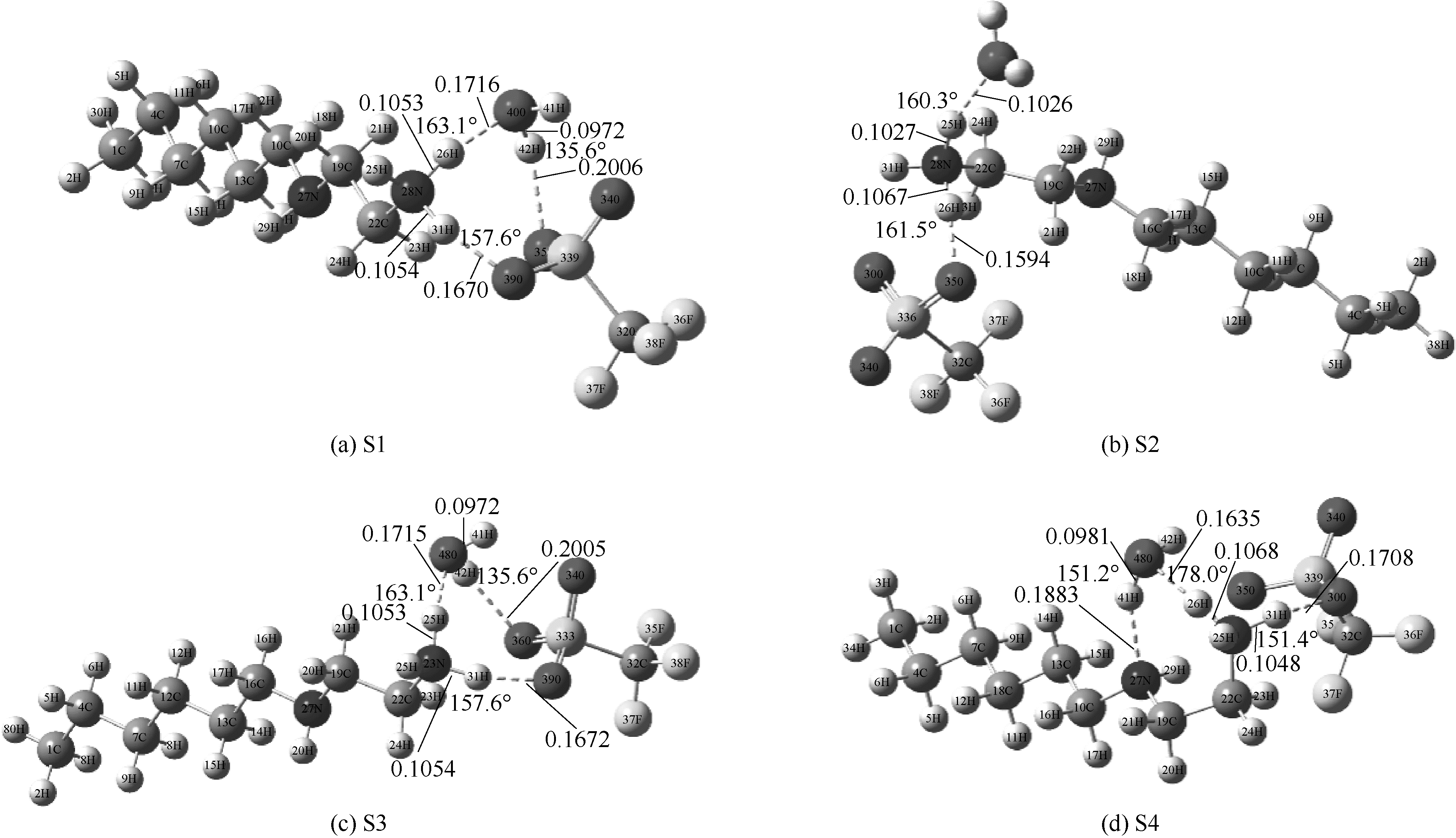
图2 在M06-2X/6-311G(d,p)水平下优化得到[HHex][TFS]-H2O分子构型(图中显示了主要氢键部位的键长键角)
Fig.2 Optimized structures of [HHex][TFS]-H2O at M06-2X/6-311G(d,p) level(main hydrogen bond is shown and it’s bond length and angles are in nanometer and degree, respectively)

图3 在M06-2X/6-311G(d,p)水平下优化得到[HHex][TFS]-2H2O分子构型(图中显示了主要氢键部位的键长键角)
Fig.3 Optimized structures of [HHex][TFS]-2H2O at M06-2X/6-311G(d,p) level(main hydrogen bond is shown and it’s bond length and angles are in nanometer and degree, respectively)
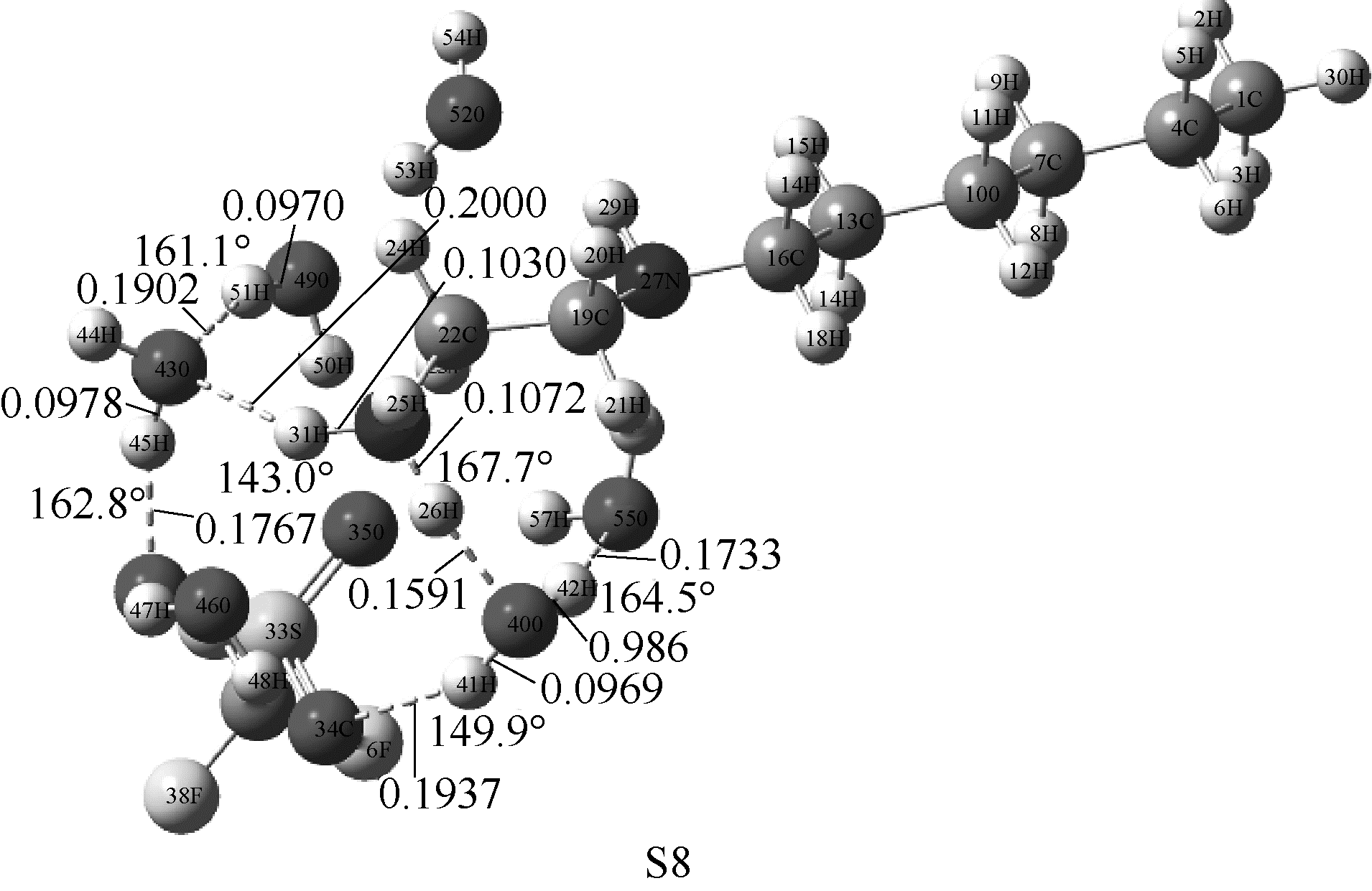
图4 在M06-2X/6-311G(d,p)水平下优化得到[HHex][TFS]-6H2O分子构型(图中显示了主要氢键部位的键长键角)
Fig.4 Optimized structures of [HHex][TFS]-6H2O at M06-2X/6-311G(d,p) level(main hydrogen bond is shown and it’s bond length and angles are in nanometer and degree, respectively)
| Structures | ΔE 0/(kcal·mol-1) | ΔE 0 BSSE/(kcal·mol-1) |
|---|---|---|
| [HHex][TFS]-H2O-S1 | -16.92 | -11.27 |
| [HHex][TFS]-H2O-S2 | -11.70 | -9.38 |
| [HHex][TFS]-H2O-S3 | -16.91 | -12.19 |
| [HHex][TFS]-H2O-S4 | -23.02 | -17.31 |
| [HHex][TFS]-2H2O-S5 | -22.14 | -14.82 |
| [HHex][TFS]-2H2O-S6 | -38.77 | -31.06 |
| [HHex][TFS]-2H2O-S7 | -27.32 | -19.45 |
| [HHex][TFS]-6H2O-S8 | -91.81 | -71.71 |
表1 在M06-2X/6-311G(d, p)水平下优化得到[HHex][TFS]-nH2O(n=1, 2, 6)的相互作用能
Table 1 Interaction energies of [HHex][TFS]-nH2O(n=1, 2, 6)at M06-2X/6-311G(d, p) level
| Structures | ΔE 0/(kcal·mol-1) | ΔE 0 BSSE/(kcal·mol-1) |
|---|---|---|
| [HHex][TFS]-H2O-S1 | -16.92 | -11.27 |
| [HHex][TFS]-H2O-S2 | -11.70 | -9.38 |
| [HHex][TFS]-H2O-S3 | -16.91 | -12.19 |
| [HHex][TFS]-H2O-S4 | -23.02 | -17.31 |
| [HHex][TFS]-2H2O-S5 | -22.14 | -14.82 |
| [HHex][TFS]-2H2O-S6 | -38.77 | -31.06 |
| [HHex][TFS]-2H2O-S7 | -27.32 | -19.45 |
| [HHex][TFS]-6H2O-S8 | -91.81 | -71.71 |
| Structures | N—H bond | ν/cm-1 | Δν/cm-1 | r/nm | Δr/nm |
|---|---|---|---|---|---|
| H2O | 1O—2H | 3893.61 | 0.0959 | ||
| 1O—3H | 3893.61 | 0.0959 | |||
| [HHex][TFS] | 28N—31H | 2389.55 | 0.1091 | ||
| 28N—26H | 3406.22 | 0.1027 | |||
| 28N—25H | 3560.49 | 0.1017 | |||
| 27N—29H | 3536.44 | 0.1016 | |||
| [HHex][TFS]-H2O-S1 | 28N—31H | 2872.39 | -482.84 | 0.1054 | -0.0036 |
| 28N—26H | 2938.01 | +468.21 | 0.1053 | 0.0026 | |
| [HHex][TFS]-H2O-S2 | 28N—31H | 3498.83 | -1109.28 | 0.1022 | -0.0069 |
| 28N—25H | 3379.68 | +180.81 | 0.1027 | 0.0010 | |
| [HHex][TFS]-H2O-S3 | 28N—31H | 2873.71 | -484.16 | 0.1054 | -0.0037 |
| 28N—26H | 2937.13 | +469.09 | 0.1053 | 0.0026 | |
| [HHex][TFS]-H2O-S4 | 28N—31H | 3039.79 | -650.24 | 0.1048 | -0.0043 |
| 28N—26H | 2654.10 | +906.39 | 0.1068 | 0.0051 | |
| [HHex][TFS]-2H2O-S5 | 28N—31H | 3500.96 | -1111.41 | 0.1022 | -0.0069 |
| 28N—25H | 3380.25 | +180.24 | 0.1027 | 0.0010 | |
| 43O—45H | 3691.90 | +201.71 | 0.0971 | 0.0012 | |
| [HHex][TFS]-2H2O-S6 | 28N—31H | 3178.23 | -788.68 | 0.1040 | -0.0051 |
| 28N—26H | 2278.36 | +1127.86 | 0.1094 | 0.0067 | |
| 40O—41H | 3520.43 | +373.18 | 0.0980 | 0.0021 | |
| 40O—42H | 3554.48 | +339.13 | 0.0980 | 0.0021 | |
| 43O—44H | 3820.36 | +73.25 | 0.0965 | 0.0006 | |
| 43O—45H | 3820.36 | +73.25 | 0.0967 | 0.0008 | |
| [HHex][TFS]-2H2O-S7 | 28N—31H | 3101.66 | -712.11 | 0.1044 | -0.0046 |
| 28N—25H | 3389.28 | +171.21 | 0.1027 | 0.0010 | |
| 40O—42H | 3748.55 | +145.06 | 0.0971 | 0.0012 | |
| [HHex][TFS]-6H2O-S8 | 28N—31H | 3370.45 | -980.90 | 0.1030 | -0.0061 |
| 28N—26H | 2600.30 | +805.92 | 0.1072 | 0.0044 | |
| 40O—42H | 3358.27 | +535.34 | 0.0986 | 0.0027 | |
| 43O—45H | 3569.23 | +324.38 | 0.0978 | 0.0019 | |
| 49O—51H | 3773.38 | +120.23 | 0.0970 | 0.0011 | |
| 52O—53H | 3546.59 | +347.02 | 0.0978 | 0.0019 | |
| 55O—56H | 3358.27 | +535.34 | 0.0983 | 0.0024 |
表2 H2O、[HHex][TFS]和[HHex][TFS]-n(H2O)构型的键长r (nm)和振动频率ν(cm-1)
Table 2 Bond lengths (r, nm) and N-H/O-H vibrational frequencies (cm-1) for H2O, [HHex][TFS] and [HHex][TFS]-n(H2O) structures
| Structures | N—H bond | ν/cm-1 | Δν/cm-1 | r/nm | Δr/nm |
|---|---|---|---|---|---|
| H2O | 1O—2H | 3893.61 | 0.0959 | ||
| 1O—3H | 3893.61 | 0.0959 | |||
| [HHex][TFS] | 28N—31H | 2389.55 | 0.1091 | ||
| 28N—26H | 3406.22 | 0.1027 | |||
| 28N—25H | 3560.49 | 0.1017 | |||
| 27N—29H | 3536.44 | 0.1016 | |||
| [HHex][TFS]-H2O-S1 | 28N—31H | 2872.39 | -482.84 | 0.1054 | -0.0036 |
| 28N—26H | 2938.01 | +468.21 | 0.1053 | 0.0026 | |
| [HHex][TFS]-H2O-S2 | 28N—31H | 3498.83 | -1109.28 | 0.1022 | -0.0069 |
| 28N—25H | 3379.68 | +180.81 | 0.1027 | 0.0010 | |
| [HHex][TFS]-H2O-S3 | 28N—31H | 2873.71 | -484.16 | 0.1054 | -0.0037 |
| 28N—26H | 2937.13 | +469.09 | 0.1053 | 0.0026 | |
| [HHex][TFS]-H2O-S4 | 28N—31H | 3039.79 | -650.24 | 0.1048 | -0.0043 |
| 28N—26H | 2654.10 | +906.39 | 0.1068 | 0.0051 | |
| [HHex][TFS]-2H2O-S5 | 28N—31H | 3500.96 | -1111.41 | 0.1022 | -0.0069 |
| 28N—25H | 3380.25 | +180.24 | 0.1027 | 0.0010 | |
| 43O—45H | 3691.90 | +201.71 | 0.0971 | 0.0012 | |
| [HHex][TFS]-2H2O-S6 | 28N—31H | 3178.23 | -788.68 | 0.1040 | -0.0051 |
| 28N—26H | 2278.36 | +1127.86 | 0.1094 | 0.0067 | |
| 40O—41H | 3520.43 | +373.18 | 0.0980 | 0.0021 | |
| 40O—42H | 3554.48 | +339.13 | 0.0980 | 0.0021 | |
| 43O—44H | 3820.36 | +73.25 | 0.0965 | 0.0006 | |
| 43O—45H | 3820.36 | +73.25 | 0.0967 | 0.0008 | |
| [HHex][TFS]-2H2O-S7 | 28N—31H | 3101.66 | -712.11 | 0.1044 | -0.0046 |
| 28N—25H | 3389.28 | +171.21 | 0.1027 | 0.0010 | |
| 40O—42H | 3748.55 | +145.06 | 0.0971 | 0.0012 | |
| [HHex][TFS]-6H2O-S8 | 28N—31H | 3370.45 | -980.90 | 0.1030 | -0.0061 |
| 28N—26H | 2600.30 | +805.92 | 0.1072 | 0.0044 | |
| 40O—42H | 3358.27 | +535.34 | 0.0986 | 0.0027 | |
| 43O—45H | 3569.23 | +324.38 | 0.0978 | 0.0019 | |
| 49O—51H | 3773.38 | +120.23 | 0.0970 | 0.0011 | |
| 52O—53H | 3546.59 | +347.02 | 0.0978 | 0.0019 | |
| 55O—56H | 3358.27 | +535.34 | 0.0983 | 0.0024 |
| Atom | H2O | [HHex][TFS] | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|---|---|
| N27 | -0.676 | -0.676 | -0.684 | -0.676 | -0.736 | -0.709 | -0.737 | -0.677 | -0.738 | |
| N28 | -0.744 | -0.757 | -0.754 | -0.757 | -0.762 | -0.749 | -0.767 | -0.772 | -0.751 | |
| H29 | 0.344 | 0.342 | 0.342 | 0.342 | 0.394 | 0.355 | 0.398 | 0.340 | 0.412 | |
| H30 | 0.200 | 0.200 | 0.199 | 0.200 | 0.200 | 0.199 | 0.199 | 0.199 | 0.199 | |
| H31 | 0.482 | 0.476 | 0.439 | 0.476 | 0.477 | 0.441 | 0.471 | 0.469 | 0.475 | |
| O34 | -0.919 | -0.981 | -0.928 | -0.981 | -0.927 | -0.926 | -0.966 | -0.984 | -0.999 | |
| O35 | -1.026 | -1.023 | -1.035 | -1.023 | -1.060 | -1.037 | -1.057 | -1.025 | -1.048 | |
| O39 | -1.023 | -1.021 | -1.021 | -1.021 | -1.031 | -1.019 | -1.022 | -1.022 | -1.023 | |
| O40 | -0.899 | -0.962 | -0.934 | -0.962 | -0.958 | -0.935 | -0.981 | -0.965 | -0.988 | |
| H41 | 0.449 | 0.500 | 0.478 | 0.500 | 0.501 | 0.478 | 0.503 | 0.496 | 0.504 | |
| H42 | 0.449 | 0.503 | 0.478 | 0.503 | 0.497 | 0.479 | 0.517 | 0.500 | 0.514 | |
| O43 | -0.947 | -0.953 | -0.932 | -0.982 | ||||||
| H44 | 0.451 | 0.490 | 0.476 | 0.476 | ||||||
| H45 | 0.481 | 0.487 | 0.477 | 0.513 | ||||||
| O46 | -0.954 | |||||||||
| H47 | 0.479 | |||||||||
| H48 | 0.477 | |||||||||
| O49 | -0.976 | |||||||||
| H50 | 0.495 | |||||||||
| H51 | 0.494 | |||||||||
| O52 | -0.978 | |||||||||
| H53 | 0.506 | |||||||||
| H54 | 0.460 | |||||||||
| O55 | -0.986 | |||||||||
| H56 | 0.501 | |||||||||
| H57 | 0.487 |
表3 在M06-2X/6-311G(d,p)水平下从NPA优化得到[HHex][TFS]-nH2O(n=1, 2, 6)的部分电荷
Table 3 Selected partial charges from NPA of [HHex][TFS]-nH2O(n=1, 2, 6)at M06-2X/6-311G(d, p) level
| Atom | H2O | [HHex][TFS] | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|---|---|
| N27 | -0.676 | -0.676 | -0.684 | -0.676 | -0.736 | -0.709 | -0.737 | -0.677 | -0.738 | |
| N28 | -0.744 | -0.757 | -0.754 | -0.757 | -0.762 | -0.749 | -0.767 | -0.772 | -0.751 | |
| H29 | 0.344 | 0.342 | 0.342 | 0.342 | 0.394 | 0.355 | 0.398 | 0.340 | 0.412 | |
| H30 | 0.200 | 0.200 | 0.199 | 0.200 | 0.200 | 0.199 | 0.199 | 0.199 | 0.199 | |
| H31 | 0.482 | 0.476 | 0.439 | 0.476 | 0.477 | 0.441 | 0.471 | 0.469 | 0.475 | |
| O34 | -0.919 | -0.981 | -0.928 | -0.981 | -0.927 | -0.926 | -0.966 | -0.984 | -0.999 | |
| O35 | -1.026 | -1.023 | -1.035 | -1.023 | -1.060 | -1.037 | -1.057 | -1.025 | -1.048 | |
| O39 | -1.023 | -1.021 | -1.021 | -1.021 | -1.031 | -1.019 | -1.022 | -1.022 | -1.023 | |
| O40 | -0.899 | -0.962 | -0.934 | -0.962 | -0.958 | -0.935 | -0.981 | -0.965 | -0.988 | |
| H41 | 0.449 | 0.500 | 0.478 | 0.500 | 0.501 | 0.478 | 0.503 | 0.496 | 0.504 | |
| H42 | 0.449 | 0.503 | 0.478 | 0.503 | 0.497 | 0.479 | 0.517 | 0.500 | 0.514 | |
| O43 | -0.947 | -0.953 | -0.932 | -0.982 | ||||||
| H44 | 0.451 | 0.490 | 0.476 | 0.476 | ||||||
| H45 | 0.481 | 0.487 | 0.477 | 0.513 | ||||||
| O46 | -0.954 | |||||||||
| H47 | 0.479 | |||||||||
| H48 | 0.477 | |||||||||
| O49 | -0.976 | |||||||||
| H50 | 0.495 | |||||||||
| H51 | 0.494 | |||||||||
| O52 | -0.978 | |||||||||
| H53 | 0.506 | |||||||||
| H54 | 0.460 | |||||||||
| O55 | -0.986 | |||||||||
| H56 | 0.501 | |||||||||
| H57 | 0.487 |
| Structures | Charge transfer | E (2) | E (2) sum |
|---|---|---|---|
| [HHex][TFS]-H2O-S1 | LP(O34)→BD*(O40—H41) | 1.95 | 36.78 |
| LP(O35)→BD*(O40—H42) | 5.92 | ||
| LP(O40)→BD*(H26—N28) | 28.91 | ||
| [HHex][TFS]-H2O-S2 | LP(O40)→BD*(H25—N28) | 11.31 | 11.31 |
| [HHex][TFS]-H2O-S3 | LP(O34)→BD*(O40—H41) | 1.93 | 36.84 |
| LP(O35)→BD*(O40—H42) | 5.92 | ||
| LP(O40)→BD*(H26—N28) | 28.99 | ||
| [HHex][TFS]-H2O-S4 | LP(O40)→BD*(H26—N28) | 39.21 | 54.93 |
| LP(N27)→BD*(O40—H41) | 15.72 | ||
| [HHex][TFS]-2H2O-S5 | LP(O40)→BD*(H25—N28) | 11.18 | 21.85 |
| LP(N27)→BD*(O43—H45) | 10.67 | ||
| [HHex][TFS]-2H2O-S6 | LP(O34)→BD*(O43—H44) | 1.76 | 65.62 |
| LP(O34)→BD*(O43—H45) | 4.46 | ||
| LP(O40)→BD*(H26—N28) | 59.37 | ||
| [HHex][TFS]-2H2O-S7 | LP(O34)→BD*(O40—H41) | 2.04 | 43.12 |
| LP(O35)→BD*(O40—H42) | 5.66 | ||
| LP(O40)→BD*(H26—N28) | 23.58 | ||
| LP(O43)→BD*(H25—N28) | 11.84 | ||
| [HHex][TFS]-6H2O-S8 | LP(O34)→BD*(O40—H41) | 7.65 | 105.39 |
| LP(O39)→BD*(O43—H45) | 17.45 | ||
| LP(O39)→BD*(O46—H47) | 3.26 | ||
| LP(O35)→BD*(O49—H50) | 4.70 | ||
| LP(O35)→BD*(O55—H57) | 4.02 | ||
| LP(O40)→BD*(H26—N28) | 46.39 | ||
| LP(O43)→BD*(N28—H31) | 8.33 | ||
| LP(O43)→BD*(O49—H51) | 10.2 | ||
| LP(O46)→BD*(N28—H31) | 3.39 |
表4 在M06-2X/6-311G(d,p)水平下优化得到[HHex][TFS]-nH2O的NBO中的二阶微扰能E (2) (kcal?mol-1)
Table 4 NBO of [HHex][TFS]-nH2O and their second order stabilization energies E (2) (kcal?mol-1) calculated at M06-2X/6-311G(d,p) level
| Structures | Charge transfer | E (2) | E (2) sum |
|---|---|---|---|
| [HHex][TFS]-H2O-S1 | LP(O34)→BD*(O40—H41) | 1.95 | 36.78 |
| LP(O35)→BD*(O40—H42) | 5.92 | ||
| LP(O40)→BD*(H26—N28) | 28.91 | ||
| [HHex][TFS]-H2O-S2 | LP(O40)→BD*(H25—N28) | 11.31 | 11.31 |
| [HHex][TFS]-H2O-S3 | LP(O34)→BD*(O40—H41) | 1.93 | 36.84 |
| LP(O35)→BD*(O40—H42) | 5.92 | ||
| LP(O40)→BD*(H26—N28) | 28.99 | ||
| [HHex][TFS]-H2O-S4 | LP(O40)→BD*(H26—N28) | 39.21 | 54.93 |
| LP(N27)→BD*(O40—H41) | 15.72 | ||
| [HHex][TFS]-2H2O-S5 | LP(O40)→BD*(H25—N28) | 11.18 | 21.85 |
| LP(N27)→BD*(O43—H45) | 10.67 | ||
| [HHex][TFS]-2H2O-S6 | LP(O34)→BD*(O43—H44) | 1.76 | 65.62 |
| LP(O34)→BD*(O43—H45) | 4.46 | ||
| LP(O40)→BD*(H26—N28) | 59.37 | ||
| [HHex][TFS]-2H2O-S7 | LP(O34)→BD*(O40—H41) | 2.04 | 43.12 |
| LP(O35)→BD*(O40—H42) | 5.66 | ||
| LP(O40)→BD*(H26—N28) | 23.58 | ||
| LP(O43)→BD*(H25—N28) | 11.84 | ||
| [HHex][TFS]-6H2O-S8 | LP(O34)→BD*(O40—H41) | 7.65 | 105.39 |
| LP(O39)→BD*(O43—H45) | 17.45 | ||
| LP(O39)→BD*(O46—H47) | 3.26 | ||
| LP(O35)→BD*(O49—H50) | 4.70 | ||
| LP(O35)→BD*(O55—H57) | 4.02 | ||
| LP(O40)→BD*(H26—N28) | 46.39 | ||
| LP(O43)→BD*(N28—H31) | 8.33 | ||
| LP(O43)→BD*(O49—H51) | 10.2 | ||
| LP(O46)→BD*(N28—H31) | 3.39 |
| Structures | BCP | G(r) | H(r) | V(r) | ?2 ρ c | ρ c | ρ c(sum) |
|---|---|---|---|---|---|---|---|
| S1 | 28N—26H···40O | 23.85 | 3.04 | -26.89 | 83.25 | 28.79 | 53.20 |
| 40O—41H···34O | 7.89 | -0.68 | -7.22 | 34.27 | 9.61 | ||
| 40O—42H···35O | 13.15 | -0.88 | -12.28 | 56.12 | 14.80 | ||
| S2 | 28N—25H···40O | 24.98 | -0.06 | -24.92 | 100.18 | 24.86 | 24.86 |
| S3 | 28N—26H···40O | 23.89 | 3.06 | -26.95 | 83.30 | 28.83 | 53.24 |
| 40O—41H···34O | 13.16 | -0.87 | -12.28 | 56.14 | 14.80 | ||
| 40O—42H···35O | 7.88 | -0.68 | -7.21 | 34.24 | 9.61 | ||
| S4 | 28N—26H···40O | 28.04 | 7.71 | -35.75 | 81.30 | 35.94 | 71.20 |
| 40O—41H···27N | 17.62 | 1.73 | -19.35 | 63.53 | 23.18 | ||
| 40O—42H···35O | 10.75 | -0.93 | -9.82 | 46.71 | 12.09 | ||
| S5 | 43O—45H···27N | 13.96 | -0.31 | -13.65 | 57.08 | 18.07 | 36.26 |
| 28N—26H···40O | 17.16 | -1.45 | -15.71 | 74.43 | 18.19 | ||
| S6 | 43O—44H···34O | 8.05 | -0.76 | -7.29 | 35.23 | 9.62 | 93.03 |
| 43O—45H···35O | 11.41 | -0.93 | -10.48 | 49.37 | 13.01 | ||
| 28N—26H···40O | 36.85 | 15.53 | -52.38 | 85.29 | 47.77 | ||
| 40O—41H···27N | 17.33 | 1.45 | -18.78 | 63.49 | 22.64 | ||
| S7 | 28N—25H···43O | 16.97 | -1.37 | -15.61 | 73.37 | 18.20 | 67.42 |
| 28N—26H···40O | 21.15 | 1.31 | -22.46 | 79.35 | 25.12 | ||
| 40O—41H···34O | 7.79 | -0.67 | -7.12 | 33.84 | 9.55 | ||
| 40O—42H···35O | 12.91 | -0.88 | -12.03 | 55.15 | 14.55 | ||
| S8 | 28N—26H···40O | 31.90 | 9.29 | -41.19 | 90.42 | 39.52 | 184.83 |
| 28N—31H···43O | 12.55 | -1.36 | -11.19 | 55.63 | 14.43 | ||
| 27N—29H···55O | 13.48 | -1.49 | -11.99 | 59.90 | 15.11 | ||
| 40O—41H···34O | 14.42 | -1.30 | -13.13 | 62.89 | 15.50 | ||
| 43O—45H···39O | 21.28 | -0.19 | -21.09 | 85.91 | 22.36 | ||
| 49O—50H···35O | 11.94 | -1.31 | -10.63 | 53.00 | 13.05 | ||
| 49O—51H···43O | 15.60 | -1.25 | -14.34 | 67.42 | 16.86 | ||
| 52O—54H···29N | 18.29 | 2.04 | -20.32 | 65.01 | 24.08 | ||
| 46O—47H···39O | 10.01 | -0.82 | -9.19 | 43.33 | 11.68 | ||
| 55O—57H···35O | 11.20 | -1.42 | -9.78 | 50.50 | 12.24 |
表5 在M06-2X/6-311G(d,p)水平下优化得到[HHex][TFS]-nH2O分子间的相互作用BCPs的电子密度(kcal?mol-1)
Table 5 Properties of electron density (kcal?mol-1) of BCPs for intermolecular interactions in configurations [HHex][TFS]-nH2O calculated at M06-2X/6-311G(d,p) level
| Structures | BCP | G(r) | H(r) | V(r) | ?2 ρ c | ρ c | ρ c(sum) |
|---|---|---|---|---|---|---|---|
| S1 | 28N—26H···40O | 23.85 | 3.04 | -26.89 | 83.25 | 28.79 | 53.20 |
| 40O—41H···34O | 7.89 | -0.68 | -7.22 | 34.27 | 9.61 | ||
| 40O—42H···35O | 13.15 | -0.88 | -12.28 | 56.12 | 14.80 | ||
| S2 | 28N—25H···40O | 24.98 | -0.06 | -24.92 | 100.18 | 24.86 | 24.86 |
| S3 | 28N—26H···40O | 23.89 | 3.06 | -26.95 | 83.30 | 28.83 | 53.24 |
| 40O—41H···34O | 13.16 | -0.87 | -12.28 | 56.14 | 14.80 | ||
| 40O—42H···35O | 7.88 | -0.68 | -7.21 | 34.24 | 9.61 | ||
| S4 | 28N—26H···40O | 28.04 | 7.71 | -35.75 | 81.30 | 35.94 | 71.20 |
| 40O—41H···27N | 17.62 | 1.73 | -19.35 | 63.53 | 23.18 | ||
| 40O—42H···35O | 10.75 | -0.93 | -9.82 | 46.71 | 12.09 | ||
| S5 | 43O—45H···27N | 13.96 | -0.31 | -13.65 | 57.08 | 18.07 | 36.26 |
| 28N—26H···40O | 17.16 | -1.45 | -15.71 | 74.43 | 18.19 | ||
| S6 | 43O—44H···34O | 8.05 | -0.76 | -7.29 | 35.23 | 9.62 | 93.03 |
| 43O—45H···35O | 11.41 | -0.93 | -10.48 | 49.37 | 13.01 | ||
| 28N—26H···40O | 36.85 | 15.53 | -52.38 | 85.29 | 47.77 | ||
| 40O—41H···27N | 17.33 | 1.45 | -18.78 | 63.49 | 22.64 | ||
| S7 | 28N—25H···43O | 16.97 | -1.37 | -15.61 | 73.37 | 18.20 | 67.42 |
| 28N—26H···40O | 21.15 | 1.31 | -22.46 | 79.35 | 25.12 | ||
| 40O—41H···34O | 7.79 | -0.67 | -7.12 | 33.84 | 9.55 | ||
| 40O—42H···35O | 12.91 | -0.88 | -12.03 | 55.15 | 14.55 | ||
| S8 | 28N—26H···40O | 31.90 | 9.29 | -41.19 | 90.42 | 39.52 | 184.83 |
| 28N—31H···43O | 12.55 | -1.36 | -11.19 | 55.63 | 14.43 | ||
| 27N—29H···55O | 13.48 | -1.49 | -11.99 | 59.90 | 15.11 | ||
| 40O—41H···34O | 14.42 | -1.30 | -13.13 | 62.89 | 15.50 | ||
| 43O—45H···39O | 21.28 | -0.19 | -21.09 | 85.91 | 22.36 | ||
| 49O—50H···35O | 11.94 | -1.31 | -10.63 | 53.00 | 13.05 | ||
| 49O—51H···43O | 15.60 | -1.25 | -14.34 | 67.42 | 16.86 | ||
| 52O—54H···29N | 18.29 | 2.04 | -20.32 | 65.01 | 24.08 | ||
| 46O—47H···39O | 10.01 | -0.82 | -9.19 | 43.33 | 11.68 | ||
| 55O—57H···35O | 11.20 | -1.42 | -9.78 | 50.50 | 12.24 |
| 1 | 王昊迪 . 离子液体的性质及研究进展[J]. 当代化工研究, 2019, (1): 121-122. |
| Wang H D . Properties and research progress of ionic liquids [J]. Modern Chemical Research, 2019, (1): 121-122. | |
| 2 | 邹卫红, 张颖, 阎子峰 . 离子液体中电沉积法制备不同形貌材料的研究进展[J]. 石油化工, 2018, 47(10): 1149-1157. |
| Zou W H , Zhang Y , Yan Z F . Progress in preparation of different morphological materials by electrodeposition in ionic liquids [J]. Petrochemical Technology, 2018, 47(10): 1149-1157. | |
| 3 | Wishart J F , Castner J E W . The physical chemistry of ionic liquids[J]. J. Phys. Chem. B, 2007, 111: 201-208. |
| 4 | Egorova K S , Gordeev E G , Ananikov V P . Biological activity of ionic liquids and their application in pharmaceutics and medicine[J].Chem. Rev., 2017, 117(10): 7132-7189. |
| 5 | Vekariya R L . A review of ionic liquids: applications towards catalytic organic transformations[J]. J. Mol. Liq., 2017, 227: 44-60. |
| 6 | Lei Z G , Dai C N , Chen B H . Gas solubility in ionic liquids[J]. Chem. Rev., 2014, 114(2): 1289-1326. |
| 7 | Zeng S J , Zhang X P , Bai L , et al . Ionic liquid-based CO2 capture systems: structure, interaction and process[J]. Chem. Rev., 2017, 117(14): 9625-9673. |
| 8 | Plechkovan V , Seddon K R . Applications of ionic liquids in the chemical industry[J]. Chem. Soc. Rev., 2008, 37: 123-150. |
| 9 | Iida M , Kawakami S , Syouno E , et al . Properties of ionic liquids containing silver (I) or protic alkylethylenediamine cations with a bis(trifluoromethanesulfonyl)amide anion[J]. Colloid. Interf. SCI., 2011, 356: 630-638. |
| 10 | Wasserscheid P , Welton T . Ionic Liquids in Synthesis[M]. New York: Wiley-VCH, 2007. |
| 11 | Hallett G P , Welton T . Room-temperature ionic liquids: solvents for synthesis and catalysis[J]. Chem. Rev., 2011, 111: 3508-3576. |
| 12 | 屈建莹, 康世平, 娄童芳, 等 . 基于聚乙烯醇离子液体负载HRP修饰石墨烯/纳米金复合膜的过氧化氢生物传感器[J]. 高等学校化学学报, 2013, 34: 2097-2101. |
| Qu J Y , Kang S P , Lou T F , et al . Hydrogen peroxide biosensor based on polyvinyl alcohol ionic liquid load HRP modified graphene/nano-gold composite film[J]. Chem. J. Chin. Univ., 2013, 34: 2097-2101. | |
| 13 | Hua E , Xu Y , Zhao H . Properties of mono-protic ionic liquids composed of hexylammonium and hexylethylenediaminium cations with trifluoroacetate and bis (trifluoromethylsulfonyl) imide anions[J]. Mol. Liq., 2019, 276: 379-384. |
| 14 | Zeng S J , Gao H , Zhang X , et al . Efficient and reversible capture of SO2 by pyridinium-based ionic liquids[J]. Chem. Eng. J., 2014, 251: 248-256. |
| 15 | Huang K , Cai D N , Chen Y , et al . Thermodynamic validation of 1-alkyl-3-methylimidazolium carboxylates as task-specific ionic liquids for H2S absorption[J]. AIChE J., 2013, 59(6): 2227-2235. |
| 16 | Luo X Y , Guo Y , Ding F , et al . Significant improvements in CO2 capture by pyridine-containing anion-functionalized ionic liquids through multiple-site cooperative interactions[J]. Angew Chem. Int. Edit., 2014, 53(27): 7053-7057. |
| 17 | 曾少娟, 尚大伟, 余敏, 等 . 离子液体在氨气分离回收中的应用及展望[J]. 化工学报, 2019, 70(3): 791-800. |
| Zeng S J , Shang D W , Yu M , et al . Application and prospect of ionic liquid in ammonia separation and recovery [J]. CIESC Journal, 2019, 70(3): 791-800. | |
| 18 | Cammarata L , Kazarian S G , Salter P A , et al . Molecular states of water in room temperature ionic liquids[J]. Physical Chemistry Chemical Physics, 2001, 3(23): 5192-5200. |
| 19 | 李艳霞,邵正隆,彭宇 . 高效运行技术在现代化教学支撑平台中的应用[J].计算机科学, 2014, 41(10): 357-360. |
| Li Y X , Shao Z L , Peng Y . Application of efficient operation technology in modern teaching support platform [J]. Computer Science, 2014, 41(10): 357-360. | |
| 20 | 张琼, 吴杰颖 . Gaussian 09软件在配合物紫外-可见吸收光谱教学中的应用[J]. 赤峰学院学报(自然科学版), 2018, 34(9): 53-54. |
| Zhang Q , Wu J Y . Application of Gaussian 09 software in the teaching of UV-visible absorption spectra of complexes [J]. Journal of Chifeng University (Natural Science Edition), 2018, 34(9): 53-54. | |
| 21 | 戴国梁, 钱蕙 . Gaussian 09和Gauss View软件在结构化学教学中的应用[J]. 课程教育研究, 2018, 33: 156. |
| Dai G L , Qian H . Application of Gaussian 09 and Gauss View software in structural chemistry teaching [J]. Course Education Research, 2018, 33: 156. | |
| 22 | Zheng Q , He G S , Prasad P N . Novel two-absorbing,1,10-phenanthroline-containing π-conjugated chromophores and their nickel (Ⅱ) chelated complexes with quenched emissions[J]. J. Mater. Chem., 2005, 15, 579-587. |
| 23 | 吴丽, 李臻, 王芳, 等 . 离子液体的量化计算及分子动力学模拟研究进展[J]. 分子催化, 2012, 26(5): 456-468+390. |
| Wu L , Li Q , Wang F , et al . Advances in quantitative calculation and molecular dynamics simulation of ionic liquids [J]. Journal of Molecular Catalysis, 2012, 26(5): 456-468+390. | |
| 24 | 徐宇, 花儿 . 烷基乙二胺-CF3CO2型质子化离子液体的分子间氢键作用[J]. 高等学校化学学报, 2018, 39: 1954-1960. |
| Xu Y , Hua E . Intermolecular hydrogen bonding effect of alkyl ethyl two amine-CF3CO2 proton ionic liquid [J]. Chemical Journal of Chinese Universities, 2018, 39: 1954-1960. | |
| 25 | Isha S , Ali A , Shilendra K , et al . Experimental and theoretical DFT (B3LYP, X3LYP, CAM-B3LYP and M06-2X) study on electronic structure, spectral features, hydrogen bonding and solvent effects of 4-methylthiadiazole-5-carboxylic acid[J]. Molecular Simulation, 2019, 45: 13. |
| 26 | Hua E , Hui W . Properties of protic ionic liquids composed of N -alkyl (=hexyl, octyl and 2-ethylhexyl) ethylenediaminum cations with trifluoromethanesulfonate and trifluoroacetate anion[J]. Journal of Molecular Liquids, 2016, 220: 649-656. |
| 27 | 李晨光, 花儿 . 水分含量对异辛基乙二胺-CF3SO3型质子化离子液体物理化学性质的影响[J]. 河南师范大学学报(自然科学版), 2019, 47: 61-66. |
| Li C G , Hua E . Effect of moisture content on physical and chemical properties of -CF3SO3 type proton ionic liquid with octyl ethyl two amine[J]. Journal of Henan Normal University (Natural Science), 2019, 47: 61-66. | |
| 28 | 李巍, 张静, 戚传松 . EMIM离子液体离子簇模型的量子化学计算[J]. 物理化学学报, 2015, 31: 1690-1698. |
| Li W , Zhang J , Qi C S . Quantum chemical calculation of EMIM ion liquid ion cluster model [J]. Acta Physico-Chimica Sinica, 2015, 31: 1690-1698. | |
| 29 | 卓志昊, 郑燕升, 李军生 . N-乙基吡啶溴盐离子液体分子结构和光谱性质的量子化学计算[J]. 计算机与应用化学, 2011, 28(6): 790-792. |
| Zhuo Z H , Zheng Y S , Li J S . Quantum chemical calculation of molecular structure and spectral properties of N-ethylpyridinium bromide ionic liquids [J]. Computers and Applied Chemistry, 2011, 28(6): 790-792. | |
| 30 | Koddermann T , Wertz C , Heintz A , et al . The association of water in ionic liquids: a reliable measure of polarity[J]. Angew. Chem. Int. Ed., 2006, 45: 3697-3702. |
| [1] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [2] | 杜峰, 尹思琦, 罗辉, 邓文安, 李传, 黄振薇, 王文静. H2在Mo x S y 团簇上吸附解离的尺寸效应研究[J]. 化工学报, 2022, 73(9): 3895-3903. |
| [3] | 俞夏琪, 冯格, 赵金燕, 李嘉远, 邓声威, 郑靖楠, 李雯雯, 王亚秋, 沈榄, 刘旭, 徐威威, 王建国, 王式彬, 姚子豪, 毛成立. 基体(TDI-TMP-T313)与氧化剂(AP)相互作用的第一性原理研究[J]. 化工学报, 2022, 73(8): 3511-3517. |
| [4] | 赵继昊, 唐伟强, 徐小飞, 赵双良, 贺炅皓. 高分子复合材料中键合剂在不同纳米填料表面的吸附能计算[J]. 化工学报, 2022, 73(7): 3174-3181. |
| [5] | 罗小松, 黄金保, 周梅, 牟鑫, 徐伟伟, 吴雷. 对苯二甲酸丁二醇酯二聚体水/醇/氨解机理的理论研究[J]. 化工学报, 2022, 73(11): 4859-4871. |
| [6] | 朱先会, 王甫, 夏杰成, 袁金良. 功能型离子液体协同吸收NH3和CO2的密度泛函理论研究[J]. 化工学报, 2022, 73(10): 4324-4334. |
| [7] | 龚翔, 李林森, 姜召. PdCo/SiO2双金属催化剂用于杂环储氢载体的高效脱氢[J]. 化工学报, 2022, 73(10): 4448-4460. |
| [8] | 马生贵, 田博文, 周雨薇, 陈琳, 江霞, 高涛. 氮掺杂Stone-Wales缺陷石墨烯吸附H2S的密度泛函理论研究[J]. 化工学报, 2021, 72(9): 4496-4503. |
| [9] | 张芳芳, 韩敏, 赵娟, 凌丽霞, 章日光, 王宝俊. 单空缺石墨烯负载的Pd单原子催化剂上NO还原的密度泛函理论研究[J]. 化工学报, 2021, 72(3): 1382-1391. |
| [10] | 唐伟强, 谢鹏, 徐小飞, 赵双良. 反应密度泛函理论的构建与初步应用[J]. 化工学报, 2021, 72(2): 633-652. |
| [11] | 葛冰青, 阴义轩, 王亚溪, 张宏伟, 袁珮. 溶剂对丁腈橡胶溶解、尺寸、结构和催化加氢的影响研究[J]. 化工学报, 2021, 72(1): 543-554. |
| [12] | 刘佳鑫, 徐宇, 花儿. 异辛基乙二胺-酰基丙氨酸型质子化离子液体的分子间氢键相互作用[J]. 化工学报, 2020, 71(S1): 15-22. |
| [13] | 狄玲, 陈放, 付荣荣, 杨辰, 邢杨, 王晓宁. 富电子LMOF对有机农药的检测机理研究[J]. 化工学报, 2020, 71(8): 3830-3838. |
| [14] | 孙巍, 左然. MMAl在NH2与H混合覆盖的AlN(0001)-Al表面的吸附与扩散研究[J]. 化工学报, 2020, 71(7): 3213-3219. |
| [15] | 张红, 唐留. p型掺杂剂Cp2Mg在MOCVD气相中的反应机理研究[J]. 化工学报, 2020, 71(7): 3000-3008. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号