化工学报 ›› 2020, Vol. 71 ›› Issue (S1): 15-22.DOI: 10.11949/0438-1157.20191380
收稿日期:2019-11-13
修回日期:2019-12-19
出版日期:2020-04-25
发布日期:2020-04-25
通讯作者:
花儿
作者简介:刘佳鑫(1995—),女,硕士研究生,基金资助:
Jiaxin LIU1( ),Yu XU1,Er HUA1,2,3(
),Yu XU1,Er HUA1,2,3( )
)
Received:2019-11-13
Revised:2019-12-19
Online:2020-04-25
Published:2020-04-25
Contact:
Er HUA
摘要:
利用密度泛函理论M06-2X/6-311G(d,p)方法及基组条件下,对异辛基乙二胺-酰基丙氨酸型质子化离子液体[HEtHex]+[Acylala]-(Acyl =butanoyl, hexanoyl)的几何构型进行了优化,分别得到了5种较稳定构型S1~S5。结果显示,[HEtHex][Butlala]及[HEtHex][Hexlala]的基组重叠误差校正后的分子间相互作用能(ΔE0BSSE)均在-136.14~-117.26 kcal·mol-1(1 kcal·mol-1=4.182 kJ·mol-1)范围内,其中伯胺质子化构型(S1~S3)的相互作用能(-136.14~-127.01 kcal·mol-1)大于仲胺质子化构型(S4~S5)(-119.03~-117.26 kcal·mol-1)。由于[HEtHex][Acylala]阴阳离子间发生了质子转移而形成了较强的O—H…N型氢键,引起[HEtHex]+中N—H振动频率消失,并在2400~2815 cm-1处出现了较强的O—H的伸缩振动,即以分子与分子间的作用力键合。自然键轨道及分子中原子理论计算结果显示,[HEtHex][Acylala]间所形成氢键的稳定化能主要来源于[EtHex]分子中胺基N原子的孤对电子lp(N)与[Acylala]分子中羧基的反键轨道σ*(O—H)间的相互作用。并且分子间氢键能及二阶微扰能分别在18.69 ~ 24.19 kcal·mol-1及43.58 ~57.58 kcal?mol-1范围内,属于较强类型氢键作用。
中图分类号:
刘佳鑫, 徐宇, 花儿. 异辛基乙二胺-酰基丙氨酸型质子化离子液体的分子间氢键相互作用[J]. 化工学报, 2020, 71(S1): 15-22.
Jiaxin LIU, Yu XU, Er HUA. Structure and hydrogen bonding study on acylamino acid protic ionic liquids composed of 2-N-ethylhexylethylenediaminim cation with acylalanineate anions[J]. CIESC Journal, 2020, 71(S1): 15-22.

图1 在M06-2X/6-311G(d,p)水平下得到的[HEtHex]+和[Acylala]-的电势能面
Fig.1 Electrostatic potential surface of [HEtHex]+ cation and [Acylala]- anions at M06-2X/ 6-311G (d,p) level
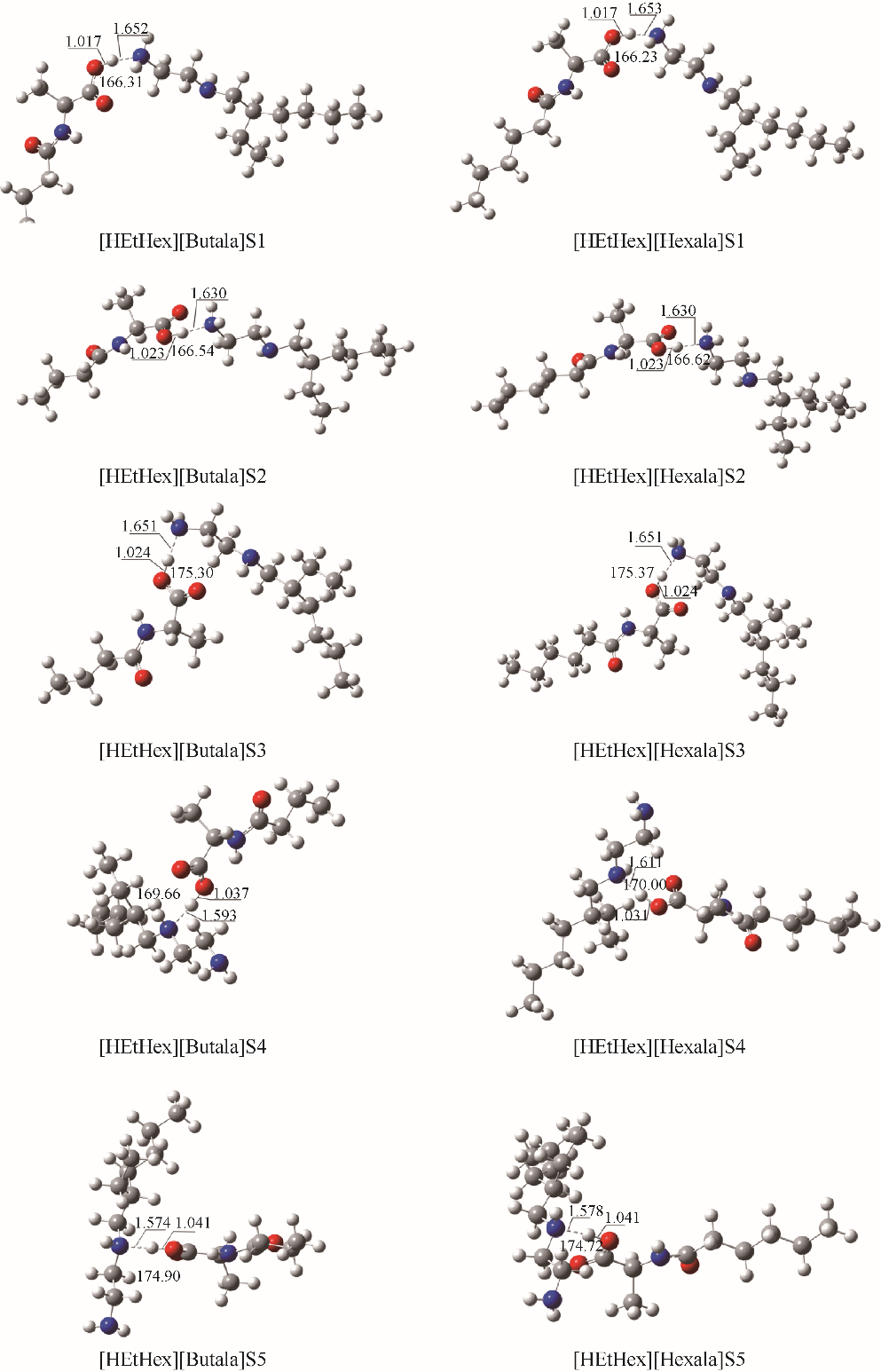
图2 在M06-2X/6-311G(d,p)水平下优化得到的[HEtHex][Acylala]分子对的较稳定构型S1-S5(图中标注了主要氢键部位的键长(?)和键角(°))
Fig.2 Optimized structures of [HEtHex][Acylala] at M06-2X/6-311G(d,p) level
| Structure | N—H bond | r/? | Δr/? |
|---|---|---|---|
| [HEtHex]1+ | N31—H33 | 1.0238 | |
| N31—H32 | 1.0245 | ||
| [HEtHex]2+ | N34—H35 | 1.0252 | |
| [HEtHex][Butala]S1 | N31—H33 | 1.6519 | 0.6281 |
| [HEtHex][Butala]S2 | N31—H33 | 1.6302 | 0.6064 |
| [HEtHex][Butala]S3 | N31—H32 | 1.6513 | 0.6268 |
| [HEtHex][Butala]S4 | N34—H35 | 1.5928 | 0.5676 |
| [HEtHex][Butala]S5 | N34—H35 | 1.5743 | 0.5491 |
| [HEtHex][Hexala]S1 | N31—H33 | 1.6528 | 0.6290 |
| [HEtHex][Hexala]S2 | N31—H33 | 1.6304 | 0.6066 |
| [HEtHex][Hexala]S3 | N31—H32 | 1.6508 | 0.6263 |
| [HEtHex][Hexala]S4 | N34—H35 | 1.6107 | 0.5855 |
| [HEtHex][Hexala]S5 | N34—H35 | 1.5775 | 0.5523 |
表1 阳离子单体及[HEtHex][Acylala]S1~S5中N—H的键长(r)及其变化值
Table 1 Bond lengths (r) for isolated cations and [HEtHex][Acylala] S1—S5 structures
| Structure | N—H bond | r/? | Δr/? |
|---|---|---|---|
| [HEtHex]1+ | N31—H33 | 1.0238 | |
| N31—H32 | 1.0245 | ||
| [HEtHex]2+ | N34—H35 | 1.0252 | |
| [HEtHex][Butala]S1 | N31—H33 | 1.6519 | 0.6281 |
| [HEtHex][Butala]S2 | N31—H33 | 1.6302 | 0.6064 |
| [HEtHex][Butala]S3 | N31—H32 | 1.6513 | 0.6268 |
| [HEtHex][Butala]S4 | N34—H35 | 1.5928 | 0.5676 |
| [HEtHex][Butala]S5 | N34—H35 | 1.5743 | 0.5491 |
| [HEtHex][Hexala]S1 | N31—H33 | 1.6528 | 0.6290 |
| [HEtHex][Hexala]S2 | N31—H33 | 1.6304 | 0.6066 |
| [HEtHex][Hexala]S3 | N31—H32 | 1.6508 | 0.6263 |
| [HEtHex][Hexala]S4 | N34—H35 | 1.6107 | 0.5855 |
| [HEtHex][Hexala]S5 | N34—H35 | 1.5775 | 0.5523 |
| Structure | ?E0/(kcal?mol-1) | ?E0BSSE/(kcal?mol-1) | Structure | ?E0/(kcal?mol-1) | ?E0BSSE/(kcal?mol-1) |
|---|---|---|---|---|---|
| [HEtHex][Butala]S1 | -133.38 | -128.68 | [HEtHex][Hexala]S1 | -133.31 | -128.63 |
| [HEtHex][Butala]S2 | -132.05 | -127.33 | [HEtHex][Hexala]S2 | -131.97 | -127.25 |
| [HEtHex][Butala]S3 | -142.15 | -136.14 | [HEtHex][Hexala]S3 | -133.11 | -127.01 |
| [HEtHex][Butala]S4 | -123.78 | -117.26 | [HEtHex][Hexala]S4 | -125.43 | -119.03 |
| [HEtHex][Butala]S5 | -124.15 | -117.66 | [HEtHex][Hexala]S5 | -124.11 | -117.57 |
表2 在M06-2X/6-311G(d,p)水平下计算得到的[HEtHex][Acylal]的相互作用能ΔE0
Table 2 Interaction energies ΔE0 for [HEtHex][Acylal] at M06-2X/6-311G(d,p) level
| Structure | ?E0/(kcal?mol-1) | ?E0BSSE/(kcal?mol-1) | Structure | ?E0/(kcal?mol-1) | ?E0BSSE/(kcal?mol-1) |
|---|---|---|---|---|---|
| [HEtHex][Butala]S1 | -133.38 | -128.68 | [HEtHex][Hexala]S1 | -133.31 | -128.63 |
| [HEtHex][Butala]S2 | -132.05 | -127.33 | [HEtHex][Hexala]S2 | -131.97 | -127.25 |
| [HEtHex][Butala]S3 | -142.15 | -136.14 | [HEtHex][Hexala]S3 | -133.11 | -127.01 |
| [HEtHex][Butala]S4 | -123.78 | -117.26 | [HEtHex][Hexala]S4 | -125.43 | -119.03 |
| [HEtHex][Butala]S5 | -124.15 | -117.66 | [HEtHex][Hexala]S5 | -124.11 | -117.57 |
| Structure | O—H bond | ν/cm-1 | Δν/cm-1 |
|---|---|---|---|
| [Butala] | O—H | 3832.81 | |
| [Hexala] | O—H | 3832.02 | |
| [HEtHex][Butala]S1 | O57—H33 | 2812.78 | 1020.03 |
| [HEtHex][Butala]S2 | O58—H33 | 2715.63 | 1117.18 |
| [HEtHex][Butala]S3 | O58—H32 | 2679.84 | 1152.97 |
| [HEtHex][Butala]S4 | O58—H35 | 2472.57 | 1360.24 |
| [HEtHex][Butala]S5 | O58—H35 | 2401.35 | 1431.46 |
| [HEtHex][Hexala]S1 | O57—H33 | 2813.06 | 1018.96 |
| [HEtHex][Hexala]S2 | O58—H33 | 2712.18 | 1119.84 |
| [HEtHex][Hexala]S3 | O58—H32 | 2686.96 | 1145.06 |
| [HEtHex][Hexala]S4 | O57—H35 | 2565.97 | 1266.05 |
| [HEtHex][Hexala]S5 | O58—H35 | 2410.52 | 1421.50 |
表3 [HEtHex][Acylala]S1~S5分子对结构中O—H的振动频率(ν)及红移值(Δν)
Table 3 O—H vibrational frequencies ν and red shift Δν for [HEtHex][Acylala]S1—S5
| Structure | O—H bond | ν/cm-1 | Δν/cm-1 |
|---|---|---|---|
| [Butala] | O—H | 3832.81 | |
| [Hexala] | O—H | 3832.02 | |
| [HEtHex][Butala]S1 | O57—H33 | 2812.78 | 1020.03 |
| [HEtHex][Butala]S2 | O58—H33 | 2715.63 | 1117.18 |
| [HEtHex][Butala]S3 | O58—H32 | 2679.84 | 1152.97 |
| [HEtHex][Butala]S4 | O58—H35 | 2472.57 | 1360.24 |
| [HEtHex][Butala]S5 | O58—H35 | 2401.35 | 1431.46 |
| [HEtHex][Hexala]S1 | O57—H33 | 2813.06 | 1018.96 |
| [HEtHex][Hexala]S2 | O58—H33 | 2712.18 | 1119.84 |
| [HEtHex][Hexala]S3 | O58—H32 | 2686.96 | 1145.06 |
| [HEtHex][Hexala]S4 | O57—H35 | 2565.97 | 1266.05 |
| [HEtHex][Hexala]S5 | O58—H35 | 2410.52 | 1421.50 |
| Bond | N31 | N34 | H32 | H33 | H35 | O57 | O58 | O59 |
|---|---|---|---|---|---|---|---|---|
| [Butala]- | -0.780 | -0.816 | -0.707 | |||||
| [Hexala]- | -0.780 | -0.816 | -0.707 | |||||
| [HEtHex]1+ | -0.690 | -0.689 | 0.437 | 0.444 | 0.358 | |||
| [HEtHex]2+ | -0.827 | -0.554 | 0.364 | 0.364 | 0.424 | |||
| [HEtHex][Butala]S1 | -0.873 | -0.687 | 0.365 | 0.514 | 0.341 | -0.734 | -0.681 | -0.66 |
| [HEtHex][Butala]S2 | -0.871 | -0.689 | 0.381 | 0.517 | 0.344 | -0.664 | -0.76 | -0.659 |
| [HEtHex][Butala]S3 | -0.869 | -0.691 | 0.517 | 0.372 | 0.365 | -0.668 | -0.767 | -0.66 |
| [HEtHex][Butala]S4 | -0.834 | -0.719 | 0.34 | 0.34 | 0.513 | -0.668 | -0.774 | -0.659 |
| [HEtHex][Butala]S5 | -0.834 | -0.708 | 0.344 | 0.34 | 0.512 | -0.663 | -0.777 | -0.659 |
| [HEtHex][Hexala]S1 | -0.873 | -0.687 | 0.365 | 0.514 | 0.341 | -0.681 | -0.734 | -0.66 |
| [HEtHex][Hexala]S2 | -0.871 | -0.689 | 0.381 | 0.517 | 0.344 | -0.664 | -0.76 | -0.659 |
| [HEtHex][Hexala]S3 | -0.869 | -0.692 | 0.517 | 0.372 | 0.368 | -0.671 | -0.765 | -0.658 |
| [HEtHex][Hexala]S4 | -0.834 | -0.719 | 0.341 | 0.34 | 0.511 | -0.746 | -0.688 | -0.661 |
| [HEtHex][Hexala]S5 | -0.834 | -0.708 | 0.344 | 0.34 | 0.512 | -0.664 | -0.776 | -0.659 |
表4 在M06-2X/6-311G(d, p)水平下NPA分析得到的[HEtHex][Acylala]主要氢键部位电荷分布
Table 4 Partial charges for main hydrogen bonding of [HEtHex][Acylala] at M06-2X/6-311G(d, p) level/e
| Bond | N31 | N34 | H32 | H33 | H35 | O57 | O58 | O59 |
|---|---|---|---|---|---|---|---|---|
| [Butala]- | -0.780 | -0.816 | -0.707 | |||||
| [Hexala]- | -0.780 | -0.816 | -0.707 | |||||
| [HEtHex]1+ | -0.690 | -0.689 | 0.437 | 0.444 | 0.358 | |||
| [HEtHex]2+ | -0.827 | -0.554 | 0.364 | 0.364 | 0.424 | |||
| [HEtHex][Butala]S1 | -0.873 | -0.687 | 0.365 | 0.514 | 0.341 | -0.734 | -0.681 | -0.66 |
| [HEtHex][Butala]S2 | -0.871 | -0.689 | 0.381 | 0.517 | 0.344 | -0.664 | -0.76 | -0.659 |
| [HEtHex][Butala]S3 | -0.869 | -0.691 | 0.517 | 0.372 | 0.365 | -0.668 | -0.767 | -0.66 |
| [HEtHex][Butala]S4 | -0.834 | -0.719 | 0.34 | 0.34 | 0.513 | -0.668 | -0.774 | -0.659 |
| [HEtHex][Butala]S5 | -0.834 | -0.708 | 0.344 | 0.34 | 0.512 | -0.663 | -0.777 | -0.659 |
| [HEtHex][Hexala]S1 | -0.873 | -0.687 | 0.365 | 0.514 | 0.341 | -0.681 | -0.734 | -0.66 |
| [HEtHex][Hexala]S2 | -0.871 | -0.689 | 0.381 | 0.517 | 0.344 | -0.664 | -0.76 | -0.659 |
| [HEtHex][Hexala]S3 | -0.869 | -0.692 | 0.517 | 0.372 | 0.368 | -0.671 | -0.765 | -0.658 |
| [HEtHex][Hexala]S4 | -0.834 | -0.719 | 0.341 | 0.34 | 0.511 | -0.746 | -0.688 | -0.661 |
| [HEtHex][Hexala]S5 | -0.834 | -0.708 | 0.344 | 0.34 | 0.512 | -0.664 | -0.776 | -0.659 |
| Structure | Charge transfer | E(2) | Structure | Charge transfer | E(2) |
|---|---|---|---|---|---|
| [HEtHex][Butala]S1 | LP(N31)→σ*(H33-O57) | 43.67 | [HEtHex][Hexala]S1 | LP(N31)→σ*(H33-O57) | 43.58 |
| [HEtHex][Butala]S2 | LP(N31)→σ*(H33-O58) | 47.74 | [HEtHex][Hexala]S2 | LP(N31)→σ*(H33-O58) | 47.72 |
| [HEtHex][Butala]S3 | LP(N31)→σ*(H32-O58) | 47.33 | [HEtHex][Hexala]S3 | LP(N31)→σ*(H32-O58) | 47.48 |
| [HEtHex][Butala]S4 | LP(N34)→σ*(H35-O58) | 54.49 | [HEtHex][Hexala]S4 | LP(N34)→σ*(H35-O57) | 50.85 |
| [HEtHex][Butala]S5 | LP(N34)→σ*(H35-O58) | 57.58 | [HEtHex][Hexala]S5 | LP(N34)→σ*(H35-O58) | 56.86 |
表5 在M06-2X/6-311G(d,p)水平下[HEtHex][Acylala]所有构型中lp(N)→σ*(H—O)轨道相互作用的稳定化能E(2)
Table 5 Second-order interaction energies E(2) between lp(N) and σ*(H—O) in all complexes [HEtHex][Acylala] calculated at M06-2X/6-311G(d,p) level/(kcal?mol-1)
| Structure | Charge transfer | E(2) | Structure | Charge transfer | E(2) |
|---|---|---|---|---|---|
| [HEtHex][Butala]S1 | LP(N31)→σ*(H33-O57) | 43.67 | [HEtHex][Hexala]S1 | LP(N31)→σ*(H33-O57) | 43.58 |
| [HEtHex][Butala]S2 | LP(N31)→σ*(H33-O58) | 47.74 | [HEtHex][Hexala]S2 | LP(N31)→σ*(H33-O58) | 47.72 |
| [HEtHex][Butala]S3 | LP(N31)→σ*(H32-O58) | 47.33 | [HEtHex][Hexala]S3 | LP(N31)→σ*(H32-O58) | 47.48 |
| [HEtHex][Butala]S4 | LP(N34)→σ*(H35-O58) | 54.49 | [HEtHex][Hexala]S4 | LP(N34)→σ*(H35-O57) | 50.85 |
| [HEtHex][Butala]S5 | LP(N34)→σ*(H35-O58) | 57.58 | [HEtHex][Hexala]S5 | LP(N34)→σ*(H35-O58) | 56.86 |
| Structure | BCP | ρc/a.u. | ?2ρc/a.u. | G(r)/a.u. | V(r)/a.u. | H(r)/a.u. | EHB/(kcal?mol-1) |
|---|---|---|---|---|---|---|---|
| [HEtHex][Butala]S1 | N31…H33—O57 | 0.061 | 0.101 | 0.043 | -0.06 | -0.017 | 18.76 |
| [HEtHex][Butala]S2 | N31…H33—O58 | 0.065 | 0.098 | 0.044 | -0.064 | -0.02 | 20.06 |
| [HEtHex][Butala]S3 | N31…H32—O58 | 0.062 | 0.095 | 0.042 | -0.06 | -0.018 | 18.69 |
| [HEtHex][Butala]S4 | N34…H35—O58 | 0.073 | 0.086 | 0.047 | -0.073 | -0.026 | 22.87 |
| [HEtHex][Butala]S5 | N34…H35—O58 | 0.076 | 0.083 | 0.049 | -0.077 | -0.028 | 24.19 |
| [HEtHex][Hexala]S1 | N31…H33—O57 | 0.061 | 0.101 | 0.042 | -0.06 | -0.017 | 18.72 |
| [HEtHex][Hexala]S2 | N31…H33—O58 | 0.065 | 0.098 | 0.044 | -0.064 | -0.02 | 20.06 |
| [HEtHex][Hexala]S3 | N31…H32—O58 | 0.062 | 0.095 | 0.042 | -0.06 | -0.018 | 18.74 |
| [HEtHex][Hexala]S4 | N34…H35—O57 | 0.070 | 0.090 | 0.046 | -0.069 | -0.023 | 21.70 |
| [HEtHex][Hexala]S5 | N34…H35—O58 | 0.076 | 0.083 | 0.049 | -0.076 | -0.028 | 23.95 |
表6 在M06-2X/6-311G(d,p)水平下计算得到的[HEtHex][Acylala]的氢键BCP的电子密度性质
Table 6 Properties of electron density of BCP for configurations of [HEtHex][Acylala] calculated at M06-2X/6-311G(d,p) level
| Structure | BCP | ρc/a.u. | ?2ρc/a.u. | G(r)/a.u. | V(r)/a.u. | H(r)/a.u. | EHB/(kcal?mol-1) |
|---|---|---|---|---|---|---|---|
| [HEtHex][Butala]S1 | N31…H33—O57 | 0.061 | 0.101 | 0.043 | -0.06 | -0.017 | 18.76 |
| [HEtHex][Butala]S2 | N31…H33—O58 | 0.065 | 0.098 | 0.044 | -0.064 | -0.02 | 20.06 |
| [HEtHex][Butala]S3 | N31…H32—O58 | 0.062 | 0.095 | 0.042 | -0.06 | -0.018 | 18.69 |
| [HEtHex][Butala]S4 | N34…H35—O58 | 0.073 | 0.086 | 0.047 | -0.073 | -0.026 | 22.87 |
| [HEtHex][Butala]S5 | N34…H35—O58 | 0.076 | 0.083 | 0.049 | -0.077 | -0.028 | 24.19 |
| [HEtHex][Hexala]S1 | N31…H33—O57 | 0.061 | 0.101 | 0.042 | -0.06 | -0.017 | 18.72 |
| [HEtHex][Hexala]S2 | N31…H33—O58 | 0.065 | 0.098 | 0.044 | -0.064 | -0.02 | 20.06 |
| [HEtHex][Hexala]S3 | N31…H32—O58 | 0.062 | 0.095 | 0.042 | -0.06 | -0.018 | 18.74 |
| [HEtHex][Hexala]S4 | N34…H35—O57 | 0.070 | 0.090 | 0.046 | -0.069 | -0.023 | 21.70 |
| [HEtHex][Hexala]S5 | N34…H35—O58 | 0.076 | 0.083 | 0.049 | -0.076 | -0.028 | 23.95 |
| Bond | H???Y/ ? | X—H???Y/ ? | Bond angle/(°) | IR shift | EHB/ (kcal?mol-1) | Density ρc/a.u. | E(2)/ (kcal?mol-1) |
|---|---|---|---|---|---|---|---|
| strong H-bond | 1.2—1.5 | 2.2—2.5 | 170—180 | ? 25% | 15—40 | ? 0.05 | ? 36 |
| H-bond for [HEtHex][Acylala] | 1.57—1.65 | 2.62—2.68 | 166—175 | 27%—37% | 18.69—24.19 | 0.061—0.076 | 43.58—57.58 |
表7 文献值中较强氢键的指标[29,30]及本研究中[HEtHex][Acylala]分子间氢键的特征值
Table 7 Criteria for strong H-bonds[29,30] and corresponding values for [HEtHex][Acylala]
| Bond | H???Y/ ? | X—H???Y/ ? | Bond angle/(°) | IR shift | EHB/ (kcal?mol-1) | Density ρc/a.u. | E(2)/ (kcal?mol-1) |
|---|---|---|---|---|---|---|---|
| strong H-bond | 1.2—1.5 | 2.2—2.5 | 170—180 | ? 25% | 15—40 | ? 0.05 | ? 36 |
| H-bond for [HEtHex][Acylala] | 1.57—1.65 | 2.62—2.68 | 166—175 | 27%—37% | 18.69—24.19 | 0.061—0.076 | 43.58—57.58 |
| 1 | Wilkes J S. A short history of ionic liquids-from molten salts to neoteric solvents[J]. Green Chemistry, 2002, 4(2): 73-80. |
| 2 | Pillai P, Pal N, Mandal A. Synthesis, characterization, surface properties and micellization behaviour of imidazolium-based ionic liquids[J]. Journal of Surfactants and Detergents, 2017, 20: 1321-1335. |
| 3 | Wishart J F, Castner J E W. The physical chemistry of ionic liquids[J]. Phys. Chem. B, 2007, 111: 201-208. |
| 4 | Egorova K S, Gordeev E G, Ananikov V P. Biological activity of ionic liquids and their application in pharmaceutics and medicine[J]. Chem. Rev., 2017, 117(10): 7132-7189. |
| 5 | Liaw H J, Chen C C, Chen Y C, et al. Relationship between flash point of ionic liquids and their thermal decomposition[J]. Green Chem., 2012, 14: 2001-2008. |
| 6 | Greaves T L, Drummond C J. Protic ionic liquids: properties and applications[J]. Chemical Reviews, 2008, 108(1): 206-237. |
| 7 | 邹卫红, 张颖, 阎子峰. 离子液体中电沉积法制备不同形貌材料的研究进展[J]. 石油化工, 2018, 47(10): 1149-1157. |
| Zou W H, Zhang Y, Yan Z F. Progress in preparation of different morphological materials by electrodeposition in ionic liquids [J]. Petrochemical Technology, 2018, 47(10): 1149-1157. | |
| 8 | Ohno H, Fukumoto K. Amino acid ionic liquids[J]. Acc. Chem. Res., 2007, 40: 1122-1129. |
| 9 | Takemura S, Kawakami S, Harada M, et al. Solvation structure of a copper (Ⅱ) ion in protic ionic liquids comprising N-hexylethylenediamine[J]. Inorg. Chem., 2014, 53: 9667-9678. |
| 10 | Nakayama C, Harada M, Iida M. Properties of protic ionic liquids comprised of N-alkyldiethylenetriamine and their complexation of copper (Ⅱ) ions[J]. Eur. J. Inorg. Chem., 2017, 31: 3744-3745. |
| 11 | Iida M, Baba C, Inoue M, et al. Ionic liquids of bis(alkylethylenediamine) silver (I) salts and the formation of silver (0) nanoparticles from the ionic liquid system[J]. Chem.Eur. J., 2008, 14: 5047-5056. |
| 12 | Zhang J M, Zhang S J, Dong K, et al. Supported absorption of CO2 by tetrabutylphosphonium amino acid ionic liquids[J]. Chem. Eur. J., 2006, 12: 4021-4026. |
| 13 | Gao H Y, Zhang Y, Wang H J, et al. Theoretical study on the structure and cation-anion interaction of amino acid cation based amino acid ionic liquid [Pro]+[NO3]-[J]. J. Phys. Chem. A, 2010, 114: 10243-10252. |
| 14 | Chen X, Zhang Y, Yu F, et al. DFT calculations on hydrogen-bonded complexes formed between guanine and acrylamide[J]. J. Solution. Chem., 2010, 39: 1341-1349. |
| 15 | Wu L, Li Q, Wang F, et al. Advances in quantitative calculation and molecular dynamics simulation of ionic liquids[J]. Journal of Molecular Catalysis, 2012, 26(5): 456-468. |
| 16 | Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1): 215-241. |
| 17 | Bsder R F W. AIM2000Program Package, Ver. 2.0[M]. Hamilton, Ontario, Canada: McMaster University, 2002. |
| 18 | Frisch M J, Trucks G W, Schlegel H B, et al. Gaussian 09[M]. Wallingford CT: Gaussian Inc., 2013. |
| 19 | Roohi H, Ghauri K. Exploring physicochemical properties of the nanostructured tunable aryl alkyl ionic liquids (TAAILs)[J]. Journal of Molecular Liquids, 2015, 209: 14-24. |
| 20 | Boys SF, Bernardi F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors[J]. Molecular Physics, 1970, 19(4): 553-566. |
| 21 | Li X H, Yin G X, Zhang X Z. Natural bond orbital (NBO) population analysis of some benzyl nitrites[J]. J. Molstruc.-Tolstruc. -Theochem., 2010, 957: 61-65. |
| 22 | Lu R, Wu C, Lin J, et al.Theoretical study on interactions between trifluoromethanesulfonate (triflate) based ionic liquid and thiophene[J]. Journal of Molecular Liquids, 2017, 237: 289‒394 |
| 23 | Bader R F W. Atom in Molecules: A Quantum Theory[M]. New York: Oxford University Press, 1990. |
| 24 | 徐宇, 花儿.烷基乙二胺‒CF3CO2型质子化离子液体的分子间氢键作用[J].高等学校化学学报, 2018, 39(9): 1954‒1960. |
| Xu Y, Hua E. Hydrogen bonding study on protic ionic liquids composed of N-alkyl ethylenediaminum cations with trifluoroacetic anion[J].Chemical Journal of Chinese Universities, 2018, 39(9): 1954‒1960. | |
| 25 | Raamat E, Kaupmees K, Ovsjannikov G, et al. Acidities of strong neutral Brønsted acids in different media[J]. J. Phys. Org. Chem., 2013, (26): 162-170. |
| 26 | Trummal A, Lipping L, Kaljurand I, et al. Acidity of strong acids in water and dimethyl sulfoxide[J]. J. Phys. Chem. A, 2016, (120): 3663-3669. |
| 27 | 张营. 氨基酸离子液体的结构和阴阳离子间相互作用的理论研究[D]. 无锡: 江南大学, 2011. |
| Zhang Y. Theoretical study on the structure and cation-anion interaction of amino acid based ionic liquids[D]. Wuxi: Jiangnan University, 2011. | |
| 28 | Steiner T.The hydrogen bond in the solid state[J]. Angewandte Chemie International Edition, 2002, 41: 48-76. |
| 29 | Hunt P A, Ashworth C R, Matthews R P. Hydrogen bonding in ionic liquids[J]. Chem. Soc. Rev., 2015, 44: 1257-1288. |
| 30 | Grabowski S J. What is the covalency of hydrogen bonding[J]. Chem. Rev., 2011, 111: 2597-2625. |
| [1] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [2] | 杜峰, 尹思琦, 罗辉, 邓文安, 李传, 黄振薇, 王文静. H2在Mo x S y 团簇上吸附解离的尺寸效应研究[J]. 化工学报, 2022, 73(9): 3895-3903. |
| [3] | 俞夏琪, 冯格, 赵金燕, 李嘉远, 邓声威, 郑靖楠, 李雯雯, 王亚秋, 沈榄, 刘旭, 徐威威, 王建国, 王式彬, 姚子豪, 毛成立. 基体(TDI-TMP-T313)与氧化剂(AP)相互作用的第一性原理研究[J]. 化工学报, 2022, 73(8): 3511-3517. |
| [4] | 赵继昊, 唐伟强, 徐小飞, 赵双良, 贺炅皓. 高分子复合材料中键合剂在不同纳米填料表面的吸附能计算[J]. 化工学报, 2022, 73(7): 3174-3181. |
| [5] | 罗小松, 黄金保, 周梅, 牟鑫, 徐伟伟, 吴雷. 对苯二甲酸丁二醇酯二聚体水/醇/氨解机理的理论研究[J]. 化工学报, 2022, 73(11): 4859-4871. |
| [6] | 朱先会, 王甫, 夏杰成, 袁金良. 功能型离子液体协同吸收NH3和CO2的密度泛函理论研究[J]. 化工学报, 2022, 73(10): 4324-4334. |
| [7] | 龚翔, 李林森, 姜召. PdCo/SiO2双金属催化剂用于杂环储氢载体的高效脱氢[J]. 化工学报, 2022, 73(10): 4448-4460. |
| [8] | 马生贵, 田博文, 周雨薇, 陈琳, 江霞, 高涛. 氮掺杂Stone-Wales缺陷石墨烯吸附H2S的密度泛函理论研究[J]. 化工学报, 2021, 72(9): 4496-4503. |
| [9] | 张芳芳, 韩敏, 赵娟, 凌丽霞, 章日光, 王宝俊. 单空缺石墨烯负载的Pd单原子催化剂上NO还原的密度泛函理论研究[J]. 化工学报, 2021, 72(3): 1382-1391. |
| [10] | 唐伟强, 谢鹏, 徐小飞, 赵双良. 反应密度泛函理论的构建与初步应用[J]. 化工学报, 2021, 72(2): 633-652. |
| [11] | 葛冰青, 阴义轩, 王亚溪, 张宏伟, 袁珮. 溶剂对丁腈橡胶溶解、尺寸、结构和催化加氢的影响研究[J]. 化工学报, 2021, 72(1): 543-554. |
| [12] | 狄玲, 陈放, 付荣荣, 杨辰, 邢杨, 王晓宁. 富电子LMOF对有机农药的检测机理研究[J]. 化工学报, 2020, 71(8): 3830-3838. |
| [13] | 孙巍, 左然. MMAl在NH2与H混合覆盖的AlN(0001)-Al表面的吸附与扩散研究[J]. 化工学报, 2020, 71(7): 3213-3219. |
| [14] | 张红, 唐留. p型掺杂剂Cp2Mg在MOCVD气相中的反应机理研究[J]. 化工学报, 2020, 71(7): 3000-3008. |
| [15] | 宫梦, 方阳, 陈伟, 陈应泉, 陆强, 杨海平, 陈汉平. 纤维素组分对氨基酸热解的影响[J]. 化工学报, 2020, 71(5): 2312-2319. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号