化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4796-4807.DOI: 10.11949/0438-1157.20201770
收稿日期:2020-12-09
修回日期:2021-08-02
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
张扬,王学重
作者简介:李闯(1994—),男,硕士研究生,基金资助:
Chuang LI1( ),Yang ZHANG1(
),Yang ZHANG1( ),Xiaojuan LIU1,Xuezhong WANG2(
),Xiaojuan LIU1,Xuezhong WANG2( )
)
Received:2020-12-09
Revised:2021-08-02
Online:2021-09-05
Published:2021-09-05
Contact:
Yang ZHANG,Xuezhong WANG
摘要:
晶体形貌作为晶体产品的重要质量指标,不仅会影响产品流动性、稳定性、溶出速率和生物可利用度等产品的质量指标,还会对过滤、干燥、压片等下游操作造成影响。通过分子模拟的方法指导阿司匹林冷却-反溶剂结晶过程的添加剂筛选,以添加剂作为晶体形貌的改性剂,降低阿司匹林晶体的长径比优化晶体形貌。通过单因素实验考查了添加剂浓度、晶种加入量、降温速率、搅拌速率和加水速率对阿司匹林晶体产品形貌、流动性和粒度分布等的影响,确定了较优的工艺条件。实验结果表明加入聚乙烯吡咯烷酮(PVP)作为添加剂可以降低阿司匹林晶体长径比,获得形貌为短棱柱状的晶体产品,能够显著改变晶体形貌优化产品的流动性。
中图分类号:
李闯, 张扬, 刘小娟, 王学重. 添加剂作用下阿司匹林结晶模拟和实验研究[J]. 化工学报, 2021, 72(9): 4796-4807.
Chuang LI, Yang ZHANG, Xiaojuan LIU, Xuezhong WANG. Modeling and experimental study of additives on solution crystallization of aspirin[J]. CIESC Journal, 2021, 72(9): 4796-4807.
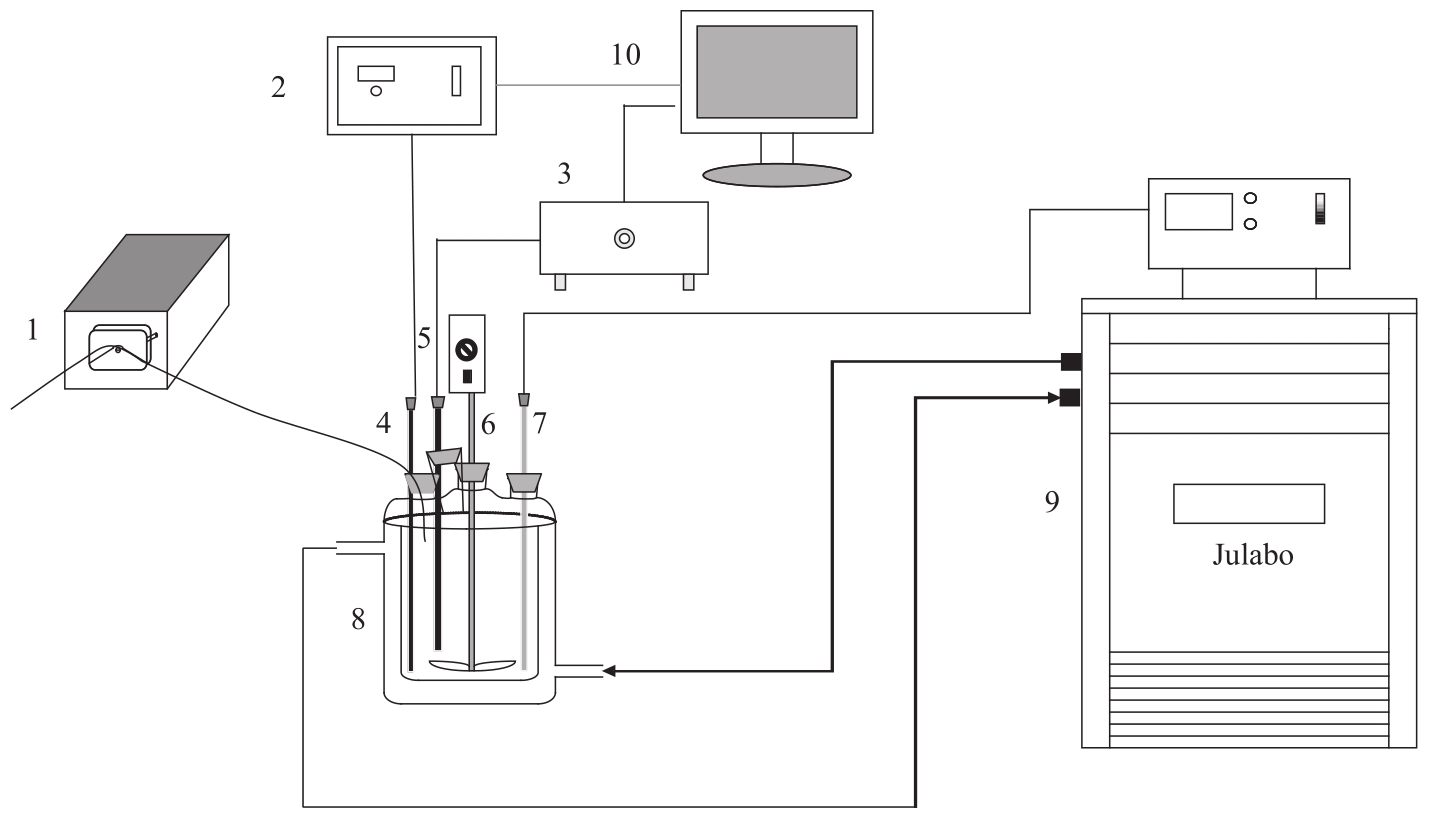
图 1 阿司匹林结晶实验装置示意图1—蠕动泵; 2—浊度仪; 3—二维成像系统; 4—浊度探头; 5—二维成像系统探头; 6—机械搅拌; 7—热电偶温度计探头; 8—250 ml四口结晶器; 9—温度控制器(Julabo FP51); 10—带有浊度和图像采集和处理软件的计算机
Fig.1 Schematic of aspirin crystallization experiments
| Face | Multiplicity | 面积占比/% |
|---|---|---|
| (1 0 0) | 2 | 51.93 |
| (1 1 0) | 4 | 10.42 |
| (0 1 1) | 4 | 11.44 |
| (0 0 2) | 2 | 25.39 |
| (1 1 -1) | 4 | 0.60 |
| (1 1 1) | 4 | 0.22 |
表1 AE模型模拟得到的阿司匹林晶体理论晶习及晶面占总晶面面积百分比
Table 1 Theoretical crystal habits and percentage of total crystal plane area obtained from AE model
| Face | Multiplicity | 面积占比/% |
|---|---|---|
| (1 0 0) | 2 | 51.93 |
| (1 1 0) | 4 | 10.42 |
| (0 1 1) | 4 | 11.44 |
| (0 0 2) | 2 | 25.39 |
| (1 1 -1) | 4 | 0.60 |
| (1 1 1) | 4 | 0.22 |
| 添加剂(聚合度) | Eads/(kcal/mol) | |||||
|---|---|---|---|---|---|---|
| (1 0 0) | (1 1 0) | (0 1 1) | (0 0 2) | (1 1 -1) | (1 1 1) | |
| PVP(45) | -122.35 | -135.69 | -159.11 | -129.30 | -120.90 | -90.74 |
| PVP(90) | -220.06 | -237.32 | -277.11 | -207.90 | -180.92 | -148.76 |
| PVP(135) | -260.23 | -303.76 | -338.57 | -256.32 | -222.13 | -195.33 |
| HPMC(100) | -301.15 | -211.30 | -197.18 | -311.26 | -268.76 | -268.81 |
表2 添加剂在阿司匹林不同晶面的吸附能
Table 2 Adsorption energy of additives on different crystal faces of aspirin
| 添加剂(聚合度) | Eads/(kcal/mol) | |||||
|---|---|---|---|---|---|---|
| (1 0 0) | (1 1 0) | (0 1 1) | (0 0 2) | (1 1 -1) | (1 1 1) | |
| PVP(45) | -122.35 | -135.69 | -159.11 | -129.30 | -120.90 | -90.74 |
| PVP(90) | -220.06 | -237.32 | -277.11 | -207.90 | -180.92 | -148.76 |
| PVP(135) | -260.23 | -303.76 | -338.57 | -256.32 | -222.13 | -195.33 |
| HPMC(100) | -301.15 | -211.30 | -197.18 | -311.26 | -268.76 | -268.81 |
| T/K | b1 | b2 | b3 | b4 | b5 | 最大溶解度Cmax×10-2/(g/g) | w×10-2 | RMSD×10-2 |
|---|---|---|---|---|---|---|---|---|
| 278.15 | -2.105 | 1.990 | -13.57 | 18.80 | -16.62 | 13.21 | 8.772 | 0.1761 |
| 283.15 | -1.921 | 1.953 | -11.01 | 13.53 | -13.98 | 16.14 | 10.66 | 0.0716 |
| 293.15 | -1.538 | 1.895 | -7.609 | 1.179 | -0.708 | 24.20 | 12.80 | 0.1114 |
| 298.15 | -1.349 | 2.144 | -9.451 | 7.815 | -7.170 | 29.65 | 13.15 | 0.1393 |
| 303.15 | -1.130 | 1.716 | -6.801 | 2.970 | -3.928 | 36.17 | 13.53 | 0.1843 |
| 313.15 | -0.790 | 1.934 | -6.496 | 3.221 | -4.178 | 52.91 | 16.31 | 0.2273 |
| 323.15 | -0.436 | 2.340 | -9.745 | 17.81 | -17.69 | 78.37 | 20.21 | 0.3460 |
| 333.15 | -0.082 | 0.551 | 4.396 | -13.09 | 6.882 | 121.0 | 34.97 | 0.6620 |
表 3 阿司匹林质量溶解度拟合参数及最大溶解度
Table 3 Mass solubility fitting parameters and maximum solubility of aspirin
| T/K | b1 | b2 | b3 | b4 | b5 | 最大溶解度Cmax×10-2/(g/g) | w×10-2 | RMSD×10-2 |
|---|---|---|---|---|---|---|---|---|
| 278.15 | -2.105 | 1.990 | -13.57 | 18.80 | -16.62 | 13.21 | 8.772 | 0.1761 |
| 283.15 | -1.921 | 1.953 | -11.01 | 13.53 | -13.98 | 16.14 | 10.66 | 0.0716 |
| 293.15 | -1.538 | 1.895 | -7.609 | 1.179 | -0.708 | 24.20 | 12.80 | 0.1114 |
| 298.15 | -1.349 | 2.144 | -9.451 | 7.815 | -7.170 | 29.65 | 13.15 | 0.1393 |
| 303.15 | -1.130 | 1.716 | -6.801 | 2.970 | -3.928 | 36.17 | 13.53 | 0.1843 |
| 313.15 | -0.790 | 1.934 | -6.496 | 3.221 | -4.178 | 52.91 | 16.31 | 0.2273 |
| 323.15 | -0.436 | 2.340 | -9.745 | 17.81 | -17.69 | 78.37 | 20.21 | 0.3460 |
| 333.15 | -0.082 | 0.551 | 4.396 | -13.09 | 6.882 | 121.0 | 34.97 | 0.6620 |
| 添加剂 | 统计数目 | 平均长径比 |
|---|---|---|
| 0 | 60 | 1.93±0.32 |
| 0.05% PVP K13-18 | 60 | 1.58±0.26 |
| 0.01% PVP K29-32 | 72 | 1.63±0.22 |
| 0.05% PVP K29-32 | 73 | 1.39±0.24 |
| 0.1% PVP K29-32 | 64 | 1.10±0.21 |
| 0.05% PVP K88-96 | 80 | 1.22±0.22 |
| 0.01% HPMC | 52 | 3.81±1.25 |
| 0.05% HPMC | 66 | 6.19±2.01 |
| 0.1% HPMC | 78 | 7.62±2.41 |
表4 不同添加剂条件下阿司匹林实验晶体长径比
Table 4 Experimental aspirin crystal aspect ratio under different additives
| 添加剂 | 统计数目 | 平均长径比 |
|---|---|---|
| 0 | 60 | 1.93±0.32 |
| 0.05% PVP K13-18 | 60 | 1.58±0.26 |
| 0.01% PVP K29-32 | 72 | 1.63±0.22 |
| 0.05% PVP K29-32 | 73 | 1.39±0.24 |
| 0.1% PVP K29-32 | 64 | 1.10±0.21 |
| 0.05% PVP K88-96 | 80 | 1.22±0.22 |
| 0.01% HPMC | 52 | 3.81±1.25 |
| 0.05% HPMC | 66 | 6.19±2.01 |
| 0.1% HPMC | 78 | 7.62±2.41 |
| 实验 编号 | 添加剂 浓度/% | 晶种量/% | 降温速率/ (K/min) | 搅拌速率/(r/min) | 加水速率/(ml/min) |
|---|---|---|---|---|---|
| 1 | 0 | 1 | 0.3 | 250 | 5 |
| 2 | 0.01 | 1 | 0.3 | 250 | 5 |
| 3 | 0.05 | 1 | 0.3 | 250 | 5 |
| 4 | 0.10 | 1 | 0.3 | 250 | 5 |
| 5 | 0.05 | 0 | 0.3 | 250 | 5 |
| 6 | 0.05 | 0.5 | 0.3 | 250 | 5 |
| 7 | 0.05 | 3 | 0.3 | 250 | 5 |
| 8 | 0.05 | 1 | 0.1 | 250 | 5 |
| 9 | 0.05 | 1 | 0.5 | 250 | 5 |
| 10 | 0.05 | 1 | 0.3 | 150 | 5 |
| 11 | 0.05 | 1 | 0.3 | 350 | 5 |
| 12 | 0.05 | 1 | 0.3 | 250 | 20 |
| 13 | 0.05 | 1 | 0.3 | 250 | 一次性倾倒 |
表5 单因素实验条件汇总
Table 5 Summary of single factor experiment conditions
| 实验 编号 | 添加剂 浓度/% | 晶种量/% | 降温速率/ (K/min) | 搅拌速率/(r/min) | 加水速率/(ml/min) |
|---|---|---|---|---|---|
| 1 | 0 | 1 | 0.3 | 250 | 5 |
| 2 | 0.01 | 1 | 0.3 | 250 | 5 |
| 3 | 0.05 | 1 | 0.3 | 250 | 5 |
| 4 | 0.10 | 1 | 0.3 | 250 | 5 |
| 5 | 0.05 | 0 | 0.3 | 250 | 5 |
| 6 | 0.05 | 0.5 | 0.3 | 250 | 5 |
| 7 | 0.05 | 3 | 0.3 | 250 | 5 |
| 8 | 0.05 | 1 | 0.1 | 250 | 5 |
| 9 | 0.05 | 1 | 0.5 | 250 | 5 |
| 10 | 0.05 | 1 | 0.3 | 150 | 5 |
| 11 | 0.05 | 1 | 0.3 | 350 | 5 |
| 12 | 0.05 | 1 | 0.3 | 250 | 20 |
| 13 | 0.05 | 1 | 0.3 | 250 | 一次性倾倒 |
| 实验编号 | D10/μm | D50/μm | D90/μm | 松装密度/(g/cm3) | 振实密度/(g/cm3) | 休止角/(°) | 收率/% | CV/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 201 | 544 | 1030 | 0.41 | 0.52 | 43 | 88.91 | 56.37 |
| 2 | 144 | 438 | 886 | 0.58 | 0.67 | 33 | 88.61 | 62.74 |
| 3 | 144 | 363 | 712 | 0.72 | 0.78 | 26 | 88.33 | 57.13 |
| 4 | 107 | 270 | 496 | 0.71 | 0.75 | 29 | 88.15 | 53.37 |
| 5 | 71.2 | 269 | 521 | 0.39 | 0.51 | 45 | 88.73 | 61.04 |
| 6 | 105 | 291 | 549 | 0.53 | 0.63 | 30 | 88.65 | 58.01 |
| 7 | 139 | 558 | 1040 | 0.65 | 0.73 | 28 | 87.85 | 56.83 |
| 8 | 279 | 579 | 1080 | 0.61 | 0.73 | 31 | 88.75 | 51.13 |
| 9 | 147 | 437 | 1170 | 0.53 | 0.68 | 35 | 87.35 | 86.71 |
| 10 | 35.3 | 472 | 1110 | 0.43 | 0.56 | 41 | 88.55 | 83.96 |
| 11 | 121 | 358 | 901 | 0.68 | 0.73 | 30 | 88.43 | 80.66 |
| 12 | 86.6 | 294 | 570 | 0.73 | 0.77 | 26 | 88.41 | 58.21 |
| 13 | 50.8 | 542 | 988 | 0.70 | 0.75 | 30 | 88.17 | 64.13 |
| 原料 | 74.5 | 459 | 916 | 0.43 | 0.55 | 40 | — | 67.81 |
表6 实验结果汇总
Table 6 Summary of experimental results
| 实验编号 | D10/μm | D50/μm | D90/μm | 松装密度/(g/cm3) | 振实密度/(g/cm3) | 休止角/(°) | 收率/% | CV/% |
|---|---|---|---|---|---|---|---|---|
| 1 | 201 | 544 | 1030 | 0.41 | 0.52 | 43 | 88.91 | 56.37 |
| 2 | 144 | 438 | 886 | 0.58 | 0.67 | 33 | 88.61 | 62.74 |
| 3 | 144 | 363 | 712 | 0.72 | 0.78 | 26 | 88.33 | 57.13 |
| 4 | 107 | 270 | 496 | 0.71 | 0.75 | 29 | 88.15 | 53.37 |
| 5 | 71.2 | 269 | 521 | 0.39 | 0.51 | 45 | 88.73 | 61.04 |
| 6 | 105 | 291 | 549 | 0.53 | 0.63 | 30 | 88.65 | 58.01 |
| 7 | 139 | 558 | 1040 | 0.65 | 0.73 | 28 | 87.85 | 56.83 |
| 8 | 279 | 579 | 1080 | 0.61 | 0.73 | 31 | 88.75 | 51.13 |
| 9 | 147 | 437 | 1170 | 0.53 | 0.68 | 35 | 87.35 | 86.71 |
| 10 | 35.3 | 472 | 1110 | 0.43 | 0.56 | 41 | 88.55 | 83.96 |
| 11 | 121 | 358 | 901 | 0.68 | 0.73 | 30 | 88.43 | 80.66 |
| 12 | 86.6 | 294 | 570 | 0.73 | 0.77 | 26 | 88.41 | 58.21 |
| 13 | 50.8 | 542 | 988 | 0.70 | 0.75 | 30 | 88.17 | 64.13 |
| 原料 | 74.5 | 459 | 916 | 0.43 | 0.55 | 40 | — | 67.81 |
| 1 | Gupta K M, Yin Y N, Poornachary S K, et al. Atomistic simulation to understand anisotropic growth behavior of naproxen crystal in the presence of polymeric additives[J]. Crystal Growth & Design, 2019, 19(7): 3768-3776. |
| 2 | Jing D D, Liu A L, Wang J K, et al. Study on crystal morphology of penicillin sulfoxide in different solvents using binding energy[J]. Organic Process Research & Development, 2015, 19(3): 410-415. |
| 3 | Liang Z Z, Yi Q H, Wang W, et al. A systematic study of solvent effect on the crystal habit of dirithromycin solvates by computer simulation[J]. Computers & Chemical Engineering, 2014, 62: 56-61. |
| 4 | An J H, Choi G J, Kim W S. Polymorphic and kinetic investigation of adefovir dipivoxil during phase transformation[J]. International Journal of Pharmaceutics, 2012, 422(1/2): 185-193. |
| 5 | Parimaladevi P, Srinivasan K. Influence of supersaturation level on the morphology of α-lactose monohydrate crystals[J]. International Dairy Journal, 2014, 39(2): 301-311. |
| 6 | Wang C, Zhang X, Du W, et al. Effects of solvent and supersaturation on crystal morphology of cefaclor dihydrate: a combined experimental and computer simulation study[J]. CrystEngComm, 2016, 18(47): 9085-9094. |
| 7 | Liang Z Z, Zhang M, Wu F, et al. Supersaturation controlled morphology and aspect ratio changes of benzoic acid crystals[J]. Computers & Chemical Engineering, 2017, 99: 296-303. |
| 8 | Tan W H, Yang X Y, Duan X Z, et al. Understanding supersaturation-dependent crystal growth of L-alanine in aqueous solution[J]. Crystal Research and Technology, 2016, 51(1): 23-29. |
| 9 | 李兰菊, 李秀喜, 徐三. 阿司匹林结晶过程的在线分析[J]. 化工学报, 2018, 69(3): 1046-1052. |
| Li L J, Li X X, Xu S. Online monitor of Aspirin crystallization process[J]. CIESC Journal, 2018, 69(3): 1046-1052. | |
| 10 | Yin Y N, Chow P S, Tan R B H. Molecular simulation study of the effect of various additives on salbutamol sulfate crystal habit[J]. Molecular Pharmaceutics, 2011, 8(5): 1910-1918. |
| 11 | Li Z H, Shi P, Yang Y, et al. Tuning crystallization and stability of the metastable polymorph of dl-methionine by a structurally similar additive[J]. CrystEngComm, 2019, 21(24): 3731-3739. |
| 12 | Zhang Y, Liu J J, Wan J, et al. Two dimensional population balance modelling of crystal growth behaviour under the influence of impurities[J]. Advanced Powder Technology, 2015, 26(2): 672-678. |
| 13 | Clydesdale G, Thomson G B, Walker E M, et al. A molecular modeling study of the crystal morphology of adipic acid and its habit modification by homologous impurities[J]. Crystal Growth & Design, 2005, 5(6): 2154-2163. |
| 14 | Weissbuch I, Lahav M, Leiserowitz L. Toward stereochemical control, monitoring, and understanding of crystal nucleation[J]. Crystal Growth & Design, 2003, 3(2): 125-150. |
| 15 | Kuvadia Z B, Doherty M F. Effect of structurally similar additives on crystal habit of organic molecular crystals at low supersaturation[J]. Crystal Growth & Design, 2013, 13(4): 1412-1428. |
| 16 | Poornachary S K, Lau G, Chow P S, et al. The effect and counter-effect of impurities on crystallization of an agrochemical active ingredient: stereochemical rationalization and nanoscale crystal growth visualization[J]. Crystal Growth & Design, 2011, 11(2): 492-500. |
| 17 | Tian F, Baldursdottir S, Rantanen J. Effects of polymer additives on the crystallization of hydrates: a molecular-level modulation[J]. Molecular Pharmaceutics, 2009, 6(1): 202-210. |
| 18 | Vetter T, Mazzotti M, Brozio J. Slowing the growth rate of ibuprofen crystals using the polymeric additive pluronic F127[J]. Crystal Growth & Design, 2011, 11(9): 3813-3821. |
| 19 | Klapwijk A R, Simone E, Nagy Z K, et al. Tuning crystal morphology of succinic acid using a polymer additive[J]. Crystal Growth & Design, 2016, 16(8): 4349-4359. |
| 20 | Ilevbare G A, Liu H Y, Edgar K J, et al. Effect of binary additive combinations on solution crystal growth of the poorly water-soluble drug, ritonavir[J]. Crystal Growth & Design, 2012, 12(12): 6050-6060. |
| 21 | Simone E, Cenzato M V, Nagy Z K. A study on the effect of the polymeric additive HPMC on morphology and polymorphism of ortho-aminobenzoic acid crystals[J]. Journal of Crystal Growth, 2016, 446: 50-59. |
| 22 | Gao Y, Olsen K W. Drug-polymer interactions at water-crystal interfaces and implications for crystallization inhibition: molecular dynamics simulations of amphiphilic block copolymer interactions with tolazamide crystals[J]. Journal of Pharmaceutical Sciences, 2015, 104(7): 2132-2141. |
| 23 | Han D D, Yu B, Liu Y M, et al. Effects of additives on the morphology of thiamine nitrate: the great difference of two kinds of similar additives[J]. Crystal Growth & Design, 2018, 18(2): 775-785. |
| 24 | Mao X L, Song X F, Lu G M, et al. Effect of additives on the morphology of calcium sulfate hemihydrate: experimental and molecular dynamics simulation studies[J]. Chemical Engineering Journal, 2015, 278: 320-327. |
| 25 | Su N N, Wang Y L, Xiao Y, et al. Mechanism of influence of organic impurity on crystallization of sodium sulfate[J]. Industrial & Engineering Chemistry Research, 2018, 57(5): 1705-1713. |
| 26 | Donnamaria M C, Xammar Oro J R. The role of hydrogen bonds in an aqueous solution of acetylsalicylic acid: a molecular dynamics simulation study[J]. Journal of Molecular Modeling, 2011, 17(10): 2485-2490. |
| 27 | 于红琴. 卡马西平多晶型的研究[D].广州:华南理工大学, 2016. |
| Yu H Q. Study on the polymorph of carbamazepine [D].Guangzhou: South China University of Technology, 2016. | |
| 28 | Zhan N X, Zhang Y, Wang X Z. Solubility of N-tert-butylbenzothiazole-2-sulfenamide in several pure and binary solvents[J]. Journal of Chemical & Engineering Data, 2019, 64(3): 1051-1062. |
| 29 | Zhang Y, Jiang Y B, Zhang D K, et al. Metastable zone width, crystal nucleation and growth kinetics measurement in anti-solvent crystallization of β-artemether in the mixture of ethanol and water[J]. Chemical Engineering Research and Design, 2015, 95: 187-194. |
| 30 | 杨丽君, 刘茜, 杨胜勇, 等. 从头算方法研究五元杂环与苯环相互作用[J]. 计算机与应用化学, 2012, 29(4): 461-464. |
| Yang L J, Liu Q, Yang S Y, et al. Ab initio calculations of interactional energies between penta-heterocycles and benzene[J]. Computers and Applied Chemistry, 2012, 29(4): 461-464. | |
| 31 | Pudasaini N, Upadhyay P P, Parker C R, et al. Downstream processability of crystal habit-modified active pharmaceutical ingredient[J]. Organic Process Research & Development, 2017, 21(4): 571-577. |
| 32 | Wu K, Ma C Y, Liu J J, et al. Measurement of crystal face specific growth kinetics[J]. Crystal Growth & Design, 2016, 16(9): 4855-4868. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [3] | 何松, 刘乔迈, 谢广烁, 王斯民, 肖娟. 高浓度水煤浆管道气膜减阻两相流模拟及代理辅助优化[J]. 化工学报, 2023, 74(9): 3766-3774. |
| [4] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [5] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [6] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [7] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [8] | 齐聪, 丁子, 余杰, 汤茂清, 梁林. 基于选择吸收纳米薄膜的太阳能温差发电特性研究[J]. 化工学报, 2023, 74(9): 3921-3930. |
| [9] | 傅予, 刘兴翀, 王瀚雨, 李海敏, 倪亚飞, 邹文静, 雷月, 彭永姗. F3EACl修饰层对钙钛矿太阳能电池性能提升的研究[J]. 化工学报, 2023, 74(8): 3554-3563. |
| [10] | 陈国泽, 卫东, 郭倩, 向志平. 负载跟踪状态下的铝空气电池堆最优功率点优化方法[J]. 化工学报, 2023, 74(8): 3533-3542. |
| [11] | 刘文竹, 云和明, 王宝雪, 胡明哲, 仲崇龙. 基于场协同和 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. 耗散的微通道拓扑优化研究[J]. 化工学报, 2023, 74(8): 3329-3341. |
| [12] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| [13] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [14] | 诸程瑛, 王振雷. 基于改进深度强化学习的乙烯裂解炉操作优化[J]. 化工学报, 2023, 74(8): 3429-3437. |
| [15] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号