化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4607-4615.DOI: 10.11949/0438-1157.20210188
收稿日期:2021-01-31
修回日期:2021-06-07
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
张早校
作者简介:刘洋(1994—),男,博士研究生,基金资助:
Yang LIU( ),Iqra AYUB,Fusheng YANG,Zhen WU,Zaoxiao ZHANG(
),Iqra AYUB,Fusheng YANG,Zhen WU,Zaoxiao ZHANG( )
)
Received:2021-01-31
Revised:2021-06-07
Online:2021-09-05
Published:2021-09-05
Contact:
Zaoxiao ZHANG
摘要:
目前大多数可再生能源如太阳能具有间歇性和不稳定性的问题,因此高效蓄热技术成为了发展太阳能的一个关键途径。金属氢化物高温蓄热技术作为热化学蓄热中最有前途的方法之一,受到了人们的广泛关注。为了实现金属氢化物高温蓄热技术的工程应用,明确其氢热耦合传递机理至关重要。本研究采用数值模拟的方法,通过建立反应器的多物理场耦合模型,讨论了不同时刻下床层内部参数的分布,得到了反应锋面的形成和移动机理以及非均匀反应的形成机理;此外,结合反应器内部氢压、接触热阻和床层热阻的变化规律,明确了不同阶段下金属氢化物高温蓄热技术的控制环节;最后,依据金属氢化物高温蓄热技术的工程应用挑战,提出了相应的研究策略。
中图分类号:
刘洋, AYUB Iqra, 杨福胜, 吴震, 张早校. 基于金属氢化物高温蓄热的氢热耦合传递机理[J]. 化工学报, 2021, 72(9): 4607-4615.
Yang LIU, Iqra AYUB, Fusheng YANG, Zhen WU, Zaoxiao ZHANG. Hydrogen thermal coupling transfer mechanism based on metal hydride high temperature heat storage technology[J]. CIESC Journal, 2021, 72(9): 4607-4615.
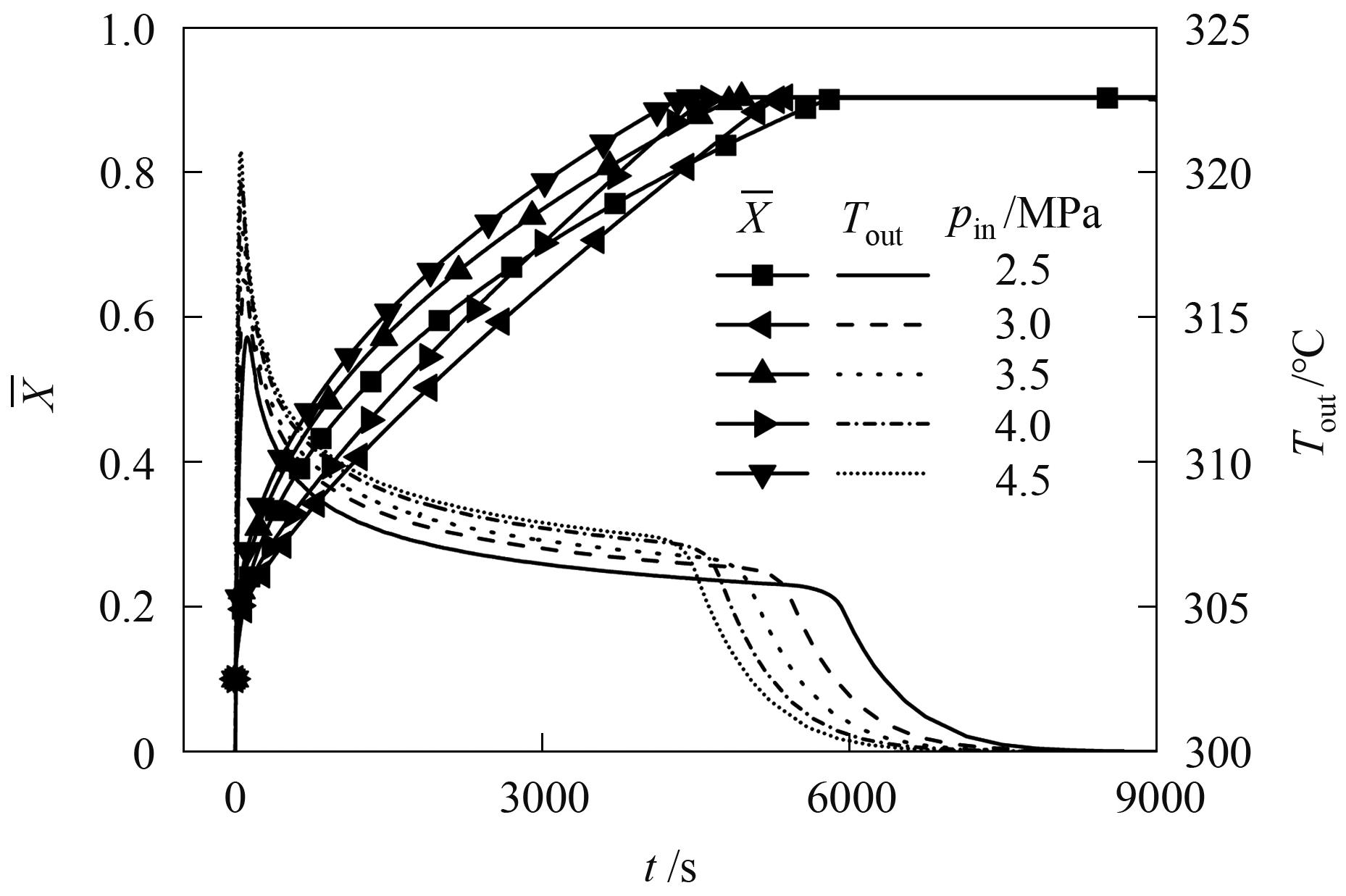
图9 不同压力下床层平均反应分数和换热流体输出温度随时间的变化曲线
Fig.9 Variation curves of average bed reaction fraction and heat transfer fluid output temperature with time under different pressures
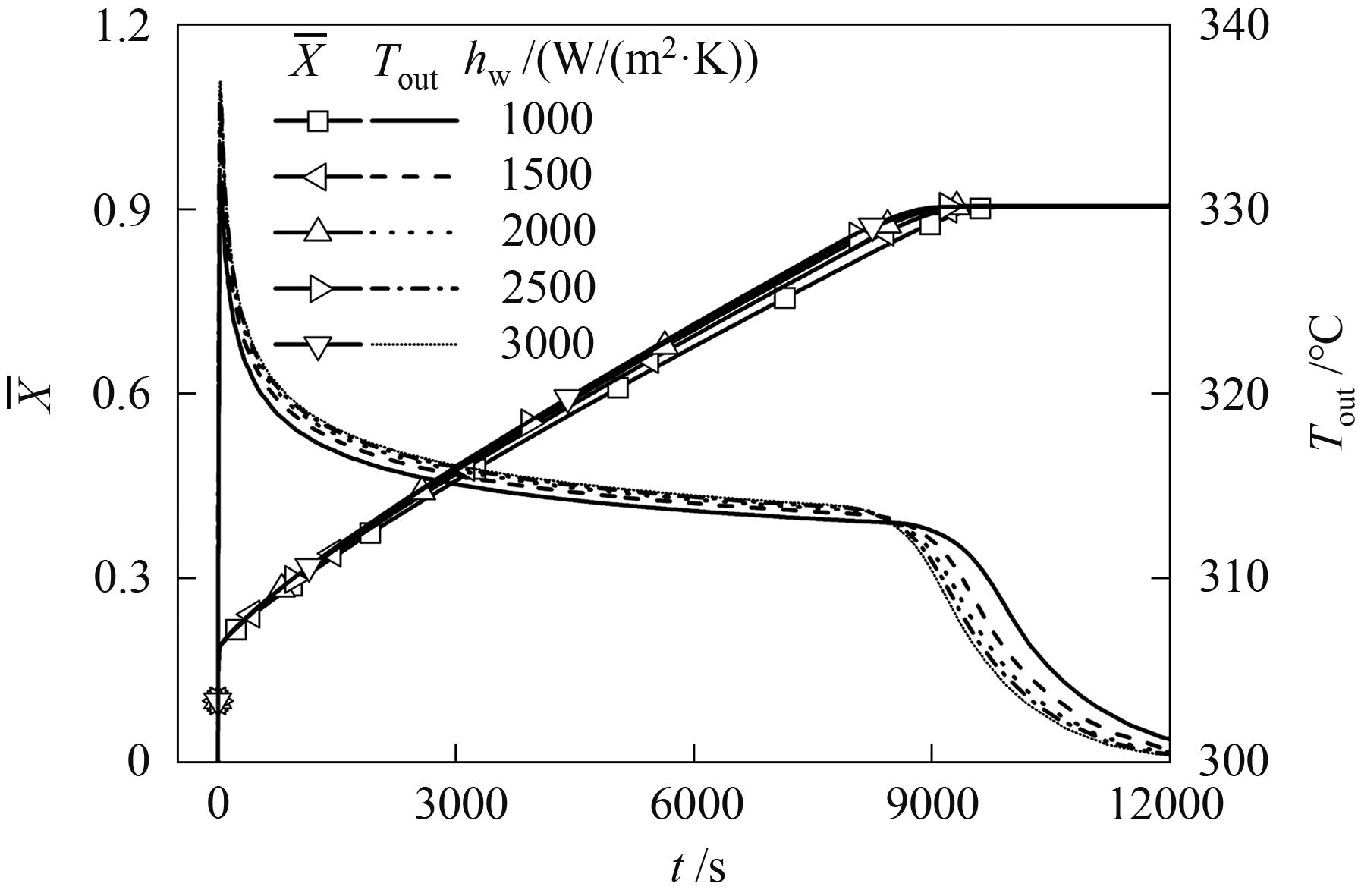
图11 不同壁面传热系数下床层平均反应分数和换热流体输出温度随时间的变化
Fig.11 Variation curves of average bed reaction fraction and heat transfer fluid output temperature with time under different wall heat transfer coefficients
| 壁面传热系数/ (W/(m2·K)) | 峰值点对应 时间/s | 峰值点对应 温度/℃ | 反应锋面形成 时间/s | 反应锋面形成 温度/℃ | 初始下降 斜率/(℃/s) | 有效输出 温度/℃ |
|---|---|---|---|---|---|---|
| 1000 | 34.60 | 331.27 | 91.66 | 327.65 | 0.063 | 313.23 |
| 1500 | 34.60 | 333.87 | 84.71 | 329.84 | 0.080 | 313.67 |
| 2000 | 32.06 | 335.32 | 80.23 | 331.19 | 0.086 | 313.96 |
| 2500 | 32.06 | 336.26 | 76.31 | 332.59 | 0.088 | 314.03 |
| 3000 | 32.06 | 336.91 | 71.35 | 333.11 | 0.097 | 314.14 |
表1 不同壁面传热系数下峰值点参数、反应锋面参数和输出温度
Table 1 Peak point, reaction front parameters and output temperature under different wall heat transfer coefficients
| 壁面传热系数/ (W/(m2·K)) | 峰值点对应 时间/s | 峰值点对应 温度/℃ | 反应锋面形成 时间/s | 反应锋面形成 温度/℃ | 初始下降 斜率/(℃/s) | 有效输出 温度/℃ |
|---|---|---|---|---|---|---|
| 1000 | 34.60 | 331.27 | 91.66 | 327.65 | 0.063 | 313.23 |
| 1500 | 34.60 | 333.87 | 84.71 | 329.84 | 0.080 | 313.67 |
| 2000 | 32.06 | 335.32 | 80.23 | 331.19 | 0.086 | 313.96 |
| 2500 | 32.06 | 336.26 | 76.31 | 332.59 | 0.088 | 314.03 |
| 3000 | 32.06 | 336.91 | 71.35 | 333.11 | 0.097 | 314.14 |

图12 不同床层热导率下床层平均反应分数和换热流体输出温度随时间的变化
Fig.12 Variation curves of average bed reaction fraction and heat transfer fluid output temperature with time under different bed thermal conductivity
| 床层热导率/ (W/(m·K)) | 峰值点对应 时间/s | 峰值点对应 温度/℃ | 有效输出 温度/℃ | 平台末端 时间/s | 反应完成 时间/s | 300℃对应 时间/s | 余热散热 时间/s | 散热段斜率/ (℃/s) |
|---|---|---|---|---|---|---|---|---|
| 1.0 | 32.06 | 336.91 | 314.14 | 8113.33 | 8901.81 | 11204.18 | 2302.37 | 0.0061 |
| 1.5 | 32.06 | 338.56 | 317.92 | 6156.81 | 7073.56 | 9174.89 | 2101.33 | 0.0085 |
| 2.0 | 34.60 | 339.56 | 322.50 | 5311.92 | 6175.61 | 8040.73 | 1865.12 | 0.0121 |
| 2.5 | 33.64 | 340.24 | 325.43 | 4912.98 | 5672.79 | 7306.30 | 1633.51 | 0.0156 |
| 3.0 | 34.78 | 340.73 | 327.11 | 4201.35 | 5324.55 | 6869.36 | 1544.81 | 0.0175 |
表2 不同床层热导率下峰值点参数、反应器输出参数
Table 2 Peak point and reactor output parameters under different bed thermal conductivity
| 床层热导率/ (W/(m·K)) | 峰值点对应 时间/s | 峰值点对应 温度/℃ | 有效输出 温度/℃ | 平台末端 时间/s | 反应完成 时间/s | 300℃对应 时间/s | 余热散热 时间/s | 散热段斜率/ (℃/s) |
|---|---|---|---|---|---|---|---|---|
| 1.0 | 32.06 | 336.91 | 314.14 | 8113.33 | 8901.81 | 11204.18 | 2302.37 | 0.0061 |
| 1.5 | 32.06 | 338.56 | 317.92 | 6156.81 | 7073.56 | 9174.89 | 2101.33 | 0.0085 |
| 2.0 | 34.60 | 339.56 | 322.50 | 5311.92 | 6175.61 | 8040.73 | 1865.12 | 0.0121 |
| 2.5 | 33.64 | 340.24 | 325.43 | 4912.98 | 5672.79 | 7306.30 | 1633.51 | 0.0156 |
| 3.0 | 34.78 | 340.73 | 327.11 | 4201.35 | 5324.55 | 6869.36 | 1544.81 | 0.0175 |
| 1 | Harries D N, Paskevicius M, Sheppard D A, et al. Concentrating solar thermal heat storage using metal hydrides[J]. Proceedings of the IEEE, 2012, 100(2): 539-549. |
| 2 | 纪军, 何雅玲. 太阳能热发电系统基础理论与关键技术战略研究[J]. 中国科学基金, 2009, 23(6): 331-336. |
| Ji J, He Y L. Strategic research on basic theory and key technology of solar thermal power generation system[J]. Bulletin of National Natural Science Foundation of China, 2009, 23(6): 331-336. | |
| 3 | Gur I, Sawyer K, Prasher R. Searching for a better thermal battery[J]. Science, 2012, 335(6075): 1454-1455. |
| 4 | 汉京晓, 杨勇平, 侯宏娟. 太阳能热发电的显热蓄热技术进展[J]. 可再生能源, 2014, 32(7): 901-905. |
| Han J X, Yang Y P, Hou H J. Review on sensible heat thermal energy storage in solar thermal generation[J]. Renewable Energy Resources, 2014, 32(7): 901-905. | |
| 5 | Koçak B, Fernandez A I, Paksoy H. Review on sensible thermal energy storage for industrial solar applications and sustainability aspects[J]. Solar Energy, 2020, 209: 135-169. |
| 6 | 白志蕊, 徐洪涛, 屈治国, 等. 相变套管式储热系统放冷性能实验研究[J]. 化工学报, 2020, 71(4): 1580-1587. |
| Bai Z R, Xu H T, Qu Z G, et al. Experimental study of phase change sleeve tube thermal storage system performance during charging[J]. CIESC Journal, 2020, 71(4): 1580-1587. | |
| 7 | Lin Y X, Alva G, Fang G Y. Review on thermal performances and applications of thermal energy storage systems with inorganic phase change materials[J]. Energy, 2018, 165: 685-708. |
| 8 | 鲍泽威, 吴震, Nyallang Nyamsi Serge, 等. 金属氢化物高温蓄热技术的研究进展[J]. 化工进展, 2012, 31(8): 1665-1670, 1676. |
| Bao Z W, Wu Z, Serge N, et al. Progress of high temperature heat storage technology using metal hydrides[J]. Chemical Industry and Engineering Progress, 2012, 31(8): 1665-1670, 1676. | |
| 9 | 闫霆, 王如竹, 李廷贤. 热化学复合吸附储热循环的理论及实验[J]. 化工学报, 2016, 67: 311-317. |
| Yan T, Wang R Z, Li T X. Theoretical analysis and experiment of thermochemical composite sorption heat storage cycle[J]. CIESC Journal, 2016, 67: 311-317. | |
| 10 | Wu S K, Zhou C, Doroodchi E, et al. A review on high-temperature thermochemical energy storage based on metal oxides redox cycle[J]. Energy Conversion and Management, 2018, 168: 421-453. |
| 11 | Ward P A, Corgnale C, Teprovich J A, et al. High performance metal hydride based thermal energy storage systems for concentrating solar power applications[J]. Journal of Alloys and Compounds, 2015, 645: S374-S378. |
| 12 | Corgnale C, Hardy B, Motyka T, et al. Screening analysis of metal hydride based thermal energy storage systems for concentrating solar power plants[J]. Renewable and Sustainable Energy Reviews, 2014, 38: 821-833. |
| 13 | Sheppard D A, Buckley C E. The potential of metal hydrides paired with compressed hydrogen as thermal energy storage for concentrating solar power plants[J]. International Journal of Hydrogen Energy, 2019, 44(18): 9143-9163. |
| 14 | Urbanczyk R, Meggouh M, Moury R, et al. Demonstration of Mg2FeH6 as heat storage material at temperatures up to 550℃[J]. Applied Physics A, 2016, 122(4): 1-5. |
| 15 | Poupin L, Humphries T D, Paskevicius M, et al. An experimental high temperature thermal battery coupled to a low temperature metal hydride for solar thermal energy storage[J]. Sustainable Energy & Fuels, 2020, 4(1): 285-292. |
| 16 | Humphries T D, Sheppard D A, Li G Q, et al. Complex hydrides as thermal energy storage materials: characterisation and thermal decomposition of Na2Mg2NiH6[J]. Journal of Materials Chemistry A, 2018, 6(19): 9099-9108. |
| 17 | Wang D, Wang Y Q, Huang Z N, et al. Design optimization and sensitivity analysis of the radiation mini-channel metal hydride reactor[J]. Energy, 2019, 173: 443-456. |
| 18 | Keshari V, Maiya M P. Design and investigation of hydriding alloy based hydrogen storage reactor integrated with a pin fin tube heat exchanger[J]. International Journal of Hydrogen Energy, 2018, 43(14): 7081-7095. |
| 19 | Ayub I, Nasir M S, Liu Y, et al. Numerical modeling and performance comparison of high-temperature metal hydride reactor equipped with bakery system for solar thermal energy storage[J]. International Journal of Hydrogen Energy, 2020, 45(56): 31612-31631. |
| 20 | Tong L, Xiao J S, Yang T Q, et al. Complete and reduced models for metal hydride reactor with coiled-tube heat exchanger[J]. International Journal of Hydrogen Energy, 2019, 44(30): 15907-15916. |
| 21 | Bao Z W, Yan D, Zhu Z Z, et al. Performance investigation of metal hydride reactors adopting multilayer bed with graded content of expanded natural graphite for thermochemical heat storage[J]. Applied Thermal Engineering, 2021, 188: 116602. |
| 22 | Nyamsi S N, Lototskyy M, Tolj I. Selection of metal hydrides-based thermal energy storage: energy storage efficiency and density targets[J]. International Journal of Hydrogen Energy, 2018, 43(50): 22568-22583. |
| 23 | Jenne S P, Jana S, Palanisamy M. Thermal and compressor-driven metal hydride based coupled system for thermal storage, cooling and thermal upgradation[J]. Thermal Science and Engineering Progress, 2021, 21: 100800. |
| 24 | d'Entremont A, Corgnale C, Hardy B, et al. Simulation of high temperature thermal energy storage system based on coupled metal hydrides for solar driven steam power plants[J]. International Journal of Hydrogen Energy, 2018, 43(2): 817-830. |
| 25 | Hahne E, Kallweit J. Thermal conductivity of metal hydride materials for storage of hydrogen: experimental investigation[J]. International Journal of Hydrogen Energy, 1998, 23(2): 107-114. |
| 26 | Sun D W, Deng S J. A theoretical model predicting the effective thermal conductivity in powdered metal hydride beds[J]. International Journal of Hydrogen Energy, 1990, 15(5): 331-336. |
| 27 | Matsushita M, Monde M, Mitsutake Y. Predictive calculation of the effective thermal conductivity in a metal hydride packed bed[J]. International Journal of Hydrogen Energy, 2014, 39(18): 9718-9725. |
| 28 | Ishido Y, Kawamura M, Ono S. Thermal conductivity of magnesium-nickel hydride powder beds in a hydrogen atmosphere[J]. International Journal of Hydrogen Energy, 1982, 7(2): 173-182. |
| 29 | 顾清之, 赵长颖. 镁-氢化镁热化学蓄热系统数值分析[J]. 化工学报, 2012, 63(12): 3776-3783. |
| Gu Q Z, Zhao C Y. Numerical study on Mg/MgH2 thermochemical heat storage system[J]. CIESC Journal, 2012, 63(12): 3776-3783. | |
| 30 | Shen D, Zhao C Y. Thermal analysis of exothermic process in a magnesium hydride reactor with porous metals[J]. Chemical Engineering Science, 2013, 98: 273-281. |
| 31 | Malleswararao K N A, Srinivasa Murthy S, et al. Performance prediction of a coupled metal hydride based thermal energy storage system[J]. International Journal of Hydrogen Energy, 2020, 45(32): 16239-16253. |
| 32 | Chaise A, de Rango P, Marty P, et al. Experimental and numerical study of a magnesium hydride tank[J]. International Journal of Hydrogen Energy, 2010, 35(12): 6311-6322. |
| 33 | Pons M, Dantzer P. Determination of thermal conductivity and wall heat transfer coefficient of hydrogen storage materials[J]. International Journal of Hydrogen Energy, 1994, 19(7): 611-616. |
| [1] | 叶展羽, 山訸, 徐震原. 用于太阳能蒸发的折纸式蒸发器性能仿真[J]. 化工学报, 2023, 74(S1): 132-140. |
| [2] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [3] | 王志国, 薛孟, 董芋双, 张田震, 秦晓凯, 韩强. 基于裂隙粗糙性表征方法的地热岩体热流耦合数值模拟与分析[J]. 化工学报, 2023, 74(S1): 223-234. |
| [4] | 宋嘉豪, 王文. 斯特林发动机与高温热管耦合运行特性研究[J]. 化工学报, 2023, 74(S1): 287-294. |
| [5] | 张思雨, 殷勇高, 贾鹏琦, 叶威. 双U型地埋管群跨季节蓄热特性研究[J]. 化工学报, 2023, 74(S1): 295-301. |
| [6] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [7] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [8] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [9] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [10] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [11] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [12] | 程小松, 殷勇高, 车春文. 不同工质在溶液除湿真空再生系统中的性能对比[J]. 化工学报, 2023, 74(8): 3494-3501. |
| [13] | 刘文竹, 云和明, 王宝雪, 胡明哲, 仲崇龙. 基于场协同和 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. |
| [14] | 洪瑞, 袁宝强, 杜文静. 垂直上升管内超临界二氧化碳传热恶化机理分析[J]. 化工学报, 2023, 74(8): 3309-3319. |
| [15] | 黄可欣, 李彤, 李桉琦, 林梅. 加装旋转叶轮T型通道流场的模态分解[J]. 化工学报, 2023, 74(7): 2848-2857. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号