化工学报 ›› 2022, Vol. 73 ›› Issue (10): 4324-4334.DOI: 10.11949/0438-1157.20220603
收稿日期:2022-04-28
修回日期:2022-08-21
出版日期:2022-10-05
发布日期:2022-11-02
通讯作者:
王甫
作者简介:朱先会(1997—),男,硕士研究生,zxh18338261771@126.com
基金资助:
Xianhui ZHU( ), Fu WANG(
), Fu WANG( ), Jiecheng XIA, Jinliang YUAN
), Jiecheng XIA, Jinliang YUAN
Received:2022-04-28
Revised:2022-08-21
Online:2022-10-05
Published:2022-11-02
Contact:
Fu WANG
摘要:
离子液体(ILs)由于其独特的结构可调性,作为添加剂可有效抑制氨法碳捕集中NH3的逃逸并同时促进CO2的吸收。揭示其吸收NH3和CO2的作用机理对于构建特定的功能型ILs结构具有重要意义。本文采用密度泛函理论(DFT),在B3LYP/6-31'++G(d,p)基组水平下对设计的五种功能型ILs进行了结构优化、频率计算以及原子电荷分析,获得了优化后的结构参数、振动频率以及原子电荷等数据。在此基础上对ILs吸收CO2和NH3进行了相互作用分析。计算结果表明:[HEBim][His]的稳定性最好,经过BSSE校正后的相互作用能为-415.73 kJ·mol-1。通过静电势和电荷分析找到了设计的ILs与气体作用的最佳位点:NH3主要与ILs阳离子的羟基形成 O—H…N型氢键,其中,[HEBim][His]吸收NH3的能力最强,形成的氢键结合能为38.52 kJ·mol-1,具有较强的氢键作用;CO2主要与阴离子中的氨基形成C—N…C型氢键,[HEBim][Ala]吸收CO2的能力最强,形成的氢键结合能为10.15 kJ·mol-1,具有较弱的氢键作用。当ILs同时与NH3和CO2相互作用时,其吸收能力均有不同程度的下降,[HEBim][His]与[HEBim][Ala]的综合吸收效果最佳。
中图分类号:
朱先会, 王甫, 夏杰成, 袁金良. 功能型离子液体协同吸收NH3和CO2的密度泛函理论研究[J]. 化工学报, 2022, 73(10): 4324-4334.
Xianhui ZHU, Fu WANG, Jiecheng XIA, Jinliang YUAN. Density functional theory investigation on the NH3 and CO2 absorption by functional ionic liquids[J]. CIESC Journal, 2022, 73(10): 4324-4334.
| Structure | BCP | ρBCP/a.u. | ∇2ρBCP/a.u. | EHB/ (kJ·mol-1) |
|---|---|---|---|---|
| [HEMim][Glu] | C1—H4—O35 | 0.04886 | 0.12196 | 42.5 |
| [HEMim][Glu] | C10—H12—O35 | 0.01331 | 0.03917 | 9.32 |
| [HEMim][Glu] | C17—H18—O34 | 0.02293 | 0.06332 | 18.33 |
| [HEMim][Asp] | C1—H6—O28 | 0.04452 | 0.12057 | 38.45 |
| [HEMim][Asp] | C9—H11—O29 | 0.02270 | 0.06867 | 18.07 |
| [HEMim][Asp] | C16—H18—O28 | 0.01305 | 0.04481 | 9.04 |
| [HEBim][Asp] | C1—H7—O37 | 0.05038 | 0.13735 | 43.92 |
| [HEBim][Asp] | C11—H13—O38 | 0.01245 | 0.04123 | 8.54 |
| [HEBim][Asp] | O14—H15—O38 | 0.02940 | 0.10492 | 24.35 |
| [HEBim][Ala] | C1—H6—O37 | 0.04807 | 0.13029 | 41.76 |
| [HEBim][Ala] | C22—H23—O38 | 0.01340 | 0.04554 | 9.41 |
| [HEBim][Ala] | O28—H29—O38 | 0.02945 | 0.10705 | 24.39 |
| [HEBim][Ala] | O37—H41—N39 | 0.01887 | 0.07515 | 14.52 |
| [HEBim][His] | C1—H9—O48 | 0.04721 | 0.12949 | 40.96 |
| [HEBim][His] | C11—H13—O47 | 0.01355 | 0.04557 | 9.54 |
| [HEBim][His] | O17—H18—O47 | 0.03139 | 0.11223 | 26.19 |
| [HEBim][His] | N44—H45—O28 | 0.01809 | 0.07527 | 13.77 |
| [HEBim][His] | N44—H46—N36 | 0.01247 | 0.04044 | 8.54 |
表1 在B3LYP/6-31’ ++G(d,p)基组水平下计算得到的五种离子液体的电子密度性质
Table 1 Properties of electron density of BCP of five ionic liquids calculated at B3LYP/6-31’++G(d,p) level
| Structure | BCP | ρBCP/a.u. | ∇2ρBCP/a.u. | EHB/ (kJ·mol-1) |
|---|---|---|---|---|
| [HEMim][Glu] | C1—H4—O35 | 0.04886 | 0.12196 | 42.5 |
| [HEMim][Glu] | C10—H12—O35 | 0.01331 | 0.03917 | 9.32 |
| [HEMim][Glu] | C17—H18—O34 | 0.02293 | 0.06332 | 18.33 |
| [HEMim][Asp] | C1—H6—O28 | 0.04452 | 0.12057 | 38.45 |
| [HEMim][Asp] | C9—H11—O29 | 0.02270 | 0.06867 | 18.07 |
| [HEMim][Asp] | C16—H18—O28 | 0.01305 | 0.04481 | 9.04 |
| [HEBim][Asp] | C1—H7—O37 | 0.05038 | 0.13735 | 43.92 |
| [HEBim][Asp] | C11—H13—O38 | 0.01245 | 0.04123 | 8.54 |
| [HEBim][Asp] | O14—H15—O38 | 0.02940 | 0.10492 | 24.35 |
| [HEBim][Ala] | C1—H6—O37 | 0.04807 | 0.13029 | 41.76 |
| [HEBim][Ala] | C22—H23—O38 | 0.01340 | 0.04554 | 9.41 |
| [HEBim][Ala] | O28—H29—O38 | 0.02945 | 0.10705 | 24.39 |
| [HEBim][Ala] | O37—H41—N39 | 0.01887 | 0.07515 | 14.52 |
| [HEBim][His] | C1—H9—O48 | 0.04721 | 0.12949 | 40.96 |
| [HEBim][His] | C11—H13—O47 | 0.01355 | 0.04557 | 9.54 |
| [HEBim][His] | O17—H18—O47 | 0.03139 | 0.11223 | 26.19 |
| [HEBim][His] | N44—H45—O28 | 0.01809 | 0.07527 | 13.77 |
| [HEBim][His] | N44—H46—N36 | 0.01247 | 0.04044 | 8.54 |
| Structure | Atoms | ESP | ADCH | Hirshfeld |
|---|---|---|---|---|
| [HEMim][Glu] | O13 | -0.7015 | -0.4896 | -0.2236 |
| [HEMim][Glu] | O31 | -0.6499 | -0.4914 | -0.1804 |
| [HEMim][Glu] | O33 | -0.5800 | -0.5392 | -0.2963 |
| [HEMim][Glu] | O34 | -0.8291 | -0.3179 | -0.3635 |
| [HEMim][Glu] | O35 | -0.7445 | -0.2780 | -0.3485 |
| [HEMim][Glu] | N36 | -0.8907 | -0.5660 | -0.2056 |
| [HEMim][Glu] | H4 | 0.3344 | 0.1135 | 0.0522 |
| [HEMim][Glu] | H5 | 0.2147 | 0.1688 | 0.0720 |
| [HEMim][Glu] | H6 | 0.2078 | 0.1686 | 0.0720 |
| [HEMim][Glu] | H14 | 0.4238 | 0.3733 | 0.1634 |
| [HEBim][Ala] | O28 | -0.6796 | -0.4611 | -0.2436 |
| [HEBim][Ala] | O37 | -0.7270 | -0.5027 | -0.3340 |
| [HEBim][Ala] | O38 | -0.7921 | -0.4611 | -0.3255 |
| [HEBim][Ala] | N39 | -1.0336 | -0.7204 | -0.2306 |
| [HEBim][Ala] | H4 | 0.2228 | 0.1274 | 0.0723 |
| [HEBim][Ala] | H5 | 0.2238 | 0.1155 | 0.0697 |
| [HEBim][Ala] | H6 | 0.2619 | 0.2064 | 0.0527 |
| [HEBim][Ala] | H29 | 0.4241 | 0.3252 | 0.1147 |
表2 在B3LYP/6-31’ ++G(d,p)基组下计算的离子液体的ESP、ADCH和Hirshfeld 电荷
Table 2 ESP, ADCH and Hirshfeld charges for ionic liquids calculated at B3LYP/6-31’ ++G(d,p) level
| Structure | Atoms | ESP | ADCH | Hirshfeld |
|---|---|---|---|---|
| [HEMim][Glu] | O13 | -0.7015 | -0.4896 | -0.2236 |
| [HEMim][Glu] | O31 | -0.6499 | -0.4914 | -0.1804 |
| [HEMim][Glu] | O33 | -0.5800 | -0.5392 | -0.2963 |
| [HEMim][Glu] | O34 | -0.8291 | -0.3179 | -0.3635 |
| [HEMim][Glu] | O35 | -0.7445 | -0.2780 | -0.3485 |
| [HEMim][Glu] | N36 | -0.8907 | -0.5660 | -0.2056 |
| [HEMim][Glu] | H4 | 0.3344 | 0.1135 | 0.0522 |
| [HEMim][Glu] | H5 | 0.2147 | 0.1688 | 0.0720 |
| [HEMim][Glu] | H6 | 0.2078 | 0.1686 | 0.0720 |
| [HEMim][Glu] | H14 | 0.4238 | 0.3733 | 0.1634 |
| [HEBim][Ala] | O28 | -0.6796 | -0.4611 | -0.2436 |
| [HEBim][Ala] | O37 | -0.7270 | -0.5027 | -0.3340 |
| [HEBim][Ala] | O38 | -0.7921 | -0.4611 | -0.3255 |
| [HEBim][Ala] | N39 | -1.0336 | -0.7204 | -0.2306 |
| [HEBim][Ala] | H4 | 0.2228 | 0.1274 | 0.0723 |
| [HEBim][Ala] | H5 | 0.2238 | 0.1155 | 0.0697 |
| [HEBim][Ala] | H6 | 0.2619 | 0.2064 | 0.0527 |
| [HEBim][Ala] | H29 | 0.4241 | 0.3252 | 0.1147 |
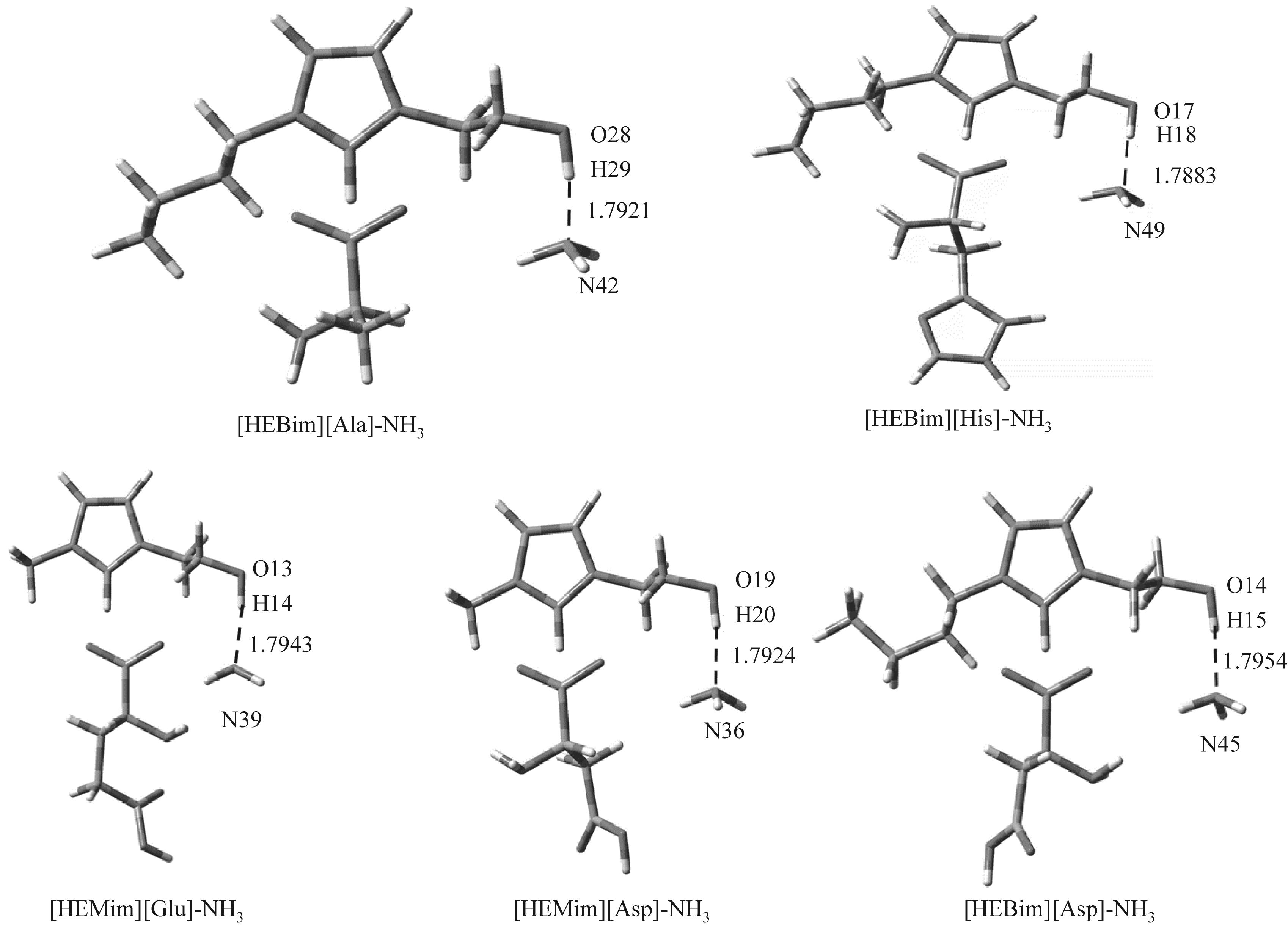
图4 在B3LYP/6-31’ ++G(d,p)基组水平下优化得到的五种离子液体和氨相互作用的稳定构型
Fig.4 Optimized structures of five ionic liquids and ammonia interaction at B3LYP/6-31’ ++G(d,p) level
| ILs-gases | BCP | ρBCP/a.u. | ∇2ρBCP/ a.u. | EHB/(kJ·mol-1) |
|---|---|---|---|---|
| [HEMim][Glu]-NH3 | O13—H14—N39 | 0.0440 | 0.1054 | 37.96 |
| [HEMim][Glu]-CO2 | C27—N36—C39 | 0.0108 | 0.0350 | 6.97 |
| [HEMim][Asp]-NH3 | O19—H20—N36 | 0.0442 | 0.1044 | 38.15 |
| [HEMim][Asp]-CO2 | C21—N39—C36 | 0.0087 | 0.0290 | 5.01 |
| [HEBim][Asp]-NH3 | O14—H15—N45 | 0.0438 | 0.1043 | 37.78 |
| [HEBim][Asp]-CO2 | C31—N37—C45 | 0.0075 | 0.0255 | 3.89 |
| [HEBim][Ala]-NH3 | O28—H29—N42 | 0.0444 | 0.1045 | 38.34 |
| [HEBim][Ala]-CO2 | C34—N37—C42 | 0.0142 | 0.0439 | 10.15 |
| [HEBim][His]-NH3 | O17—H18—N49 | 0.0446 | 0.1052 | 38.52 |
| [HEBim][His]-CO2 | C41—N44—C49 | 0.0126 | 0.0393 | 8.65 |
表3 在B3LYP/6-31’ ++G(d,p)基组水平下计算得到的五种离子液体与气体作用的键长以及电子密度性质
Table 3 The bond lengths and electron density of five ionic liquids interacting with gases calculated at B3LYP/6-31’ ++G(d,p) level
| ILs-gases | BCP | ρBCP/a.u. | ∇2ρBCP/ a.u. | EHB/(kJ·mol-1) |
|---|---|---|---|---|
| [HEMim][Glu]-NH3 | O13—H14—N39 | 0.0440 | 0.1054 | 37.96 |
| [HEMim][Glu]-CO2 | C27—N36—C39 | 0.0108 | 0.0350 | 6.97 |
| [HEMim][Asp]-NH3 | O19—H20—N36 | 0.0442 | 0.1044 | 38.15 |
| [HEMim][Asp]-CO2 | C21—N39—C36 | 0.0087 | 0.0290 | 5.01 |
| [HEBim][Asp]-NH3 | O14—H15—N45 | 0.0438 | 0.1043 | 37.78 |
| [HEBim][Asp]-CO2 | C31—N37—C45 | 0.0075 | 0.0255 | 3.89 |
| [HEBim][Ala]-NH3 | O28—H29—N42 | 0.0444 | 0.1045 | 38.34 |
| [HEBim][Ala]-CO2 | C34—N37—C42 | 0.0142 | 0.0439 | 10.15 |
| [HEBim][His]-NH3 | O17—H18—N49 | 0.0446 | 0.1052 | 38.52 |
| [HEBim][His]-CO2 | C41—N44—C49 | 0.0126 | 0.0393 | 8.65 |

图5 在B3LYP/6-31’ ++G(d,p)基组水平下优化得到的五种离子液体和二氧化碳相互作用的稳定构型
Fig.5 Optimized structures of five ionic liquids and carbon dioxide interaction at B3LYP/6-31’ ++G(d,p) level
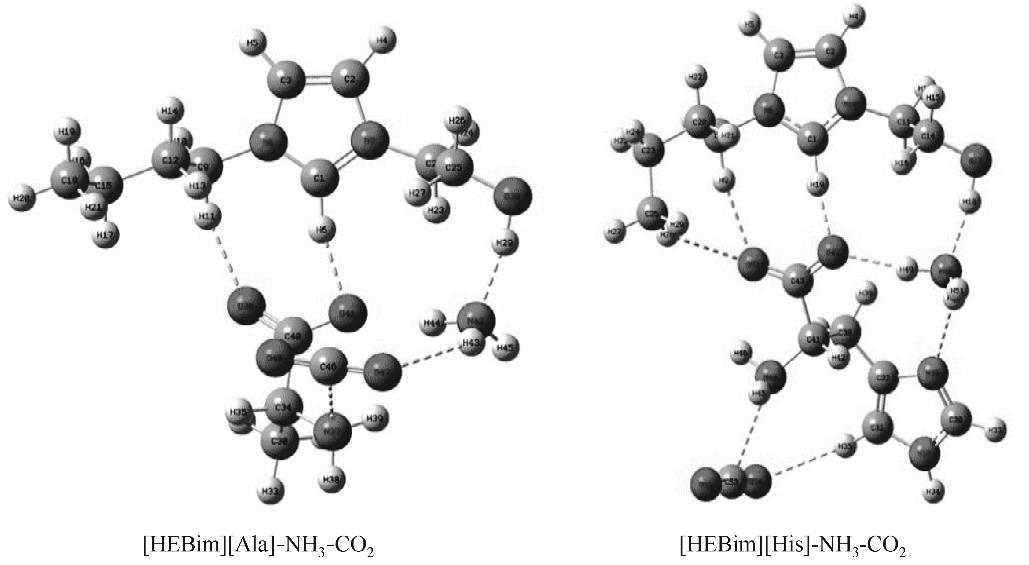
图6 在B3LYP/6-31’ ++G(d,p)基组水平下优化得到的两种离子液体与气体同时作用的稳定构型
Fig.6 Two stale configurations of two ILs interacting with gases obtained at B3LYP/6-31’ ++G(d,p) level
| ILs-gases | BCP | Bond length/ Å | ρBCP/a.u. | ∇2ρBCP/a.u. | EHB/(kJ·mol-1) |
|---|---|---|---|---|---|
| [HEBim][His]-NH3-CO2 | O17—H18—N48 | 1.7886 | 0.0445 | 0.1034 | 38.43 |
| [HEBim][His]-NH3-CO2 | C41—N44—C53 | 2.9830 | 0.0112 | 0.0345 | 7.35 |
| [HEBim][Ala]-NH3-CO2 | O28—H29—N42 | 1.8015 | 0.0436 | 0.1027 | 37.59 |
| [HEBim][Ala]-NH3-CO2 | C34—N37—C46 | 2.8661 | 0.0129 | 0.0407 | 8.93 |
表4 在B3LYP/6-31’ ++G(d,p)基组水平下计算得到的两种离子液体与气体共同作用的键长、结合能以及电子密度等性质
Table 4 The bond lengths, binding energies and electron densities of the two ILs interacting with gas calculated at the B3LYP/6-31 ++G(d,p) level
| ILs-gases | BCP | Bond length/ Å | ρBCP/a.u. | ∇2ρBCP/a.u. | EHB/(kJ·mol-1) |
|---|---|---|---|---|---|
| [HEBim][His]-NH3-CO2 | O17—H18—N48 | 1.7886 | 0.0445 | 0.1034 | 38.43 |
| [HEBim][His]-NH3-CO2 | C41—N44—C53 | 2.9830 | 0.0112 | 0.0345 | 7.35 |
| [HEBim][Ala]-NH3-CO2 | O28—H29—N42 | 1.8015 | 0.0436 | 0.1027 | 37.59 |
| [HEBim][Ala]-NH3-CO2 | C34—N37—C46 | 2.8661 | 0.0129 | 0.0407 | 8.93 |
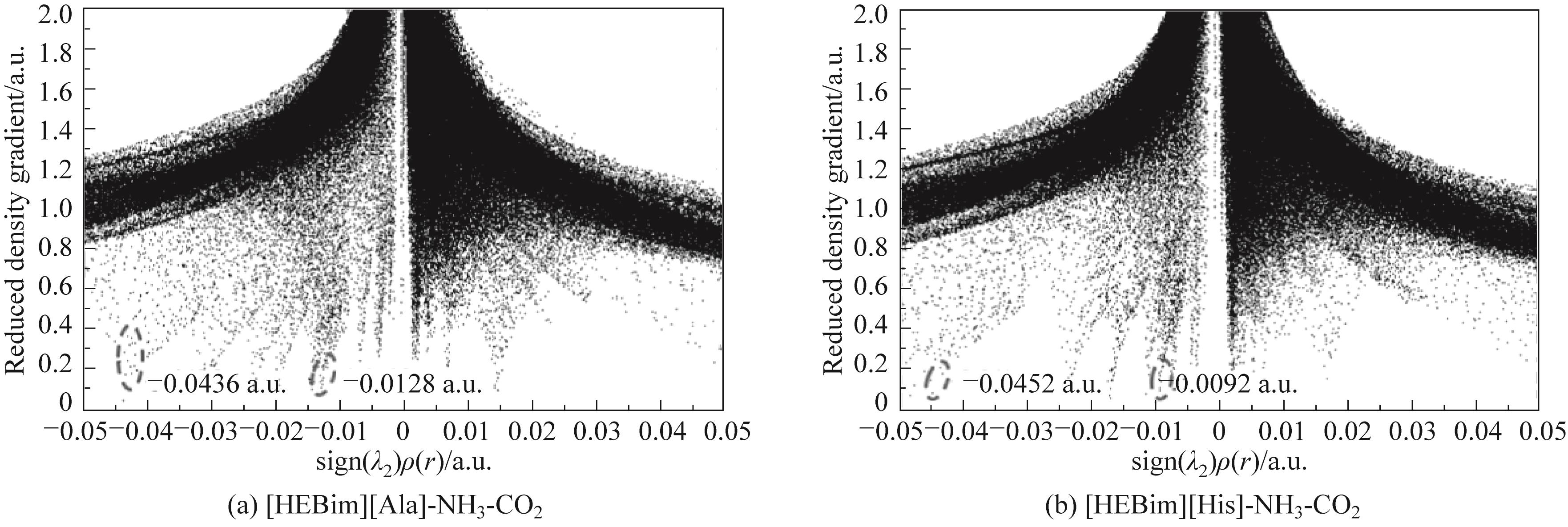
图8 通过第二个Hessian矩阵的特征值计算得出的离子液体与气体相互作用的电子密度RDG图
Fig.8 Plot of RDG versus electron density multiplied by the sign of the second Hessian eigenvalue(λ2) for ILs-gas structures
| ILs-gases | H-bonds | sign(λ2)ρ(r)/a.u. |
|---|---|---|
| [HEBim][His]-NH3 | O17—H18…N48 | -0.0452 |
| [HEBim][His]-CO2 | C41—N44…C53 | -0.0092 |
| [HEBim][Ala]-NH3 | O28—H29…N42 | -0.0436 |
| [HEBim][Ala]-CO2 | C34—N37…C46 | -0.0128 |
表5 离子液体与气体之间的氢键的sign(λ2)ρ(r)值
Table 5 sign(λ2)ρ(r) values for H-bonds between ILs and gases
| ILs-gases | H-bonds | sign(λ2)ρ(r)/a.u. |
|---|---|---|
| [HEBim][His]-NH3 | O17—H18…N48 | -0.0452 |
| [HEBim][His]-CO2 | C41—N44…C53 | -0.0092 |
| [HEBim][Ala]-NH3 | O28—H29…N42 | -0.0436 |
| [HEBim][Ala]-CO2 | C34—N37…C46 | -0.0128 |
| 1 | Guo J X, Huang C. Feasible roadmap for CCS retrofit of coal-based power plants to reduce Chinese carbon emissions by 2050[J]. Applied Energy, 2020, 259: 114112. |
| 2 | 王甫, 赵军, 邓帅, 等. 氨法碳捕集中氨逃逸抑制机制研究进展[J]. 化工进展, 2017, 36(12): 4641-4650. |
| Wang F, Zhao J, Deng S, et al. Review on development and mechanism of reducing ammonia escape from carbon dioxide capture process using ammonia method[J]. Chemical Industry and Engineering Progress, 2017, 36(12): 4641-4650. | |
| 3 | McEwen A B, McDevitt S F, Koch V R. Nonaqueous electrolytes for electrochemical capacitors: imidazolium cations and inorganic fluorides with organic carbonates[J]. Journal of the Electrochemical Society, 1997, 144(4): L84-L86. |
| 4 | Rogers R D, Seddon K R. Ionic liquids: solvents of the future? [J]. Science, 2003, 302(5646): 792-793. |
| 5 | Fung Y S, Zhou R Q. Room temperature molten salt as medium for lithium battery[J]. Journal of Power Sources, 1999, 81/82: 891-895. |
| 6 | Li G H, Zhou Q, Zhang X P, et al. Solubilities of ammonia in basic imidazolium ionic liquids [J]. Fluid Phase Equilibria, 2010, 297(1): 34-39. |
| 7 | 陈晏杰, 姚月华, 张香平, 等. 基于离子液体的合成氨驰放气中氨回收工艺模拟计算[J]. 过程工程学报, 2011, 11(4): 644-651. |
| Chen Y J, Yao Y H, Zhang X P, et al. Simulation and optimization of ammonia recovery with ionic liquid from purge gas in ammonia synthesis plant[J]. The Chinese Journal of Process Engineering, 2011, 11(4): 644-651. | |
| 8 | Zeng S J, Wang J L, Li P F, et al. Efficient adsorption of ammonia by incorporation of metal ionic liquids into silica gels as mesoporous composites[J]. Chemical Engineering Journal, 2019, 370: 81-88. |
| 9 | Xu Y, Zhao J, Wu W, et al. Experimental and theoretical studies on the influence of ionic liquids as additives on ammonia-based CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 42: 454-460. |
| 10 | Shi W, Maginn E J. Molecular simulation of ammonia absorption in the ionic liquid 1-ethyl-3-methylimidazolium bis (trifluoromethylsulfonyl) imide ([emim][Tf 2N])[J]. AIChE Journal, 2009, 55(9): 2414-2421. |
| 11 | Shang D W, Zhang X P, Zeng S J, et al. Protic ionic liquid [Bim][NTf2] with strong hydrogen bond donating ability for highly efficient ammonia absorption[J]. Green Chemistry, 2017, 19(4): 937-945. |
| 12 | 曾少娟, 尚大伟, 余敏, 等. 离子液体在氨气分离回收中的应用及展望[J]. 化工学报, 2019, 70(3): 791-800. |
| Zeng S J, Shang D W, Yu M, et al. Applications and perspectives of NH3 separation and recovery with ionic liquids[J]. CIESC Journal, 2019, 70(3): 791-800. | |
| 13 | Blanchard L A, Dan H C, Beckman E J, et al. Green processing using ionic liquids and CO2 [J]. Nature, 1999, 399(6731): 28-29. |
| 14 | Blanchard L A, Gu Z Y, Brennecke J F. High-pressure phase behavior of ionic liquid/CO2 systems[J]. The Journal of Physical Chemistry B, 2001, 105(12): 2437-2444. |
| 15 | Hallett J P, Welton T. Room-temperature ionic liquids: solvents for synthesis[J]. Chemical Reviews, 2011, 111(5): 3508-3576. |
| 16 | Cammarata L, Kazarian S, Salter P, et al. Molecular states of water in room temperature ionic liquids[J]. Physical Chemistry Chemical Physics, 2001, 3(23): 5192-5200. |
| 17 | Sharma P, Park S D, Baek I H, et al. Effects of anions on absorption capacity of carbon dioxide in acid functionalized ionic liquids[J]. Fuel Processing Technology, 2012, 100: 55-62. |
| 18 | 崔国凯, 吕书贞, 王键吉. 功能化离子液体在二氧化碳吸收分离中的应用[J]. 化工学报, 2020, 71(1): 16-25. |
| Cui G K, Lyu S Z, Wang J J. Functional ionic liquids for carbon dioxide capture and separation. [J]. CIESC Journal, 2020, 71(1): 16-25. | |
| 19 | 张慧, 张红梅, 沈锦优, 等. 氨基功能型离子液体吸收CO2的性能[J]. 化工学报, 2016, 67(12): 5057-5065. |
| Zhang H, Zhang H M, Shen J Y, et al. Absorption performance of CO2 in amino-functionalized task-specific ionic liquids[J]. CIESC Journal, 2016, 67(12): 5057-5065. | |
| 20 | Gao H S, Bai L, Han J L, et al. Functionalized ionic liquid membranes for CO2 separation[J]. Chemical Communications, 2018, 54(90): 12671-12685. |
| 21 | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 22 | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09[M]. Wallingford CT: Gaussian Inc., 2013. |
| 23 | Boys S F, Bernardi F. The calculation of small molecular interactions by the differences of separate total energies[J]. Molecular Physics, 1970, 19(4): 553-566. |
| 24 | Bader R F W. Atom in Molecules: A Quantum Theory[M]. New York: Oxford University Press, 1990. |
| 25 | He H Y, Zhang S J, Liu X M, et al. Structures and hydrogen bonds of biodegradable naphthenate ionic liquids[J]. Fluid Phase Equilibria, 2013, 360: 169-179. |
| 26 | Zhang Y Q, He H Y, Dong K, et al. A DFT study on lignin dissolution in imidazolium-based ionic liquids[J]. RSC Advances, 2017, 7(21): 12670-12681. |
| 27 | 张营. 氨基酸离子液体的结构和阴阳离子间相互作用的理论研究[D]. 无锡:江南大学, 2011. |
| Zhang Y. Theoretical study on the structure and cation-anion interaction of amino acid based ionic liquids[D]. Wuxi: Jiangnan University, 2011. | |
| 28 | Li Z J, Zhang X P, Dong H F, et al. Efficient absorption of ammonia with hydroxyl-functionalized ionic liquids[J]. RSC Advances, 2015, 5(99): 81362-81370. |
| 29 | Yuan L, Zhang X P, Ren B Z, et al. Dual-functionalized protic ionic liquids for efficient absorption of NH3 through synergistically physicochemical interaction[J]. Journal of Chemical Technology & Biotechnology, 2020, 95(6): 1815-1824. |
| 30 | Johnson E R, Keinan S, Mori-Sánchez P, et al. Revealing noncovalent interactions[J]. Journal of the American Chemical Society, 2010, 132(18): 6498-6506. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [4] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [5] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [6] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [7] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [11] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [12] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [13] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [14] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [15] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号