化工学报 ›› 2022, Vol. 73 ›› Issue (4): 1772-1780.DOI: 10.11949/0438-1157.20220021
王毅1( ),熊启钊1,陈杨1,2(
),熊启钊1,陈杨1,2( ),杨江峰1,2,李立博1,2,3,李晋平1,2,3
),杨江峰1,2,李立博1,2,3,李晋平1,2,3
收稿日期:2022-01-06
修回日期:2022-02-21
出版日期:2022-04-05
发布日期:2022-04-25
通讯作者:
陈杨
作者简介:王毅(1997—),男,硕士研究生, 基金资助:
Yi WANG1( ),Qizhao XIONG1,Yang CHEN1,2(
),Qizhao XIONG1,Yang CHEN1,2( ),Jiangfeng YANG1,2,Libo LI1,2,3,Jinping LI1,2,3
),Jiangfeng YANG1,2,Libo LI1,2,3,Jinping LI1,2,3
Received:2022-01-06
Revised:2022-02-21
Online:2022-04-05
Published:2022-04-25
Contact:
Yang CHEN
摘要:
近年来,金属有机骨架材料(MOF)在气体吸附和储存领域得到了迅速发展,但由于结构的不稳定性,其在强腐蚀性气体氨(NH3)的吸附方面并不令人满意。考虑到NH3是唯一的无碳排放的氢能源载体,开发高效的储氨技术来载氢是有效的降低二氧化碳排放的手段。利用MOF材料具有的高比表面积和结构多样的特性,在NH3的吸附和储存方面具有广阔的应用前景。而NH3具有孤对电子,会攻击金属与配体之间形成的配位键,使MOF材料的结构遭到破坏。锆基金属有机骨架材料是公认结构稳定性较好的MOF材料,但其是否能胜任干燥NH3及含水条件下的稳定性仍未深入考察,由此需探究该系列材料在NH3吸附领域的适用性。在此,通过实验和计算模拟研究锆基系列的金属有机骨架UiO-66、NU-1000、MOF-801和 MOF-808的结构特征、稳定性和NH3吸附性能。结果表明,UiO-66、NU-1000和MOF-808在纯NH3环境下的稳定性较好,并且显示出高吸附量且可循环的氨吸附性能(13.04、6.38、9.65 mmol/g)。受限于水和氨对结构的协同破坏作用,NU-1000和MOF-801的结构均不能维持,而UiO-66和MOF-808的结构非常稳定,无论在干燥NH3环境及含水NH3环境下均能胜任而应用于NH3吸附和储存。
中图分类号:
王毅, 熊启钊, 陈杨, 杨江峰, 李立博, 李晋平. 锆基金属有机骨架材料用于氨吸附性能的研究[J]. 化工学报, 2022, 73(4): 1772-1780.
Yi WANG, Qizhao XIONG, Yang CHEN, Jiangfeng YANG, Libo LI, Jinping LI. Research on Zr-based metal-organic frameworks for NH3 adsorption[J]. CIESC Journal, 2022, 73(4): 1772-1780.
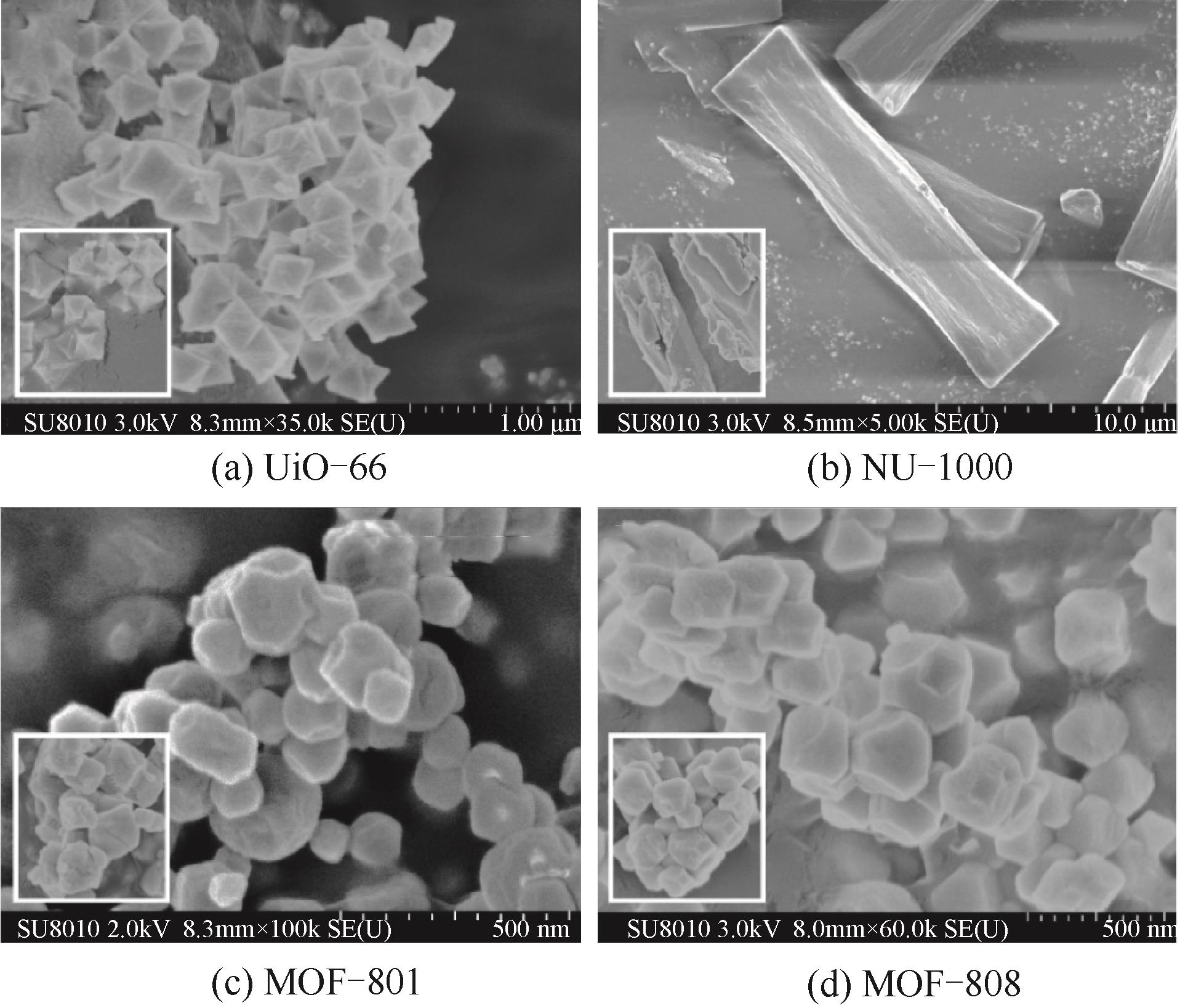
图2 合成的 UiO-66、NU-1000、MOF-801和MOF-808的形貌及其NH3/H2O共吸附之后的形貌
Fig.2 SEM images of synthesized UiO-66, NU-1000, MOF-801, and MOF-808 and after NH3/H2O co-adsorption
| 材料 | BET比表面积/(m2/g) | 孔径/? | 孔体积/(cm3/g) | 298 K NH3吸附量/(mmol/g) |
|---|---|---|---|---|
| UiO-66 | 916 | 8.4,7.4 | 0.49 | 13.04 |
| NU-1000 | 1567 | 31 | 1.4 | 6.38 |
| MOF-801 | 873 | 7.4,5.6,4.8 | 0.45 | 9.85 |
| MOF-808 | 1512 | 4.8,18.4 | 0.84 | 9.65 |
表1 孔结构及吸附性能
Table 1 Pore structure and adsorption performance of the samples
| 材料 | BET比表面积/(m2/g) | 孔径/? | 孔体积/(cm3/g) | 298 K NH3吸附量/(mmol/g) |
|---|---|---|---|---|
| UiO-66 | 916 | 8.4,7.4 | 0.49 | 13.04 |
| NU-1000 | 1567 | 31 | 1.4 | 6.38 |
| MOF-801 | 873 | 7.4,5.6,4.8 | 0.45 | 9.85 |
| MOF-808 | 1512 | 4.8,18.4 | 0.84 | 9.65 |

图7 在1 bar、25℃下使用 GCMC 计算的UiO-66、NU-1000、MOF-801和MOF-808中NH3分子(红点)的吸附分布
Fig.7 The adsorbed NH3 molecule density (red points) in UiO-66, NU-1000, MOF-801 and MOF-808 obtained from the GCMC simulations at 1 bar and 25℃
| 1 | Li K, Andersen S Z, Statt M J, et al. Enhancement of lithium-mediated ammonia synthesis by addition of oxygen[J]. Science, 2021, 374(6575): 1593-1597. |
| 2 | Zhang Y Y, Zhang X, Chen Z J, et al. A flexible interpenetrated zirconium-based metal-organic framework with high affinity toward ammonia[J]. ChemSusChem, 2020, 13(7): 1710-1714. |
| 3 | MacFarlane D R, Choi J, Suryanto B H, et al. Liquefied sunshine: transforming renewables into fertilizers and energy carriers with electromaterials[J]. Advanced Materials, 2020, 32(18): e1904804. |
| 4 | MacFarlane D R, Cherepanov P V, Choi J, et al. A roadmap to the ammonia economy[J]. Joule, 2020, 4(6): 1186-1205. |
| 5 | Guo J P, Chen P. Catalyst: NH3 as an energy carrier[J]. Chem, 2017, 3(5): 709-712. |
| 6 | Chen Y, Zhang F, Wang Y, et al. Recyclable ammonia uptake of a MIL series of metal-organic frameworks with high structural stability[J]. Microporous and Mesoporous Materials, 2018, 258: 170-177. |
| 7 | Zhao Y, Setzler B P, Wang J, et al. An efficient direct ammonia fuel cell for affordable carbon-neutral transportation[J]. Joule, 2019, 3(10): 2472-2484 |
| 8 | 王均利, 曾少娟, 陈能, 等. 氨气吸附材料的研究进展[J]. 过程工程学报, 2019, 19(1): 14-24. |
| Wang J L, Zeng S J, Chen N, et al. Research progress of ammonia adsorption materials[J]. The Chinese Journal of Process Engineering, 2019, 19(1): 14-24. | |
| 9 | 金青青, 梁晓怿, 张佳楠, 等. 改性球形活性炭对氨气吸附性能的研究[J]. 无机盐工业, 2021, 53(4): 61-66. |
| Jin Q Q, Liang X Y, Zhang J N, et al. Study on adsorption performance of modified spherical activated carbon for ammonia[J]. Inorganic Chemicals Industry, 2021, 53(4): 61-66. | |
| 10 | 杨江峰, 欧阳坤, 陈杨, 等. 柔性MOFs材料Cu(BDC)的氨气吸附及可逆转化性能[J]. 化工学报, 2017, 68(1): 418-423. |
| Yang J F, Ouyang K, Chen Y, et al. NH3 adsorption on flexy reversible metal-organic frameworks Cu(BDC)[J]. CIESC Journal, 2017, 68(1): 418-423. | |
| 11 | Sharonov V E, Aristov Y I. Ammonia adsorption by MgCl2, CaCl2 and BaCl2 confined to porous alumina: the fixed bed adsorber[J]. Reaction Kinetics and Catalysis Letters, 2005, 85(1): 183-188. |
| 12 | Helminen J, Helenius J, Paatero E, et al. Adsorption equilibria of ammonia gas on inorganic and organic sorbents at 298.15 K[J]. Journal of Chemical & Engineering Data, 2001, 46(2): 391-399. |
| 13 | Furukawa H, Ko N, Go Y B, et al. Ultrahigh porosity in metal-organic frameworks[J]. Science, 2010, 329(5990): 424-428. |
| 14 | Farha O K, Eryazici I, Jeong N C, et al. Metal-organic framework materials with ultrahigh surface areas: is the sky the limit? [J]. Journal of the American Chemical Society, 2012, 134(36): 15016-15021. |
| 15 | Li J R, Kuppler R J, Zhou H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| 16 | Chen Y, Xiong Q Z, Wang Y, et al. Boosting molecular recognition of acetylene in UiO-66 framework through pore environment functionalization[J]. Chemical Engineering Science, 2021, 237:116572 |
| 17 | Zhou H C, Long J R, Yaghi O M. Introduction to metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 673-674. |
| 18 | Zhou H C J, Kitagawa S. Metal-organic frameworks (MOFs)[J]. Chemical Society Reviews, 2014, 43(16): 5415-5418. |
| 19 | Furukawa H, Cordova K E, O'Keeffe M, et al. The chemistry and applications of metal-organic frameworks[J]. Science, 2013, 341(6149):1230444. |
| 20 | Li J R, Sculley J, Zhou H C. Metal-organic frameworks for separations[J]. Chemical Reviews, 2012, 112(2): 869-932. |
| 21 | Cui Y J, Yue Y F, Qian G D, et al. Luminescent functional metal-organic frameworks[J]. Chemical Reviews, 2012, 112(2): 1126-1162. |
| 22 | Li L B, Lin R-B, Krishna R, et al. Ethane/ethylene separation in a metal-organic framework with iron-peroxo sites[J]. Science, 2018, 362(6413): 443-446. |
| 23 | Travlou N A, Singh K, Rodríguez-Castellón E, et al. Cu-BTC MOF-graphene-based hybrid materials as low concentration ammonia sensors[J]. Journal of Materials Chemistry A, 2015, 3(21): 11417-11429. |
| 24 | Britt D, Tranchemontagne D, Yaghi O M. Metal-organic frameworks with high capacity and selectivity for harmful gases[J]. Proceedings of the National Academy of Sciences of the United States of America, 2008, 105(33): 11623-11627. |
| 25 | Doonan C J, Tranchemontagne D J, Glover T G, et al. Exceptional ammonia uptake by a covalent organic framework[J]. Nature Chemistry, 2010, 2(3): 235-238. |
| 26 | Glover T G, Peterson G W, Schindler B J, et al. MOF-74 building unit has a direct impact on toxic gas adsorption[J]. Chemical Engineering Science, 2011, 66(2): 163-170. |
| 27 | Petit C, Bandosz T J. Enhanced adsorption of ammonia on metal-organic framework/graphite oxide composites: analysis of surface interactions[J]. Advanced Functional Materials, 2010, 20(1): 111-118. |
| 28 | Petit C, Mendoza B, Bandosz T J. Reactive adsorption of ammonia on Cu-based MOF/graphene composites[J]. Langmuir, 2010, 26(19): 15302-15309. |
| 29 | Petit C, Bandosz T J. Synthesis, characterization, and ammonia adsorption properties of mesoporous metal–organic framework (MIL(Fe))-graphite oxide composites: exploring the limits of materials fabrication[J]. Advanced Functional Materials, 2011, 21(11): 2108-2117. |
| 30 | Bai Y, Dou Y B, Xie L H, et al. Zr-based metal-organic frameworks: design, synthesis, structure, and applications[J]. Chemical Society Reviews, 2016, 45(8): 2327-2367. |
| 31 | Cavka J H, Jakobsen S, Olsbye U, et al. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability[J]. Journal of the American Chemical Society, 2008, 130(42): 13850-13851. |
| 32 | Deria P, Mondloch J E, Tylianakis E, et al. Perfluoroalkane functionalization of NU-1000 via solvent-assisted ligand incorporation: synthesis and CO2 adsorption studies[J]. Journal of the American Chemical Society, 2013, 135(45): 16801-16804. |
| 33 | Furukawa H, Gándara F, Zhang Y B, et al. Water adsorption in porous metal-organic frameworks and related materials[J]. Journal of the American Chemical Society, 2014, 136(11): 4369-4381. |
| 34 | Chen Y, Yang C Y, Wang X Q, et al. Vapor phase solvents loaded in zeolite as the sustainable medium for the preparation of Cu-BTC and ZIF-8[J]. Chemical Engineering Journal, 2017, 313: 179-186. |
| 35 | Gelb L D, Gubbins K E. Characterization of porous glasses: simulation models, adsorption isotherms, and the Brunauer- Emmett-Teller analysis method[J]. Langmuir, 1998, 14(8): 2097-2111. |
| 36 | Chen Y, Wang Y, Yang C Y, et al. Antenna-protected metal-organic squares for water/ammonia uptake with excellent stability and regenerability[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(6): 5082-5089. |
| 37 | Liu Y, Liu J, Lin Y S, et al. Effects of water vapor and trace gas impurities in flue gas on CO2/N2 separation using ZIF-68[J]. The Journal of Physical Chemistry C, 2014, 118(13): 6744-6751. |
| 38 | Moghadam P Z, Ghosh P, Snurr R Q. Understanding the effects of preadsorbed perfluoroalkanes on the adsorption of water and ammonia in MOFs[J]. The Journal of Physical Chemistry C, 2015, 119(6): 3163-3170. |
| 39 | Nijem N, Fürsich K, Bluhm H, et al. Ammonia adsorption and co-adsorption with water in HKUST-1: spectroscopic evidence for cooperative interactions[J]. The Journal of Physical Chemistry C, 2015, 119(44): 24781-24788. |
| 40 | Huang C C, Li H S, Chen C H. Effect of surface acidic oxides of activated carbon on adsorption of ammonia[J]. Journal of Hazardous Materials, 2008, 159(2/3): 523-527. |
| 41 | Saha D, Deng S G. Ammonia adsorption and its effects on framework stability of MOF-5 and MOF-177[J]. Journal of Colloid and Interface Science, 2010, 348(2): 615-620. |
| 42 | Kajiwara T, Higuchi M, Watanabe D, et al. A systematic study on the stability of porous coordination polymers against ammonia[J]. Chemistry-A European Journal, 2014, 20(47): 15611-15617. |
| 43 | Petit C, Huang L L, Jagiello J, et al. Toward understanding reactive adsorption of ammonia on Cu-MOF/graphite oxide nanocomposites[J]. Langmuir, 2011, 27(21): 13043-13051. |
| 44 | Rieth A J, Tulchinsky Y, Dincă M. High and reversible ammonia uptake in mesoporous azolate metal-organic frameworks with open Mn, Co, and Ni sites[J]. Journal of the American Chemical Society, 2016, 138(30): 9401-9404. |
| 45 | Han X, Lu W P, Chen Y L, et al. High ammonia adsorption in mfm-300 materials: dynamics and charge transfer in host-guest binding[J]. Journal of the American Chemical Society, 2021, 143(8): 3153-3161. |
| [1] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [2] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [3] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [4] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [5] | 蔡斌, 张效林, 罗倩, 党江涛, 左栗源, 刘欣梅. 导电薄膜材料的研究进展[J]. 化工学报, 2023, 74(6): 2308-2321. |
| [6] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [7] | 王子健, 柯明, 李佳涵, 李舒婷, 孙巾茹, 童燕兵, 赵治平, 刘加英, 任璐. 短b轴ZSM-5分子筛制备方法及应用研究进展[J]. 化工学报, 2023, 74(4): 1457-1473. |
| [8] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [9] | 陈向上, 马振杰, 任希华, 贾悦, 吕晓龙, 陈华艳. 三维网络萃取膜的制备及传质效率研究[J]. 化工学报, 2023, 74(3): 1126-1133. |
| [10] | 杨宏欣, 李兴亚, 葛亮, 徐铜文. 含哌啶阳离子侧长链型一/二价阴离子选择性分离膜的制备[J]. 化工学报, 2022, 73(8): 3739-3748. |
| [11] | 朱江伟, 马鹏飞, 杜晓, 杨言言, 郝晓刚, 罗善霞. 基于可变价NiFe-LDH/rGO对磷酸根离子的特异性电控分离[J]. 化工学报, 2022, 73(7): 3057-3067. |
| [12] | 宋健斐, 孙立强, 解明, 魏耀东. 旋风分离器内气相旋转流不稳定性的实验研究[J]. 化工学报, 2022, 73(7): 2858-2864. |
| [13] | 徐珂, 史国强, 薛冬峰. 无机杂化钙钛矿团簇材料:介尺度钙钛矿材料发光性质研究[J]. 化工学报, 2022, 73(6): 2748-2756. |
| [14] | 任玉鑫, 徐润峰, 王婉颖, 陈鹏忠, 彭孝军. 彩色光刻胶用蒽醌染料的合成及稳定性研究[J]. 化工学报, 2022, 73(5): 2251-2261. |
| [15] | 宋超宇, 熊亚选, 张金花, 金宇贺, 药晨华, 王辉祥, 丁玉龙. 污泥焚烧炉渣基定型复合相变储热材料的制备和性能[J]. 化工学报, 2022, 73(5): 2279-2287. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号