化工学报 ›› 2019, Vol. 70 ›› Issue (6): 2110-2116.DOI: 10.11949/0438-1157.20190123
收稿日期:2019-02-18
修回日期:2019-03-22
出版日期:2019-06-05
发布日期:2019-06-05
通讯作者:
彭昌军
作者简介:<named-content content-type="corresp-name">李进龙</named-content>(1976—),男,博士,副教授,<email>lijinlong@cczu.edu.cn</email>
基金资助:
Jinlong LI1( ),Jiashu LI1,Qing YANG2,Changjun PENG2(
),Jiashu LI1,Qing YANG2,Changjun PENG2( ),Honglai LIU2
),Honglai LIU2
Received:2019-02-18
Revised:2019-03-22
Online:2019-06-05
Published:2019-06-05
Contact:
Changjun PENG
摘要:
采用改进的Ellis平衡蒸馏仪测定了乙腈+水+1-乙基-3-甲基咪唑磷酸二乙酯盐([EMIM][DEP])、乙腈+水+{1-乙基-3-甲基咪唑醋酸盐([EMIM][OAC])+[EMIM][DEP]}常压(101.3 kPa)等压汽液平衡(VLE)数据。实验结果表明,备选离子液体可促进水+乙腈混合物的分离并消除其共沸点。借助NRTL模型成功关联了含离子液体的三元和四元VLE实验数据,获得了乙腈-[EMIM][DEP]、水-[EMIM][DEP]和[EMIM][OAC]-[EMIM][DEP]二元交互作用参数。应用COSMO-SAC预测了实验VLE,结果令人满意。量化计算表明可与水形成强相互作用的离子液体更易促进乙腈与水的分离。
中图分类号:
李进龙, 李佳书, 杨青, 彭昌军, 刘洪来. 乙腈+水+离子液体等压汽液平衡测定与计算[J]. 化工学报, 2019, 70(6): 2110-2116.
Jinlong LI, Jiashu LI, Qing YANG, Changjun PENG, Honglai LIU. Determination and calculation of isobaric vapor equilibrium for acetonitrile + water + ionic liquid[J]. CIESC Journal, 2019, 70(6): 2110-2116.
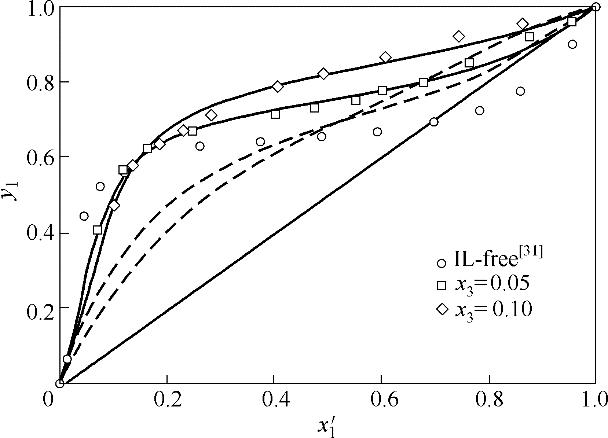
图1 乙腈(1)+水(2)+[EMIM][DEP]( 3)体系x′-y相图
Fig.1 x 1′-y 1 diagram for acetonitrile (1) + water (2) + [EMIM][DEP] (3) (solid lines, NRTL; dash lines, COSMO-SAC)
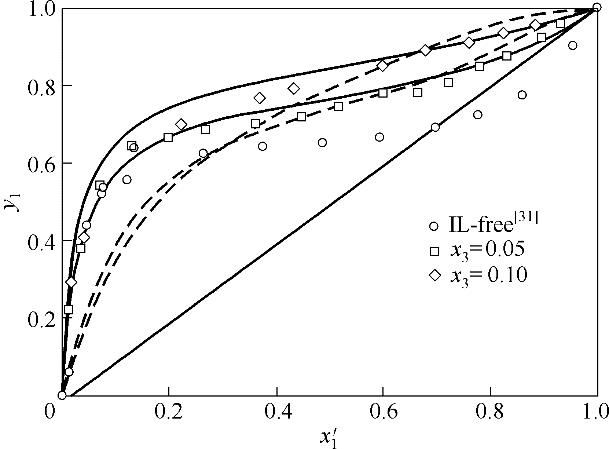
图2 乙腈(1)+水(2)+{[EMIM][OAC]+[EMIM][DEP]}( 3)体系x′-y相图
Fig.2 x 1′-y 1 diagram for acetonitrile(1) + water(2) + {[EMIM][OAC]+[EMIM][DEP]}(3) (solid lines, NRTL; dash lines, COSMO-SAC)
| x 3 | T exp / K | x 1' | y 1 | T cal / K | y cal 1 |
|---|---|---|---|---|---|
| 0.05 | 367.34 | 0.0411 | 0.2247 | 369.44 | 0.2178 |
| 360.35 | 0.0656 | 0.4110 | 364.08 | 0.3764 | |
| 355.08 | 0.1212 | 0.5615 | 356.95 | 0.5496 | |
| 352.33 | 0.1653 | 0.6242 | 354.05 | 0.6123 | |
| 350.26 | 0.2489 | 0.6650 | 351.36 | 0.6731 | |
| 350.13 | 0.4063 | 0.7102 | 350.06 | 0.7262 | |
| 350.23 | 0.4772 | 0.7277 | 350.11 | 0.7436 | |
| 350.10 | 0.5507 | 0.7475 | 350.38 | 0.7618 | |
| 350.82 | 0.6028 | 0.7774 | 350.68 | 0.7759 | |
| 351.07 | 0.6788 | 0.8044 | 351.27 | 0.7994 | |
| 351.72 | 0.7627 | 0.8453 | 352.13 | 0.8316 | |
| 352.38 | 0.8750 | 0.9207 | 353.73 | 0.8912 | |
| 354.02 | 0.9509 | 0.9619 | 355.26 | 0.9498 | |
| 0.10 | 368.97 | 0.0688 | 0.2624 | 371.79 | 0.3066 |
| 361.90 | 0.0972 | 0.4758 | 366.51 | 0.4525 | |
| 358.50 | 0.1392 | 0.5778 | 361.23 | 0.5762 | |
| 355.44 | 0.1861 | 0.6334 | 357.62 | 0.6527 | |
| 354.57 | 0.2293 | 0.6757 | 355.51 | 0.6971 | |
| 353.28 | 0.2811 | 0.7112 | 353.91 | 0.7337 | |
| 352.34 | 0.4098 | 0.7822 | 352.31 | 0.7908 | |
| 352.38 | 0.4907 | 0.8257 | 352.28 | 0.8165 | |
| 352.04 | 0.6075 | 0.8714 | 352.98 | 0.8499 | |
| 353.59 | 0.7434 | 0.9202 | 354.61 | 0.8908 | |
| 355.20 | 0.8629 | 0.9584 | 356.63 | 0.9338 |
表1 乙腈(1)+水(2)+[EMIM][DEP](3)三元汽液平衡数据
Table 1 Isobaric VLE data for acetonitrile (1)+water (2)+[EMIM][DEP] (3) at 101.3 kPa
| x 3 | T exp / K | x 1' | y 1 | T cal / K | y cal 1 |
|---|---|---|---|---|---|
| 0.05 | 367.34 | 0.0411 | 0.2247 | 369.44 | 0.2178 |
| 360.35 | 0.0656 | 0.4110 | 364.08 | 0.3764 | |
| 355.08 | 0.1212 | 0.5615 | 356.95 | 0.5496 | |
| 352.33 | 0.1653 | 0.6242 | 354.05 | 0.6123 | |
| 350.26 | 0.2489 | 0.6650 | 351.36 | 0.6731 | |
| 350.13 | 0.4063 | 0.7102 | 350.06 | 0.7262 | |
| 350.23 | 0.4772 | 0.7277 | 350.11 | 0.7436 | |
| 350.10 | 0.5507 | 0.7475 | 350.38 | 0.7618 | |
| 350.82 | 0.6028 | 0.7774 | 350.68 | 0.7759 | |
| 351.07 | 0.6788 | 0.8044 | 351.27 | 0.7994 | |
| 351.72 | 0.7627 | 0.8453 | 352.13 | 0.8316 | |
| 352.38 | 0.8750 | 0.9207 | 353.73 | 0.8912 | |
| 354.02 | 0.9509 | 0.9619 | 355.26 | 0.9498 | |
| 0.10 | 368.97 | 0.0688 | 0.2624 | 371.79 | 0.3066 |
| 361.90 | 0.0972 | 0.4758 | 366.51 | 0.4525 | |
| 358.50 | 0.1392 | 0.5778 | 361.23 | 0.5762 | |
| 355.44 | 0.1861 | 0.6334 | 357.62 | 0.6527 | |
| 354.57 | 0.2293 | 0.6757 | 355.51 | 0.6971 | |
| 353.28 | 0.2811 | 0.7112 | 353.91 | 0.7337 | |
| 352.34 | 0.4098 | 0.7822 | 352.31 | 0.7908 | |
| 352.38 | 0.4907 | 0.8257 | 352.28 | 0.8165 | |
| 352.04 | 0.6075 | 0.8714 | 352.98 | 0.8499 | |
| 353.59 | 0.7434 | 0.9202 | 354.61 | 0.8908 | |
| 355.20 | 0.8629 | 0.9584 | 356.63 | 0.9338 |
| x 3 | T exp / K | x 1' | y 1 | T cal / K | y cal 1 |
|---|---|---|---|---|---|
| 0.05 | 369.44 | 0.0097 | 0.2244 | 370.15 | 0.1783 |
| 363.08 | 0.0343 | 0.3822 | 363.22 | 0.3837 | |
| 356.20 | 0.0705 | 0.5409 | 358.05 | 0.5133 | |
| 350.66 | 0.1288 | 0.6457 | 353.69 | 0.6113 | |
| 350.13 | 0.1975 | 0.6635 | 351.16 | 0.6683 | |
| 349.71 | 0.2671 | 0.6828 | 349.94 | 0.7016 | |
| 349.75 | 0.3576 | 0.7036 | 349.37 | 0.7302 | |
| 349.79 | 0.4456 | 0.7214 | 349.38 | 0.7523 | |
| 350.30 | 0.5188 | 0.7427 | 349.65 | 0.7701 | |
| 350.51 | 0.6000 | 0.7804 | 350.14 | 0.7915 | |
| 350.51 | 0.6671 | 0.7809 | 350.70 | 0.8116 | |
| 351.05 | 0.7223 | 0.8078 | 351.25 | 0.8308 | |
| 351.70 | 0.7810 | 0.8446 | 351.94 | 0.8544 | |
| 352.29 | 0.8315 | 0.8759 | 352.65 | 0.8787 | |
| 353.34 | 0.8942 | 0.9269 | 353.69 | 0.9149 | |
| 354.04 | 0.9303 | 0.9571 | 354.39 | 0.9398 | |
| 0.10 | 370.86 | 0.0128 | 0.2920 | 372.20 | 0.2630 |
| 366.54 | 0.0369 | 0.4089 | 364.25 | 0.4741 | |
| 359.12 | 0.0719 | 0.5444 | 358.72 | 0.5968 | |
| 354.24 | 0.1315 | 0.6394 | 354.27 | 0.6880 | |
| 353.48 | 0.2226 | 0.7001 | 351.31 | 0.7540 | |
| 353.45 | 0.3668 | 0.7673 | 349.99 | 0.8091 | |
| 353.40 | 0.4340 | 0.7927 | 350.05 | 0.8277 | |
| 353.26 | 0.5986 | 0.8552 | 351.18 | 0.8688 | |
| 353.36 | 0.6800 | 0.8843 | 352.12 | 0.8896 | |
| 353.05 | 0.7627 | 0.9058 | 353.29 | 0.9126 | |
| 353.90 | 0.8265 | 0.9399 | 354.32 | 0.9322 | |
| 354.01 | 0.8846 | 0.9544 | 355.38 | 0.9521 |
表2 乙腈(1)+水(2)+{[EMIM][OAC]+[EMIM][DEP]}(3)四元汽液平衡数据
Table 2 Isobaric VLE data for acetonitrile(1)+water(2)+{[EMIM][OAC]+[EMIM][DEP]}(3) at 101.3 kPa
| x 3 | T exp / K | x 1' | y 1 | T cal / K | y cal 1 |
|---|---|---|---|---|---|
| 0.05 | 369.44 | 0.0097 | 0.2244 | 370.15 | 0.1783 |
| 363.08 | 0.0343 | 0.3822 | 363.22 | 0.3837 | |
| 356.20 | 0.0705 | 0.5409 | 358.05 | 0.5133 | |
| 350.66 | 0.1288 | 0.6457 | 353.69 | 0.6113 | |
| 350.13 | 0.1975 | 0.6635 | 351.16 | 0.6683 | |
| 349.71 | 0.2671 | 0.6828 | 349.94 | 0.7016 | |
| 349.75 | 0.3576 | 0.7036 | 349.37 | 0.7302 | |
| 349.79 | 0.4456 | 0.7214 | 349.38 | 0.7523 | |
| 350.30 | 0.5188 | 0.7427 | 349.65 | 0.7701 | |
| 350.51 | 0.6000 | 0.7804 | 350.14 | 0.7915 | |
| 350.51 | 0.6671 | 0.7809 | 350.70 | 0.8116 | |
| 351.05 | 0.7223 | 0.8078 | 351.25 | 0.8308 | |
| 351.70 | 0.7810 | 0.8446 | 351.94 | 0.8544 | |
| 352.29 | 0.8315 | 0.8759 | 352.65 | 0.8787 | |
| 353.34 | 0.8942 | 0.9269 | 353.69 | 0.9149 | |
| 354.04 | 0.9303 | 0.9571 | 354.39 | 0.9398 | |
| 0.10 | 370.86 | 0.0128 | 0.2920 | 372.20 | 0.2630 |
| 366.54 | 0.0369 | 0.4089 | 364.25 | 0.4741 | |
| 359.12 | 0.0719 | 0.5444 | 358.72 | 0.5968 | |
| 354.24 | 0.1315 | 0.6394 | 354.27 | 0.6880 | |
| 353.48 | 0.2226 | 0.7001 | 351.31 | 0.7540 | |
| 353.45 | 0.3668 | 0.7673 | 349.99 | 0.8091 | |
| 353.40 | 0.4340 | 0.7927 | 350.05 | 0.8277 | |
| 353.26 | 0.5986 | 0.8552 | 351.18 | 0.8688 | |
| 353.36 | 0.6800 | 0.8843 | 352.12 | 0.8896 | |
| 353.05 | 0.7627 | 0.9058 | 353.29 | 0.9126 | |
| 353.90 | 0.8265 | 0.9399 | 354.32 | 0.9322 | |
| 354.01 | 0.8846 | 0.9544 | 355.38 | 0.9521 |
| component i | component j | (g ij -g jj )/ (J·mol-1) | (g ji -g ii )/ (J·mol-1) | αij |
|---|---|---|---|---|
| acetonitrile | water | 2214.21 | 5202.99 | 0.3545 |
| acetonitrile | [EMIM][OAC] | 11936.91 | -4743.74 | 0.3 |
| water | [EMIM][OAC] | 24633.47 | -13615.84 | 0.3 |
| acetonitrile | [EMIM][DEP] | -16256.15 | -3321.27 | 0.9 |
| water | [EMIM][DEP] | -8388.62 | -18920.54 | 0.1 |
| [EMIM][OAC] | [EMIM][DEP] | -25040.97 | 44917.49 | 0.6750 |
表3 NRTL模型二元交互参数
Table 3 Binary interaction parameters for NRTL equation
| component i | component j | (g ij -g jj )/ (J·mol-1) | (g ji -g ii )/ (J·mol-1) | αij |
|---|---|---|---|---|
| acetonitrile | water | 2214.21 | 5202.99 | 0.3545 |
| acetonitrile | [EMIM][OAC] | 11936.91 | -4743.74 | 0.3 |
| water | [EMIM][OAC] | 24633.47 | -13615.84 | 0.3 |
| acetonitrile | [EMIM][DEP] | -16256.15 | -3321.27 | 0.9 |
| water | [EMIM][DEP] | -8388.62 | -18920.54 | 0.1 |
| [EMIM][OAC] | [EMIM][DEP] | -25040.97 | 44917.49 | 0.6750 |
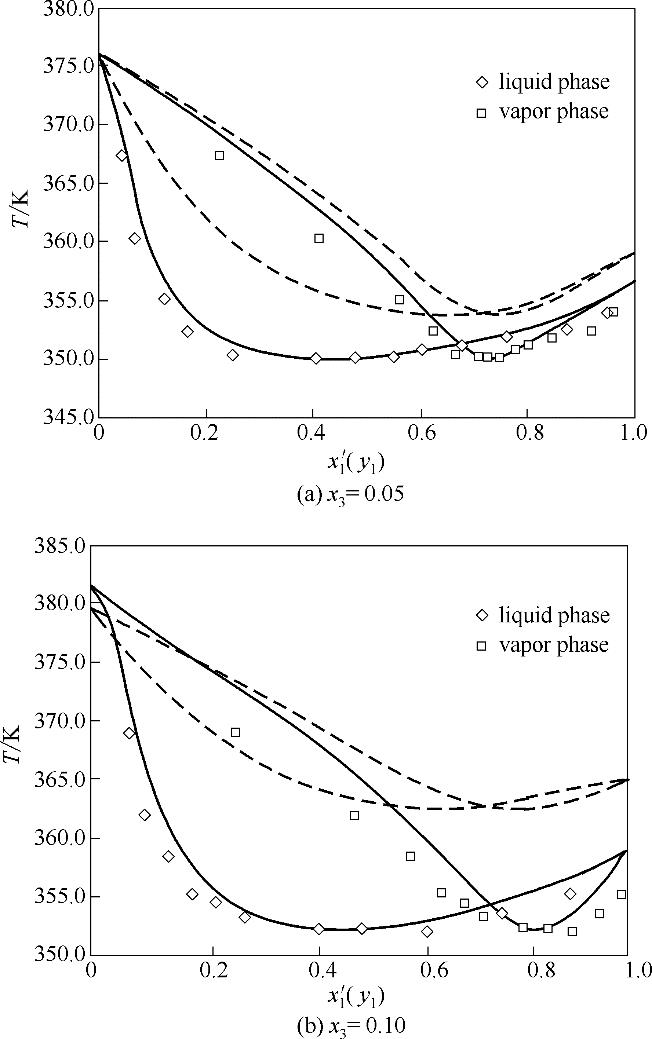
图3 x 3=0.05和0.10时乙腈(1)+水(2)+[EMIM][DEP](3)体系T-x′(y)相图
Fig.3 T-x′ (y) diagram for acetonitrile (1) + water (2) + [EMIM][DEP](3) at x 3=0.05 and 0.10(solid lines, NRTL; dash lines, COSMO-SAC)
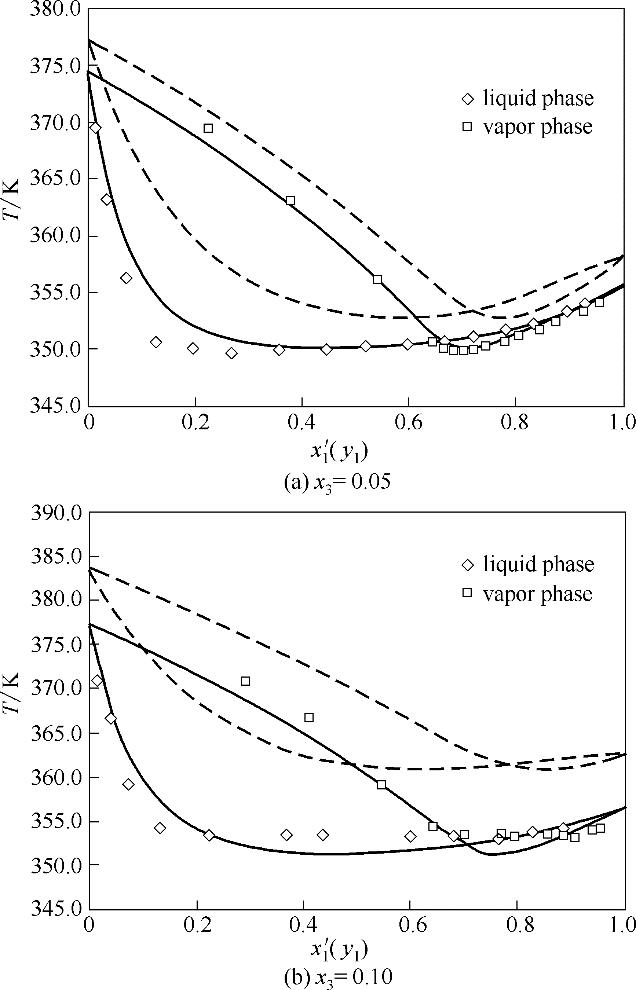
图4 x 3=0.05和0.10时乙腈(1)+水(2)+ {[EMIM][OAC]+[EMIM][DEP]}(3)体系T-x′(y)相图
Fig.4 T-x′(y) diagram for acetonitrile(1) + water(2) + {[EMIM][DEP]+[EMIM][OAC]}(3) at x 3=0.05 and 0.10(solid lines, NRTL; dash lines, COSMO-SAC)
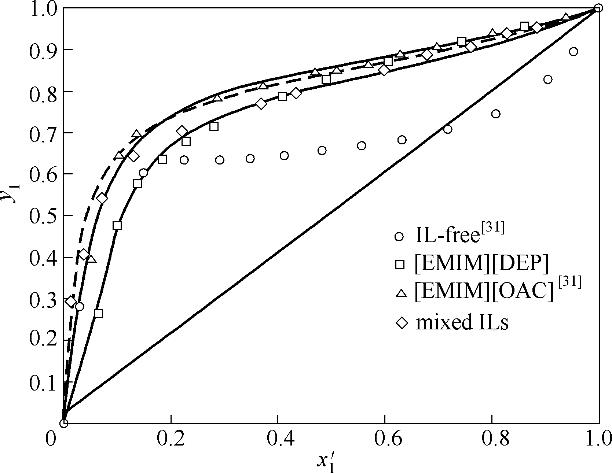
图5 离子液体促进分离性能比较
Fig.5 Effects of different ILs (x 3=0.10) on separation ability of acetonitrile (1) and water (2)(lines, NRTL; dash line, mixed ILs from NRTL)
| Component | Acetonitrile/(kJ·mol-1) | Water/(kJ·mol-1) |
|---|---|---|
| [EMIM][OAC] | -4.41 | -25.96 |
| [EMIM][DEP] | -3.15 | -11.94 |
| water | -10.16 | — |
表4 离子液体与水、乙腈相互作用能
Table 4 Interaction energy between ILs and water/acetonitrile
| Component | Acetonitrile/(kJ·mol-1) | Water/(kJ·mol-1) |
|---|---|---|
| [EMIM][OAC] | -4.41 | -25.96 |
| [EMIM][DEP] | -3.15 | -11.94 |
| water | -10.16 | — |
| 1 | McConvey I F , Woods D , Lewis M , et al . The importance of acetonitrile in the pharmaceutical industry and opportunities for its recovery from waste[J]. Org. Process Res. Dev., 2012, 16: 612-624. |
| 2 | Rosen J , Hellenas K E . Analysis of acrylamide in cooked foods by liquid chromatography tandem mass spectrometry[J]. Analyst, 2002, 127: 880-882. |
| 3 | 贾自成, 董满祥, 童俊国, 等 . 乙腈法抽提丁二烯装置的改扩建[J]. 石油炼制与化工, 1998, 29(9): 36-39. |
| Jia Z C , Dong M X , Tong J G , et al . Revamping and enlargement of a butadiene extraction unit with acetonitrile method[J]. Petroleum Process. Petrochem., 1998, 29(9): 36-39. | |
| 4 | Gmehling J , Menke J , Krafczyk J , et al . Azeotropic Data[M]. Weinheim, Germany: VCH, 1994. |
| 5 | Liang K , Li W S , Luo H T , et al . Energy-efficient extractive distillation process by combining preconcentration column and entrainer recovery column[J]. Ind. Eng. Chem. Res., 2014, 53: 7121-7131. |
| 6 | Acosta J , Arce A , Rodil E , et al . A thermodynamic study on binary and ternary mixtures of acetonitrile, water and butyl acetate[J]. Fluid Phase Equilib., 2002, 203: 83-98. |
| 7 | Yu B Y , Huang R , Zhong X Y , et al . Energy-efficient extraction-distillation process for separating diluted acetonitrile-water mixture: rigorous design with experimental verification from ternary liquid-liquid equilibrium data[J]. Ind. Eng. Chem. Res., 2017, 56: 15112-15121. |
| 8 | 许文友 . 乙腈-水-氟化钾及乙腈-水-碳酸钾液液相平衡数据的测定和关联[J]. 化工学报, 2001, 52(8): 742-745. |
| Xu W Y . Measurement and correlation of liquid-liquid equilibrium data for acetonitrile-water-potassium fluoride and acetonitrile-water-potassium carbonate systems[J]. Journal of Chemical Industry and Engineering (China), 2001, 52(8): 742-745. | |
| 9 | 崔现宝, 李杨, 冯天扬, 等 . 加盐萃取精馏分离乙腈-水物系[J]. 石油化工, 2007, 36(12): 1229-1233. |
| Cui X B , Li Y , Feng T Y , et al . Separation of acetonitrile-water by saline extractive distillation[J]. Petrochem. Technol., 2007, 36(12): 1229-1233. | |
| 10 | Wishart J F , Castner J E W . The physical chemistry of ionic liquids[J]. J.Phys.Chem.B, 2007, 111: 201-208. |
| 11 | Welton T . Room-temperature ionic liquids. Solvents for synthesis and catalysis[J]. Chem. Rev., 1999, 99: 2071-2083. |
| 12 | Vogl T , Menne S , Kuhnel R S , et al . The beneficial effect of protic ionic liquids on the lithium environment in electrolytes for battery applications[J]. J.Mater.Chem.A, 2014, 2: 8258-8265. |
| 13 | Ye C F , Liu W M , Chen Y X , et al . Room-temperature ionic liquids: a novel versatile lubricant[J]. Chem. Commun., 2001, 2244-2245. |
| 14 | Seiler M , Jork C , Kavarnou A , et al . Separation of azeotropic mixtures using hyperbranched polymers or ionic liquids[J]. AIChE J., 2004, 50(10): 2439-2454. |
| 15 | Lei Z G , Xi X M , Dai C N , et al . Extractive distillation with the mixture of ionic liquid and solid inorganic salt as entrainers[J]. AIChE J., 2014, 60(8): 2994-3004. |
| 16 | Pereiro A B , Araujo J M M , Esperanca J M S S , et al . Ionic liquids in separations of azeotropic systems — a review[J]. J. Chem. Thermodyn., 2012, 46: 2-28. |
| 17 | Artemenko S , Haddad S , Mazur V . Azeotrope breaking potential of ionic liquids in separation processes[J]. J. Mol. Liq., 2017, 235: 49-52. |
| 18 | Boli E , Dimou E , Voutsas E . Separation of the isopropanol-water azeotropic mixture using ionic liquids[J]. Fluid Phase Equilib., 2018, 456: 77-83. |
| 19 | Li W X , Du Y , Li J P , et al . Isobaric vapor-liquid equilibrium for acetone + methanol system containing different ionic liquids at 101.3 kPa[J]. Fluid Phase Equilib., 2018, 459: 10-17. |
| 20 | Wang P , Xu D M , Yan P S , et al . Separation of azeotrope (ethanol and ethyl methyl carbonate) by different imidazolium-based ionic liquids: ionic liquids interaction analysis and phase equilibrium measurements[J]. J. Mol. Liq., 2018, 261: 89-95. |
| 21 | Ma Y X , Xu X C , Wen G L , et al . Separation of azeotropes hexane+ ethanol/1-propanol by ionic liquid extraction: liquid-liquid phase equilibrium measurements and thermodynamic modeling[J]. J.Chem. Eng. Data, 2017, 62(12): 4296-4300. |
| 22 | Zhang Z G , Zhang D B , Li W X , et al . Separation of acetonitrile + ethanol mixture using imidazolium-based ionic liquids as entrainers[J]. Fluid Phase Equilib., 2018, 474: 43-49. |
| 23 | Navarro P , Larriba M , Garcia J , et al . Design of the recovery section of the extracted aromatics in the sparation of BTEX from naphtha feed to ethylene crackers using [4empy][Tf2N] and [emim][DCA] mixed ionic liquids as solvent[J]. Sep. Purif. Technol., 2017, 180: 149-156. |
| 24 | Pereiro A B , Rodriguez A . Phase equilibria of the azeotropic mixture hexane + ethyl acetate with ionic liquids at 298.15 K[J]. J.Chem. Thermodyn., 2008, 53(6): 1360-1366. |
| 25 | Kurzin A V , Evdokimov A N , Poltoratskiy G M , et al . Isothermal vapor-liquid equilibrium data for the systems 1,4-dioxane + water + tetrabutylammonium nitrate and acetonitrile + water + tetrabutylammonium bromide[J]. J.Chem. Eng. Data, 2004, 49(2): 208-211. |
| 26 | Fang J , Liu J , Li C L , et al . Isobaric vapor-liquid equilibrium for the acetonitrile + water system containing different ionic liquids at atmospheric pressure[J]. J. Chem. Eng. Data, 2013, 58: 1483-1489. |
| 27 | Fang J , Zhao R , Wang H , et al . Salting-out effect of ionic liquids on isobaric vapor-liquid equilibrium of acetonitrile-water system[J]. Chin. J.Chem. Eng., 2015, 23(8): 1369-1373. |
| 28 | Kurzin A V , Evdokimov A N , Antipina V B , et al . Measurement and correlation of isothermal vapor-liquid equilibrium data for the system acetonitrile + water + tetrapropylammonium bromide[J]. J. Chem. Eng. Data, 2006, 51: 1361-1363. |
| 29 | Kurzin A V , Evdokimov A N , Antipina V B , et al . Vapor pressures for the acetonitrile + tetrabutylammonium bromide, water + tetrabutylammonium bromide, and acetonitrile + water +tetrabutylammonium bromide systems[J]. J. Chem. Eng. Data, 2009, 54: 1049-1051. |
| 30 | Li T T , Yang Q , Ding H R , et al . Amino acid based ionic liquids as additives for separation of acetonitrile and water azeotropic mixture: COSMO-RS prediction and experimental verification[J]. Ind. Eng. Chem. Res., 2015, 54: 12143-12149. |
| 31 | Li J L , Li T T , Peng C J , et al . Extractive distillation with ionic liquid entrainers for the separation of acetonitrile and water[J]. Ind. Eng. Chem. Res., 2019, 58: 5602-5612. |
| 32 | Lu Q L , Li J L , Peng C J , et al . Experimental determination of vapor liquid equilibrium for methanol + methyl propionate + 1-butyl-3-methylimidazo-lium bis(trifluoromethylsulfonyl)imide at atmospheric pressure[J]. J. Chem. Thermodyn., 2019, 132: 289-294. |
| 33 | Hiaki T , Kawai A . Vapor-liquid equilibria determination for a hydrofluoroether with several alcohols[J]. Fluid Phase Equilib., 1999, 158/159/160: 979-989. |
| 34 | Renon H , Prausnitz J M . Local compositions in thermodynamic excess functions for liquid mixtures[J]. AIChE J., 1968, 14: 135-144. |
| 35 | Klamt A , Eckert F . COSMO-RS: a novel and efficient method for the a priori prediction of thermophysical data of liquids[J]. Fluid Phase Equilib., 2000, 172: 43-72. |
| 36 | Hsieh C M , Sandler S I , Lin S T . Improvements of COSMO-SAC for vapor-liquid and liquid-liquid equilibrium predictions[J]. Fluid Phase Equilib., 2010, 297: 90-97. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [6] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [7] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [8] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [9] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [10] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [11] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [12] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [13] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [14] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [15] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号