化工学报 ›› 2020, Vol. 71 ›› Issue (1): 361-367.DOI: 10.11949/0438-1157.20191261
收稿日期:2019-10-23
修回日期:2019-11-20
出版日期:2020-01-05
发布日期:2020-01-05
通讯作者:
王从敏
作者简介:肖俏欣(1994—),女,硕士研究生,基金资助:
Qiaoxin XIAO( ),Wenjun LIN,Haoran LI,Congmin WANG(
),Wenjun LIN,Haoran LI,Congmin WANG( )
)
Received:2019-10-23
Revised:2019-11-20
Online:2020-01-05
Published:2020-01-05
Contact:
Congmin WANG
摘要:
发展高效、经济、绿色的SO2吸收剂不但具有较强的学术价值,而且有良好的应用前景。设计并制备了一系列含醚的阴离子功能化离子液体,系统地研究了阴离子上引入醚基团对离子液体SO2吸收容量的影响。结果表明,在阴离子的苯环上引入甲氧基,对离子液体的吸收容量有明显提升。当阳离子为摩尔质量更小的三丁基乙基磷[P4442]时,所得离子液体的吸收容量没有明显下降,20℃、105 Pa SO2下,[P4442][2-CH3OPhCOO]有效吸收量为每摩尔离子液体吸收3.32 mol SO2,有效质量吸收量是每克离子液体吸收0.56 g SO2。六次吸收解吸循环,表明[P4442][2-CH3OPhCOO]可以高效可逆地捕集SO2。基于含醚阴离子功能化离子液体的加强效应进行气体捕集的方法,可进一步应用于分离、催化等领域。
中图分类号:
肖俏欣, 林文俊, 李浩然, 王从敏. 含醚阴离子功能化离子液体高效捕集SO2[J]. 化工学报, 2020, 71(1): 361-367.
Qiaoxin XIAO, Wenjun LIN, Haoran LI, Congmin WANG. Efficient SO2 capture by ether-containing anion-functionalized ionic liquids[J]. CIESC Journal, 2020, 71(1): 361-367.
| 离子液体 | 吸收量/(mol·mol-1) | 解吸残余量/(mol·mol-1) | 净吸收量/(mol·mol-1) | 吸收焓①/(kJ·mol-1) |
|---|---|---|---|---|
| [P66614][PhCOO] | 3.56 | 0.51 | 3.05 | -81.21 |
| [P66614][3-CH3OPhCOO] | 4.29 | 0.51 | 3.78 | -96.50 |
| [P66614][2-CH3OPhCOO] | 4.49 | 0.56 | 3.93 | -97.30 |
| [P66614][4-CH3OPhCOO] | 4.22 | 0.53 | 3.69 | -104.30 |
| [P4442][PhCOO] | 3.33 | 0.55 | 2.78 | -81.21 |
| [P4442][2-CH3OPhCOO] | 4.00 | 0.68 | 3.32 | -97.30 |
表1 含醚阴离子功能化的离子液体在105 Pa下的SO2吸收效果
Table 1 SO2 absorption of ionic liquid functionalized with ether anion at 105 Pa
| 离子液体 | 吸收量/(mol·mol-1) | 解吸残余量/(mol·mol-1) | 净吸收量/(mol·mol-1) | 吸收焓①/(kJ·mol-1) |
|---|---|---|---|---|
| [P66614][PhCOO] | 3.56 | 0.51 | 3.05 | -81.21 |
| [P66614][3-CH3OPhCOO] | 4.29 | 0.51 | 3.78 | -96.50 |
| [P66614][2-CH3OPhCOO] | 4.49 | 0.56 | 3.93 | -97.30 |
| [P66614][4-CH3OPhCOO] | 4.22 | 0.53 | 3.69 | -104.30 |
| [P4442][PhCOO] | 3.33 | 0.55 | 2.78 | -81.21 |
| [P4442][2-CH3OPhCOO] | 4.00 | 0.68 | 3.32 | -97.30 |
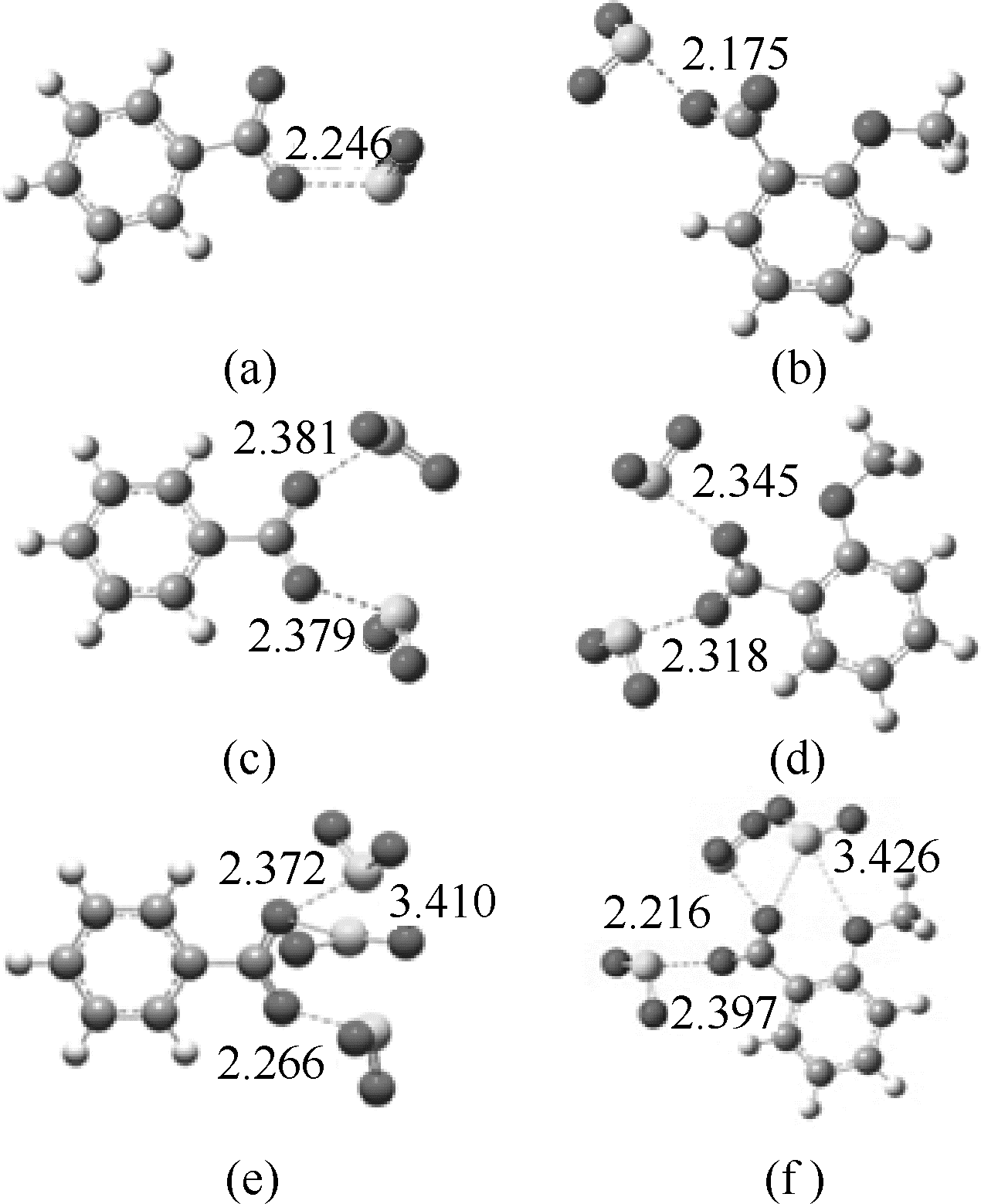
图9 [P4442][PhCOO]、[P4442][2-CH3OPhCOO]吸收SO2的DFT计算(键长单位:10-10m)
Fig.9 DFT calculations of interaction between [P4442][PhCOO] or [P4442][2-CH3OPhCOO] with SO2 molecule

图10 [P4442][2-CH3OPhCOO]、[P4442][ PhCOO]吸收SO2前后的红外光谱 a—吸收SO2前的[P4442][PhCOO];b—吸收SO2后的[P4442][PhCOO]; c—吸收SO2前的[P4442][2-CH3OPhCOO];d—吸收SO2后的[P4442][2-CH3OPhCOO]
Fig.10 IR spectra of [P4442][2-CH3OPhCOO] or [P4442][ PhCOO]
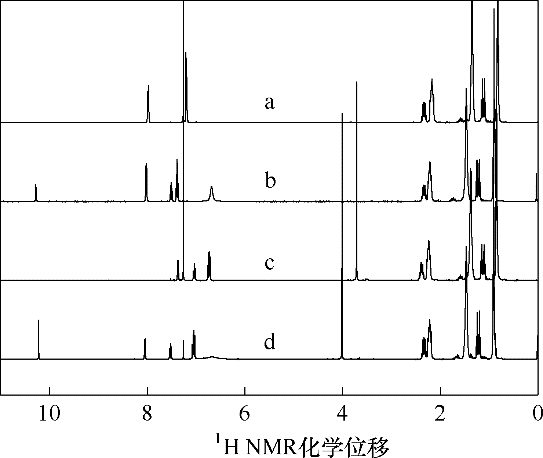
图11 [P4442][2-CH3OPhCOO]、[P4442][ PhCOO]吸收SO2前后的1H NMR谱(溶剂氘代氯仿) a—吸收SO2前的[P4442][PhCOO];b—吸收SO2后的[P4442][PhCOO]; c—吸收SO2前的[P4442][2-CH3OPhCOO];d—吸收SO2后的[P4442][2-CH3OPhCOO]
Fig.11 1H NMR spectra of [P4442][2-CH3OPhCOO] or [P4442][PhCOO]
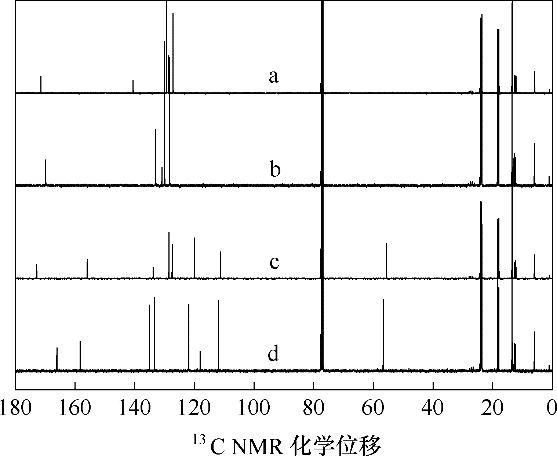
图12 [P4442][2-CH3OPhCOO]、[P4442][ PhCOO]吸收SO2前后的13C NMR谱(溶剂氘代氯仿) a—吸收SO2前的[P4442][PhCOO];b—吸收SO2后的[P4442][PhCOO]; c—吸收SO2前的[P4442][2-CH3OPhCOO];d—吸收SO2后的[P4442][2-CH3OPhCOO]
Fig.12 13C NMR spectra of [P4442][2-CH3OPhCOO] or [P4442][PhCOO]
| 1 | Wu W , Han B , Gao H , et al . Desulfurization of flue gas: SO2 absorption by an ionic liquid[J]. Angew. Chem. Int. Ed. , 2004, 43: 2415-2417. |
| 2 | Nickolay A K , Chris A M , Li C , et al . Streets aura OMI observations of regional SO2 and NO2 pollution changes from 2005 to 2015[J]. Atmos. Chem. Phys., 2016, 16: 4605-4629. |
| 3 | Bao J , Yang X , Zhao Z , et al . The spatial-temporal characteristics of air pollution in China from 2001—2014[J]. Int. J. Environ. Res. Public Health, 2015, 12: 15875-15887. |
| 4 | Hu H , Yang Q , Lu X , et al . Air pollution and control in different areas of China[J]. Critical Reviews in Environmental Science and Technology, 2010, 40(6): 452-518. |
| 5 | Ma X , Takao K , Tsutomu T , et al . Use of limestone for SO2 removal from flue gas in the semidry FGD process with a powder-particle spouted bed[J]. Chemical Engineering Science, 2000, 55: 4643-4652. |
| 6 | Tilly J . Flue gas desulfurization: cost and functional analysis of large scale proven plants[D]. Massachusetts: Massachusetts Institute of Technology, 1983. |
| 7 | Hirofumi K , Takanori N , Masanori M , et al . New wet FGD process using granular limestone[J]. Ind. Eng. Chem. Res. , 2002, 41(12): 3028-3036. |
| 8 | Han D H , Sohn H Y . Calcined calcium magnesium acetate as a superior SO2 sorbent (Ⅰ): Thermal decomposition[J]. AIChE J., 2002, 48: 2971-2977. |
| 9 | Sohn H Y , Han D H . Ca-Mg acetate as dry SO2 sorbent (Ⅱ): Sulfation of CaO in calcination product[J]. AIChE J. , 2002, 48: 2978-2984. |
| 10 | Sohn H Y , Han D H . Ca-Mg acetate as dry SO2 sorbent(Ⅲ): Sulfation of MgO + CaO[J]. AIChE J. , 2002, 48: 2985-2991. |
| 11 | Blanchard L A , Hancu D , Beckman E J , et al . Green processing using ionic liquids and CO2 [J]. Nature, 1999, 399 (6731): 28-29. |
| 12 | Bausach M , Pera-Titus M , Fite C , et al . Kinetic modeling of the reaction between hydrated lime and SO2 at low temperature[J]. AIChE J., 2005, 51: 1455-1466. |
| 13 | Zhao T , Hu X , Wu D , et al . Direct synthesis of dimethyl carbonate from carbon dioxide and methanol at room temperature using imidazolium hydrogen carbonate ionic liquid as a recyclable catalyst and dehydrant[J]. ChemSusChem, 2017, 10: 2046-2052. |
| 14 | Xu Y . CO2 absorption behavior of azole-based protic ionic liquids: influence of the alkalinity and physicochemical properties[J]. Journal of CO2 Utilization, 2017, 19: 1-8. |
| 15 | Liu X , Gao B , Deng D . SO2 absorption/desorption performance of renewable phenol-based deep eutectic solvents[J]. Separation Science and Technology, 2018, 53(14): 2150-2158. |
| 16 | Gurkan B E , De la fuente J C , Mindrup E M , et al . Equimolar CO2 absorption by anion-functionalized ionic liquids[J]. J. Am. Chem. Soc., 2010, 132: 2116-2117. |
| 17 | Joan F B , Burcu E G . Ionic liquids for CO2 capture and emission reduction[J]. J. Phys. Chem. Lett., 2010, 1(24): 3459-3464. |
| 18 | Brett F G , Juan C F , Burcu E G , et al . Experimental measurements of amine-functionalized anion-tethered ionic liquids with carbon dioxide[J]. Ind. Eng. Chem. Res., 2011, 50: 111-118. |
| 19 | Goodrich B F , de la Fuente J C , Gurkan B E , et al . Effect of water and temperature on absorption of CO2 by amine-functionalized anion-tethered ionic liquids[J]. J. Phys. Chem. B, 2011, 115: 9140-9150. |
| 20 | Lin W , Zhou X , Cai J , et al . Anion-functionalized pillararenes for efficient sulfur dioxide capture: significant effect of the anion and the cavity[J]. Chem. Eur. J., 2017, 23: 14143-14148. |
| 21 | Huang K , Zhang X , Hu X , et al . Hydrophobic protic ionic liquids tethered with tertiary amine group for highly efficient and selective absorption of H2S from CO2 [J]. AIChE J., 2016, 62(12): 4480-4490. |
| 22 | Eleanor D B , Rebecca D M , Ioanna N , et al . CO2 capture by a task-specific ionic liquid[J]. J. Am. Chem. Soc. , 2002, 124(6): 926-927. |
| 23 | Kenta F , Masahiro Y , Hiroyuki O . Room temperature ionic liquids from 20 natural amino acids[J]. J. Am. Chem. Soc. , 2005, 127(8): 2398-2399. |
| 24 | Shiflett M B , Yokozeki A . Chemical absorption of sulfur dioxide in room-temperature ionic liquids[J]. Ind. Eng. Chem. Res., 2010, 49(3): 1370-1377. |
| 25 | Lin W , Cai Z , Lv X , et al . Significantly enhanced carbon dioxide capture by anion-functionalized liquid pillar[5]arene through multiple-site interactions[J]. Ind. Eng. Chem. Res., 2019, 58: 16894-16900. |
| 26 | Gurkan B , Goodrich B F , Mindrup E M , et al . Molecular design of high capacity, low viscosity, chemically tunable ionic liquids for CO2 capture[J]. J. Phys. Chem. Lett., 2010, 1(24): 3494-3499. |
| 27 | Wang C , Luo H , Li H , et al . Tuning the physicochemical properties of diverse phenolic ionic liquids for equimolar CO2 capture by the substituent on the anion[J]. Chem. Eur. J. , 2012, 18: 2153-2160. |
| 28 | Zhang Y Q , Zhang S J , Lu X M , et al . Dual amino-functionalised phosphonium ionic liquids for CO2 capture[J]. Chem. Eur. J., 2009, 15: 3003-3011. |
| 29 | Lin W , Pan M , Xiao Q , et al . Tuning the capture of CO2 through entropic effect induced by reversible trans-cis isomerization of light-responsive ionic liquids[J]. J. Phys. Chem. Lett. , 2019, 10: 3346-3351. |
| 30 | Jiang Y , Liu X , Deng D . Absorption of SO2 in fuloroate ionic liquids/PEG200 mixtures and thermodynamic analysis [J]. J. Chem. Eng. Data, 2018, 63: 259-268. |
| 31 | Stewart A F , Jennifer M P , Douglas R M . Ionic liquids—an overview[J]. Aust. J. Chem., 2004, 57: 113. |
| 32 | Wang C , Luo X , Luo X , et al . Tuning the basicity of ionic liquids for equimolar CO2 capture[J]. Angew. Chem., 2011, 123: 5020. |
| 33 | Craig M T , Dai S , Jiang D . Computational investigation of reactive to nonreactive capture of carbon dioxide by oxygen-containing Lewis bases[J]. J. Phys. Chem. A, 2010, 114: 11761. |
| 34 | Cui G , Zheng J , Luo X , et al . Tuning anion-functionalized ionic liquids for improved SO2 capture[J]. Angew. Chem. , 2013, 125: 10814-10818. |
| 35 | Cui G , Wang C , Zheng J , et al . Highly efficient SO2 capture by dual functionalized ionic liquids through a combination of chemical and physical absorption[J]. Chem. Commun., 2012, 48: 2633-2635. |
| 36 | Chen K , Lin W , Yu X , et al . Designing of anion-functionalized ionic liquids for efficient capture of SO2 from flue gas[J]. AIChE J., 2015, 61: 2028-2034. |
| 37 | Cui G , Zheng J , Luo X , et al . Tuning anion-functionalized ionic liquids for improved SO2 capture[J]. Angew. Chem. , 2013, 125: 10814-10818. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [10] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [11] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [12] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [13] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [14] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [15] | 杨灿, 孙雪琦, 尚明华, 张建, 张香平, 曾少娟. 相变离子液体体系吸收分离CO2的研究现状及展望[J]. 化工学报, 2023, 74(4): 1419-1432. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号