化工学报 ›› 2021, Vol. 72 ›› Issue (7): 3466-3477.DOI: 10.11949/0438-1157.20210062
王宗旭1,2( ),李紫欣1,2,白璐1,董海峰1,3,张香平1,2,3(
),李紫欣1,2,白璐1,董海峰1,3,张香平1,2,3( )
)
收稿日期:2021-01-11
修回日期:2021-05-04
出版日期:2021-07-05
发布日期:2021-07-05
通讯作者:
张香平
作者简介:王宗旭(1990—),男,博士研究生,基金资助:
WANG Zongxu1,2( ),LI Zixin1,2,BAI Lu1,DONG Haifeng1,3,ZHANG Xiangping1,2,3(
),LI Zixin1,2,BAI Lu1,DONG Haifeng1,3,ZHANG Xiangping1,2,3( )
)
Received:2021-01-11
Revised:2021-05-04
Online:2021-07-05
Published:2021-07-05
Contact:
ZHANG Xiangping
摘要:
固/液界面上形成界面纳米气泡(SNBs),广泛存在于电催化、流体输送、矿物浮选等领域中,并影响各个过程的效率,因此明确其形成及稳定机理对过程调控具有重要意义。首先从实验观察和模拟计算两个角度,对纳米气泡的研究方法进行探讨,综述了不同气体类型、固体界面性质、液相添加剂下纳米气泡的形成规律。由于目前纳米气泡形成后的稳定性尚不十分明确,主要总结了现阶段广为接受的接触线钉扎稳定机制,并分析了该领域的研究现状。此外,考虑到离子液体作为重要的化工溶剂,概述了该体系中微纳气泡的相关研究。最后简要对未来工作进行了展望,以期为离子液体体系中纳米气泡的研究提供新思路。
中图分类号:
王宗旭,李紫欣,白璐,董海峰,张香平. 固/液界面纳米气泡形成及稳定性研究进展[J]. 化工学报, 2021, 72(7): 3466-3477.
WANG Zongxu,LI Zixin,BAI Lu,DONG Haifeng,ZHANG Xiangping. Formation and stability of nanobubble at solid/liquid interface[J]. CIESC Journal, 2021, 72(7): 3466-3477.
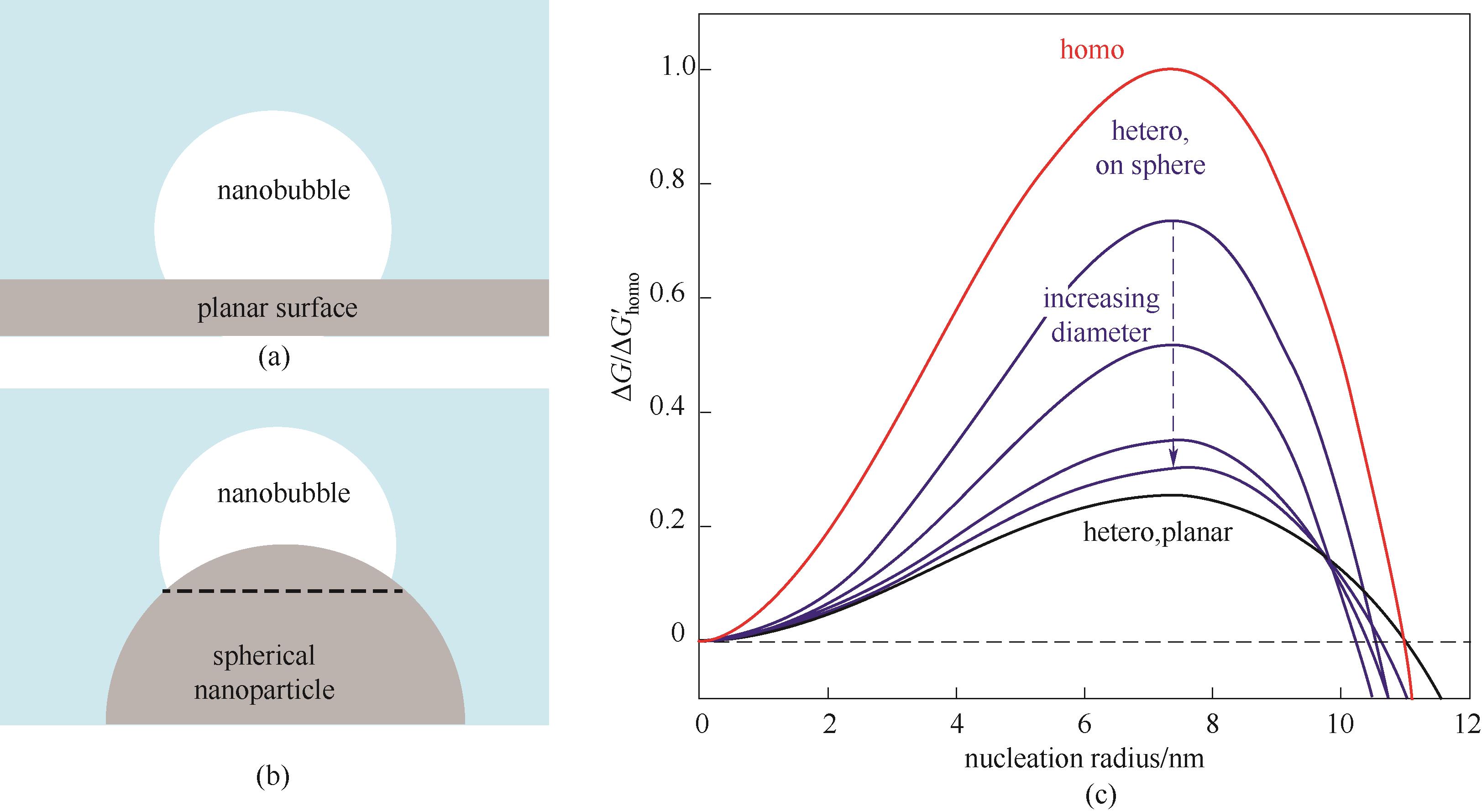
图2 液相中纳米气泡成核类型及能量图示[64](a)平面上的非均质成核;(b)球形纳米颗粒上的非均质成核;(c)均质成核(红色线)、平面上的非均质成核(黑色线)、球形纳米颗粒上的非均质成核(蓝色线)
Fig.2 Nucleation type and energy diagram of nanobubbles in liquid phase[64](a) Heterogeneous nucleation on the plane; (b) Heterogeneous nucleation on spherical nanoparticles; (c) Homogeneous nucleation (red line), heterogeneous nucleation on the plane (black line), heterogeneous nucleation on spherical nanoparticles (blue line)
| 1 | Zhang X, Winnik F M. Preface to the nanobubbles special issue[J]. Langmuir, 2016, 32(43): 11071. |
| 2 | 张雪花, 胡钧. 固液界面纳米气泡的研究进展[J]. 化学进展, 2004, 16(5): 673-681. |
| Zhang X H, Hu J. Nanobubbles at the solid/water interface[J]. Progress in Chemistry, 2004, 16(5): 673-681. | |
| 3 | Attard P, Moody M P, Tyrrell J W G. Nanobubbles: the big picture[J]. Physica A: Statistical Mechanics and Its Applications, 2002, 314(1/2/3/4): 696-705. |
| 4 | Chan C U, Ohl C D. Total-internal-reflection-fluorescence microscopy for the study of nanobubble dynamics[J]. Physical Review Letters, 2012, 109(17): 174501. |
| 5 | Alheshibri M, Qian J, Jehannin M, et al. A history of nanobubbles[J]. Langmuir, 2016, 32(43): 11086-11100. |
| 6 | Zou J, Zhang H, Guo Z, et al. Surface nanobubbles nucleate liquid boiling[J]. Langmuir, 2018, 34(46): 14096-14101. |
| 7 | Ke S, Xiao W, Quan N N, et al. Formation and stability of bulk nanobubbles in different solutions[J]. Langmuir, 2019, 35(15): 5250-5256. |
| 8 | Zhang X H, Maeda N. Interfacial gaseous states on crystalline surfaces[J]. The Journal of Physical Chemistry C, 2011, 115(3): 736-743. |
| 9 | Zhang Y, Zhao L, Deng S, et al. Effect of nanobubble evolution on hydrate process: a review[J]. Journal of Thermal Science, 2019, 28(5): 948-961. |
| 10 | Angulo A, van der Linde P, Gardeniers H, et al. Influence of bubbles on the energy conversion efficiency of electrochemical reactors[J]. Joule, 2020, 4(3): 555-579. |
| 11 | Hu H B, Wang D Z, Ren F, et al. A comparative analysis of the effective and local slip lengths for liquid flows over a trapped nanobubble[J]. International Journal of Multiphase Flow, 2018, 104: 166-173. |
| 12 | Wang Y F, Pan Z C, Luo X M, et al. Effect of nanobubbles on adsorption of sodium oleate on calcite surface[J]. Minerals Engineering, 2019, 133: 127-137. |
| 13 | Azevedo A, Oliveira H, Rubio J. Bulk nanobubbles in the mineral and environmental areas: updating research and applications[J]. Advances in Colloid and Interface Science, 2019, 271: 101992. |
| 14 | Calgaroto S, Wilberg K Q, Rubio J. On the nanobubbles interfacial properties and future applications in flotation[J]. Minerals Engineering, 2014, 60: 33-40. |
| 15 | Kalacheva A V, Medvedev V A, Serkov A T. Continuous deaeration of spinning solutions of polyacrylonitrile in dimethyl acetamide[J]. Fibre Chemistry, 2001, 33(1): 9-11. |
| 16 | 王丽娟, 党晓波, 郑桂宁. 碳纤维原丝纺丝液脱单、脱泡工艺及装置技术研究[J]. 合成纤维, 2017, 46(11): 16-19. |
| Wang L J, Dang X B, Zheng G N. Study on the process and device of de-monomer and de-bubble of carbon fiber precursor spinning solution[J]. Synthetic Fiber in China, 2017, 46(11): 16-19. | |
| 17 | Pereiro I, Fomitcheva K A, Petrini L, et al. Nip the bubble in the bud: a guide to avoid gas nucleation in microfluidics[J]. Lab on a Chip, 2019, 19(14): 2296-2314. |
| 18 | Fatemi N, Dong Z Y, van Gerven T, et al. Microbubbles as heterogeneous nucleation sites for crystallization in continuous microfluidic devices[J]. Langmuir, 2019, 35(1): 60-69. |
| 19 | Zhu J, An H, Alheshibri M, et al. Cleaning with bulk nanobubbles[J]. Langmuir, 2016, 32(43): 11203-11211. |
| 20 | Etchepare R, Azevedo A, Calgaroto S, et al. Removal of ferric hydroxide by flotation with micro and nanobubbles[J]. Separation and Purification Technology, 2017, 184: 347-353. |
| 21 | Cho S H, Kim J Y, Chun J H, et al. Ultrasonic formation of nanobubbles and their zeta-potentials in aqueous electrolyte and surfactant solutions[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2005, 269(1/2/3): 28-34. |
| 22 | Postnikov A V, Uvarov I V, Penkov N V, et al. Collective behavior of bulk nanobubbles produced by alternating polarity electrolysis[J]. Nanoscale, 2018, 10(1): 428-435. |
| 23 | Yang J W, Duan J M, Fornasiero D, et al. Very small bubble formation at the solid-water interface[J]. The Journal of Physical Chemistry B, 2003, 107(25): 6139-6147. |
| 24 | Li D Y, Qi L T, Liu Y B, et al. Study on the formation and properties of trapped nanobubbles and surface nanobubbles by spontaneous and temperature difference methods[J]. Langmuir, 2019, 35(37): 12035-12041. |
| 25 | Lou S T, Ouyang Z Q, Zhang Y, et al. Nanobubbles on solid surface imaged by atomic force microscopy[J]. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures, 2000, 18(5): 2573. |
| 26 | Walczyk W, Schönherr H. Closer look at the effect of AFM imaging conditions on the apparent dimensions of surface nanobubbles[J]. Langmuir, 2013, 29(2): 620-632. |
| 27 | Teshima H, Takahashi K, Takata Y, et al. Wettability of AFM tip influences the profile of interfacial nanobubbles[J]. Journal of Applied Physics, 2018, 123(5): 054303. |
| 28 | Kundu P, Liu S Y, Chen F R, et al. In-situ generation of highly stable, sub 10-nm oxygen nanobubbles in liquid environmental tem[C]//2016 IEEE 29th International Conference on Micro Electro Mechanical Systems (MEMS). Shanghai, China, 2016: 133-136. |
| 29 | Chen J, Zhou K, Wang Y, et al. Measuring the activation energy barrier for the nucleation of single nanosized vapor bubbles[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(26): 12678-12683. |
| 30 | Lemay S G. Noise as data: nucleation of electrochemically generated nanobubbles[J]. ACS Nano, 2019, 13(6): 6141-6144. |
| 31 | Luo L, White H S. Electrogeneration of single nanobubbles at sub-50-nm-radius platinum nanodisk electrodes[J]. Langmuir, 2013, 29(35): 11169-11175. |
| 32 | Chen Q J, Luo L, Faraji H, et al. Electrochemical measurements of single H2 nanobubble nucleation and stability at Pt nanoelectrodes[J]. The Journal of Physical Chemistry Letters, 2014, 5(20): 3539-3544. |
| 33 | Chen Q J, Wiedenroth H S, German S R, et al. Electrochemical nucleation of stable N2 nanobubbles at Pt nanoelectrodes[J]. Journal of the American Chemical Society, 2015, 137(37): 12064-12069. |
| 34 | Ren H, German S R, Edwards M A, et al. Electrochemical generation of individual O2 nanobubbles via H2O2 oxidation[J]. The Journal of Physical Chemistry Letters, 2017, 8(11): 2450-2454. |
| 35 | Ren H, Edwards M A, Wang Y F, et al. Electrochemically controlled nucleation of single CO2 nanobubbles via formate oxidation at Pt nanoelectrodes[J]. The Journal of Physical Chemistry Letters, 2020, 11(4): 1291-1296. |
| 36 | German S R, Edwards M A, Ren H, et al. Critical nuclei size, rate, and activation energy of H2 gas nucleation[J]. Journal of the American Chemical Society, 2018, 140(11): 4047-4053. |
| 37 | Soto Á M, German S R, Ren H, et al. The nucleation rate of single O2 nanobubbles at Pt nanoelectrodes[J]. Langmuir, 2018, 34(25): 7309-7318. |
| 38 | Bentley C L, Edmondson J, Meloni G N, et al. Nanoscale electrochemical mapping[J]. Analytical Chemistry, 2019, 91(1): 84-108. |
| 39 | Wang Y F, Gordon E, Ren H. Mapping the nucleation of H2 bubbles on polycrystalline Pt via scanning electrochemical cell microscopy[J]. The Journal of Physical Chemistry Letters, 2019, 10(14): 3887-3892. |
| 40 | Che Z, Theodorakis P E. Formation, dissolution and properties of surface nanobubbles [J]. Journal of Colloid and Interface Science, 2017, 487: 123-129. |
| 41 | Liu Y W, Zhang X R. Molecular dynamics simulation of nanobubble nucleation on rough surfaces[J]. The Journal of Chemical Physics, 2017, 146(16): 164704. |
| 42 | Xiao Q X, Liu Y W, Guo Z J, et al. Solvent exchange leading to nanobubble nucleation: a molecular dynamics study[J]. Langmuir, 2017, 33(32): 8090-8096. |
| 43 | Liu Y W, Edwards M A, German S R, et al. The dynamic steady state of an electrochemically generated nanobubble[J]. Langmuir, 2017, 33(8): 1845-1853. |
| 44 | Liu H B, Pan L M, Wen J. Numerical simulation of hydrogen bubble growth at an electrode surface[J]. The Canadian Journal of Chemical Engineering, 2016, 94(1): 192-199. |
| 45 | Wu C J, Chu K C, Sheng Y J, et al. Sliding dynamic behavior of a nanobubble on a surface[J]. The Journal of Physical Chemistry C, 2017, 121(33): 17932-17940. |
| 46 | Liu Y W, Zhang X R. Nanobubble stability induced by contact line pinning[J]. The Journal of Chemical Physics, 2013, 138(1): 014706. |
| 47 | Okitsu K, Suzuki T, Takenaka N, et al. Acoustic multibubble cavitation in water: a new aspect of the effect of a rare gas atmosphere on bubble temperature and its relevance to sonochemistry[J]. The Journal of Physical Chemistry B, 2006, 110(41): 20081-20084. |
| 48 | Brotchie A, Statham T, Zhou M F, et al. Acoustic bubble sizes, coalescence, and sonochemical activity in aqueous electrolyte solutions saturated with different gases[J]. Langmuir, 2010, 26(15): 12690-12695. |
| 49 | van Limbeek M A J, Seddon J R T. Surface nanobubbles as a function of gas type[J]. Langmuir, 2011, 27(14): 8694-8699. |
| 50 | Yang J W, Duan J M, Fornasiero D, et al. Kinetics of CO2 nanobubble formation at the solid/water interface[J]. Physical Chemistry Chemical Physics, 2007, 9(48): 6327. |
| 51 | Fang C K, Ko H C, Yang C W, et al. Nucleation processes of nanobubbles at a solid/water interface[J]. Sci. Rep., 2016, 6: 24651. |
| 52 | Hasan M N, Rabbi K F, Mukut K M, et al. Nano scale dynamics of bubble nucleation in confined liquid subjected to rapid cooling: effect of solid-liquid interfacial wettability[C]// 7th Bsme International Conference on Thermal Engineering. Dhaka, Bangladesh, 2017. |
| 53 | Ye Y M, Klimchuk S, Shang M W, et al. Acoustic bubble suppression by constructing a hydrophilic coating on HDPE surface[J]. ACS Applied Materials & Interfaces, 2019, 11(18): 16944-16950. |
| 54 | Faber M S, Dziedzic R, Lukowski M A, et al. High-performance electrocatalysis using metallic cobalt pyrite (CoS2) micro- and nanostructures[J]. Journal of the American Chemical Society, 2014, 136(28): 10053-10061. |
| 55 | Lu Z Y, Zhu W, Yu X Y, et al. Ultrahigh hydrogen evolution performance of under-water“superaerophobic”MoS2 nanostructured electrodes[J]. Advanced Materials, 2014, 26(17): 2683-2687. |
| 56 | Chen Q J, Ranaweera R, Luo L. Hydrogen bubble formation at hydrogen-insertion electrodes[J]. The Journal of Physical Chemistry C, 2018, 122(27): 15421-15426. |
| 57 | German S R, Edwards M A, Chen Q J, et al. Electrochemistry of single nanobubbles. Estimating the critical size of bubble-forming nuclei for gas-evolving electrode reactions[J]. Faraday Discussions, 2016, 193: 223-240. |
| 58 | Rooze J, Rebrov E V, Schouten J C, et al. Dissolved gas and ultrasonic cavitation — a review[J]. Ultrasonics Sonochemistry, 2013, 20(1): 1-11. |
| 59 | Xiao W, Zhao Y L, Yang J, et al. Effect of sodium oleate on the adsorption morphology and mechanism of nanobubbles on the mica surface[J]. Langmuir, 2019, 35(28): 9239-9245. |
| 60 | Yasui K, Tuziuti T, Izu N, et al. Is surface tension reduced by nanobubbles (ultrafine bubbles) generated by cavitation?[J]. Ultrasonics Sonochemistry, 2019, 52: 13-18. |
| 61 | Ashokkumar M, Hodnett M, Zeqiri B, et al. Acoustic emission spectra from 515 kHz cavitation in aqueous solutions containing surface-active solutes[J]. Journal of the American Chemical Society, 2007, 129(8): 2250-2258. |
| 62 | Lee J, Kentish S, Ashokkumar M. Effect of surfactants on the rate of growth of an air bubble by rectified diffusion[J]. The Journal of Physical Chemistry B, 2005, 109(30): 14595-14598. |
| 63 | Fernández D, Maurer P, Martine M, et al. Bubble formation at a gas-evolving microelectrode[J]. Langmuir, 2014, 30(43): 13065-13074. |
| 64 | Fatemi N, Devos C, de Cordt G, et al. Effect of sodium dodecyl sulfate on the continuous crystallization in microfluidic devices using microbubbles[J]. Chemical Engineering & Technology, 2019, 42(10): 2105-2112. |
| 65 | Zhang M M, Seddon J R T. Nanobubble-nanoparticle interactions in bulk solutions[J]. Langmuir, 2016, 32(43): 11280-11286. |
| 66 | Xiao W, Wang X X, Zhou L M, et al. Influence of mixing and nanosolids on the formation of nanobubbles[J]. The Journal of Physical Chemistry B, 2019, 123(1): 317-323. |
| 67 | Olszok V, Rivas-Botero J, Wollmann A, et al. Particle-induced nanobubble generation for material-selective nanoparticle flotation[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2020, 592: 124576. |
| 68 | Borkent B M, Dammer S M, Schönherr H, et al. Superstability of surface nanobubbles[J]. Physical Review Letters, 2007, 98(20): 204502. |
| 69 | Seddon J R, Kooij E S, Poelsema B, et al. Surface bubble nucleation stability[J]. Physical Review Letters, 2011, 106(5): 056101. |
| 70 | Sun Y J, Xie G Y, Peng Y L, et al. Stability theories of nanobubbles at solid-liquid interface: a review[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2016, 495: 176-186. |
| 71 | Das S, Snoeijer J H, Lohse D. Effect of impurities in description of surface nanobubbles[J]. Physical Review E, Statistical, Nonlinear, and Soft Matter Physics, 2010, 82(5): 056310. |
| 72 | Guo Z J, Wang X, Zhang X R. Stability of surface nanobubbles without contact line pinning[J]. Langmuir, 2019, 35(25): 8482-8489. |
| 73 | Uchida T, Liu S, Enari M, et al. Effect of NaCl on the lifetime of micro- and nanobubbles[J]. Nanomaterials, 2016, 6(2): E31. |
| 74 | Meegoda J N, Hewage S A, Batagoda J H. Application of the diffused double layer theory to nanobubbles[J]. Langmuir, 2019, 35(37): 12100-12112. |
| 75 | Zhang H G, Guo Z J, Zhang X R. Surface enrichment of ions leads to the stability of bulk nanobubbles[J]. Soft Matter, 2020, 16(23): 5470-5477. |
| 76 | Zhang X H, Chan D Y C, Wang D Y, et al. Stability of interfacial nanobubbles[J]. Langmuir, 2013, 29(4): 1017-1023. |
| 77 | Liu Y, Wang J, Zhang X, et al. Contact line pinning and the relationship between nanobubbles and substrates[J]. The Journal of Chemical Physics, 2014, 140(5): 054705. |
| 78 | Liu Y W, Zhang X R. A unified mechanism for the stability of surface nanobubbles: contact line pinning and supersaturation[J]. The Journal of Chemical Physics, 2014, 141(13): 134702. |
| 79 | Lohse D, Zhang X H. Pinning and gas oversaturation imply stable single surface nanobubbles[J]. Physical Review E, 2015, 91(3): 031003. |
| 80 | Shin D, Park J B, Kim Y J, et al. Growth dynamics and gas transport mechanism of nanobubbles in graphene liquid cells[J]. Nature Communications, 2015, 6: 6068. |
| 81 | Wang Y F, Luo X M, Qin W Q, et al. New insights into the contact angle and formation process of nanobubbles based on line tension and pinning[J]. Applied Surface Science, 2019, 481: 1585-1594. |
| 82 | German S R, Chen Q J, Edwards M A, et al. Electrochemical measurement of hydrogen and nitrogen nanobubble lifetimes at Pt nanoelectrodes[J]. Journal of the Electrochemical Society, 2016, 163(4): H3160-H3166. |
| 83 | Chen Q J, Liu Y W, Edwards M A, et al. Nitrogen bubbles at Pt nanoelectrodes in a nonaqueous medium: oscillating behavior and geometry of critical nuclei[J]. Analytical Chemistry, 2020, 92(9): 6408-6414. |
| 84 | Shang D W, Zhang X P, Zeng S J, et al. Protic ionic liquid [Bim][NTf2] with strong hydrogen bond donating ability for highly efficient ammonia absorption[J]. Green Chemistry, 2017, 19(4): 937-945. |
| 85 | Feng J P, Zeng S J, Feng J Q, et al. CO2 electroreduction in ionic liquids: a review[J]. Chinese Journal of Chemistry, 2018, 36(10): 961-970. |
| 86 | 王均凤, 聂毅, 王斌琦, 等. 离子液体法再生纤维素纤维制造技术及发展趋势[J]. 化工学报, 2019, 70(10): 3836-3846. |
| Wang J F, Nie Y, Wang B Q, et al. Manufacturing technology and development direction on regenerated cellulose fibers using ionic liquids[J]. CIESC Journal, 2019, 70(10): 3836-3846. | |
| 87 | Taylor S F R, Brittle S A, Desai P, et al. Factors affecting bubble size in ionic liquids[J]. Physical Chemistry Chemical Physics, 2017, 19(22): 14306-14318. |
| 88 | Qin K, Wang K, Luo R, et al. Dispersion of supercritical carbon dioxide to [Emim][BF4] with a T-junction tubing connector[J]. Chemical Engineering and Processing - Process Intensification, 2018, 127: 58-64. |
| 89 | 张香平, 曾少娟, 冯佳奇, 等. CO2化工: 离子微环境调控的CO2绿色高效转化[J]. 中国科学: 化学, 2020, 50(2): 282-298. |
| Zhang X P, Zeng S J, Feng J Q, et al. CO2 chemical engineering: CO2 green conversion enhanced by ionic liquid microhabitat[J]. Scientia Sinica (Chimica), 2020, 50(2): 282-298. | |
| 90 | Feng J P, Zeng S J, Liu H Z, et al. Insights into carbon dioxide electroreduction in ionic liquids: carbon dioxide activation and selectivity tailored by ionic microhabitat[J]. ChemSusChem, 2018, 11(18): 3191-3197. |
| 91 | Zhao X, Ranaweera R, Luo L. Highly efficient hydrogen evolution of platinum via tuning the interfacial dissolved-gas concentration[J]. Chemical Communications, 2019, 55(10): 1378-1381. |
| 92 | Chew E K, Lee K Y, Lau E V. The role of carbon chain length in the attachment between microbubbles and aqueous solutions of ionic liquid[J]. Journal of Colloid and Interface Science, 2017, 506: 452-459. |
| 93 | 冯建朋, 张香平, 尚大伟, 等. 离子液体中电化学还原CO2研究评述与展望[J]. 化工学报, 2018, 69(1): 69-75. |
| Feng J P, Zhang X P, Shang D W, et al. Review and prospect of CO2 electro-reduction in ionic liquids[J]. CIESC Journal, 2018, 69(1): 69-75. |
| [1] | 周晓庆, 李春煜, 杨光, 蔡爱峰, 吴静怡. 液滴撞击不同曲率过冷波纹面结冰动力学行为及机理研究[J]. 化工学报, 2023, 74(S1): 141-153. |
| [2] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [3] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [4] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [5] | 金伟其, 吴月荣, 王霞, 李力, 裘溯, 袁盼, 王铭赫. 化工园区工业气体泄漏气云红外成像检测技术与国产化装备进展[J]. 化工学报, 2023, 74(S1): 32-44. |
| [6] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [7] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [11] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [12] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [13] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [14] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [15] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号