化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2889-2899.DOI: 10.11949/0438-1157.20200075
王永胜( ),兰小林,邱天,张新平,吴莹莹,陈莉,徐伟祥,郭栋杰,段正康(
),兰小林,邱天,张新平,吴莹莹,陈莉,徐伟祥,郭栋杰,段正康( )
)
收稿日期:2020-01-17
修回日期:2020-03-30
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
段正康
作者简介:王永胜(1995—),男,硕士研究生,基金资助:
Yongsheng WANG( ),Xiaolin LAN,Tian QIU,Xinping ZHANG,Yingying WU,Li CHEN,Weixiang XU,Dongjie GUO,Zhengkang DUAN(
),Xiaolin LAN,Tian QIU,Xinping ZHANG,Yingying WU,Li CHEN,Weixiang XU,Dongjie GUO,Zhengkang DUAN( )
)
Received:2020-01-17
Revised:2020-03-30
Online:2020-06-05
Published:2020-06-05
Contact:
Zhengkang DUAN
摘要:
采用原位水热法制备了Cu/rGO催化剂并引入对苯二甲酸(TPA)对Cu基石墨烯复合材料进行改性研究,探究了不同溶剂、水热时间、沉淀pH对引入TPA的Cu/rGO催化剂材料微观结构特性的影响。通过XRD、FT-IR、XPS和SEM表征技术分析了催化剂的形貌结构及物化性质。考察了催化剂用于二乙醇胺脱氢的催化性能。在V(乙醇)∶V(水)=1∶1为溶剂,沉淀pH为13.0,160℃水热10 h,制备的催化剂性能最好,亚氨基二乙酸收率为86.55%,与没有添加TPA的Cu/rGO催化剂相比收率提高20%。TPA的加入,增强了GO片层间的相互作用,增加了GO片层间的有机官能团,稳定Cu2O并使其结晶度较好,增加催化剂活性位点,以提高反应速率。且粒径约为10 nm左右的Cu纳米粒子均匀分布在褶皱状片层结构的rGO表面,提高催化剂抗烧结性能。
中图分类号:
王永胜, 兰小林, 邱天, 张新平, 吴莹莹, 陈莉, 徐伟祥, 郭栋杰, 段正康. 铜基石墨烯复合催化剂的合成与表征[J]. 化工学报, 2020, 71(6): 2889-2899.
Yongsheng WANG, Xiaolin LAN, Tian QIU, Xinping ZHANG, Yingying WU, Li CHEN, Weixiang XU, Dongjie GUO, Zhengkang DUAN. Synthesis and characterization of copper-based graphene composite catalyst[J]. CIESC Journal, 2020, 71(6): 2889-2899.
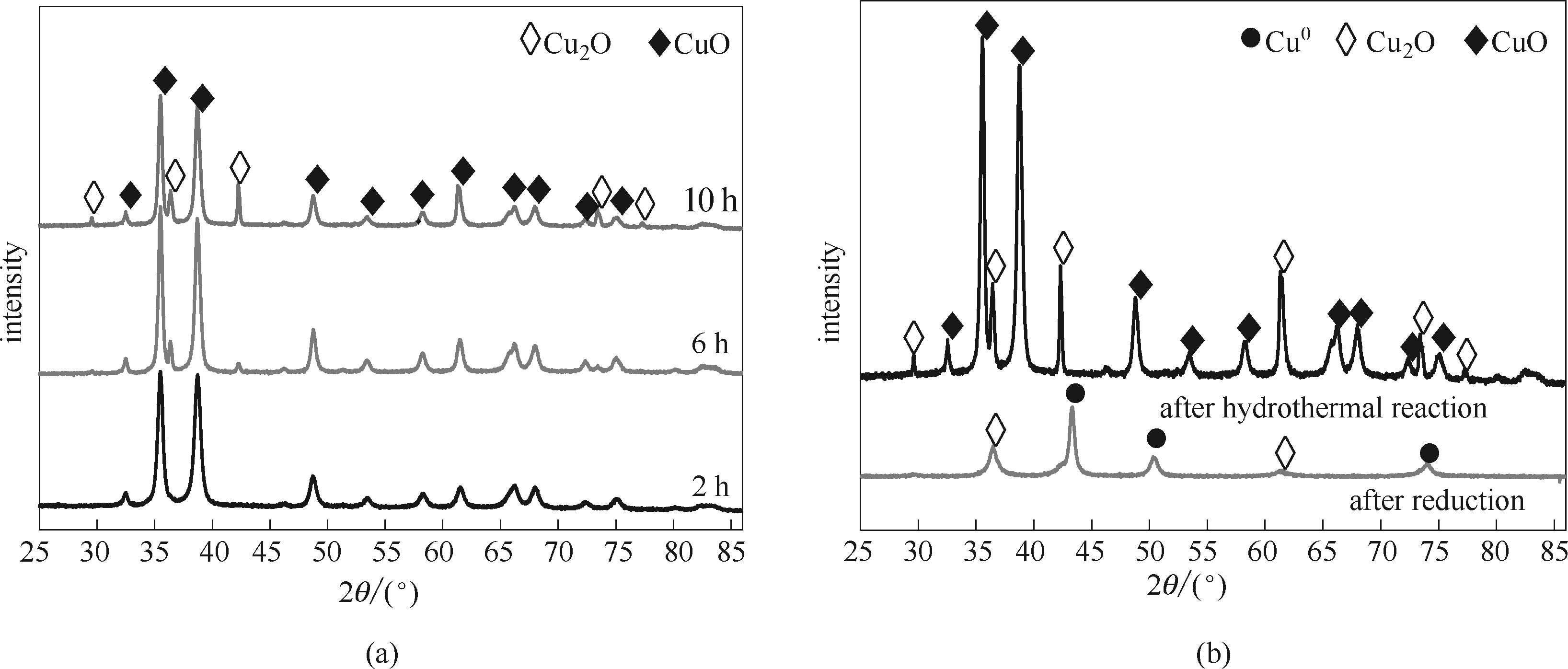
图3 不同水热时间后水热产物(a)与水热后催化剂前体及还原后 Cu/rGO催化剂(b)XRD谱图
Fig.3 XRD patterns of hydrothermal products after different hydrothermal times (a), precursor of hydrothermal catalyst and reduced Cu/rGO catalyst (b)
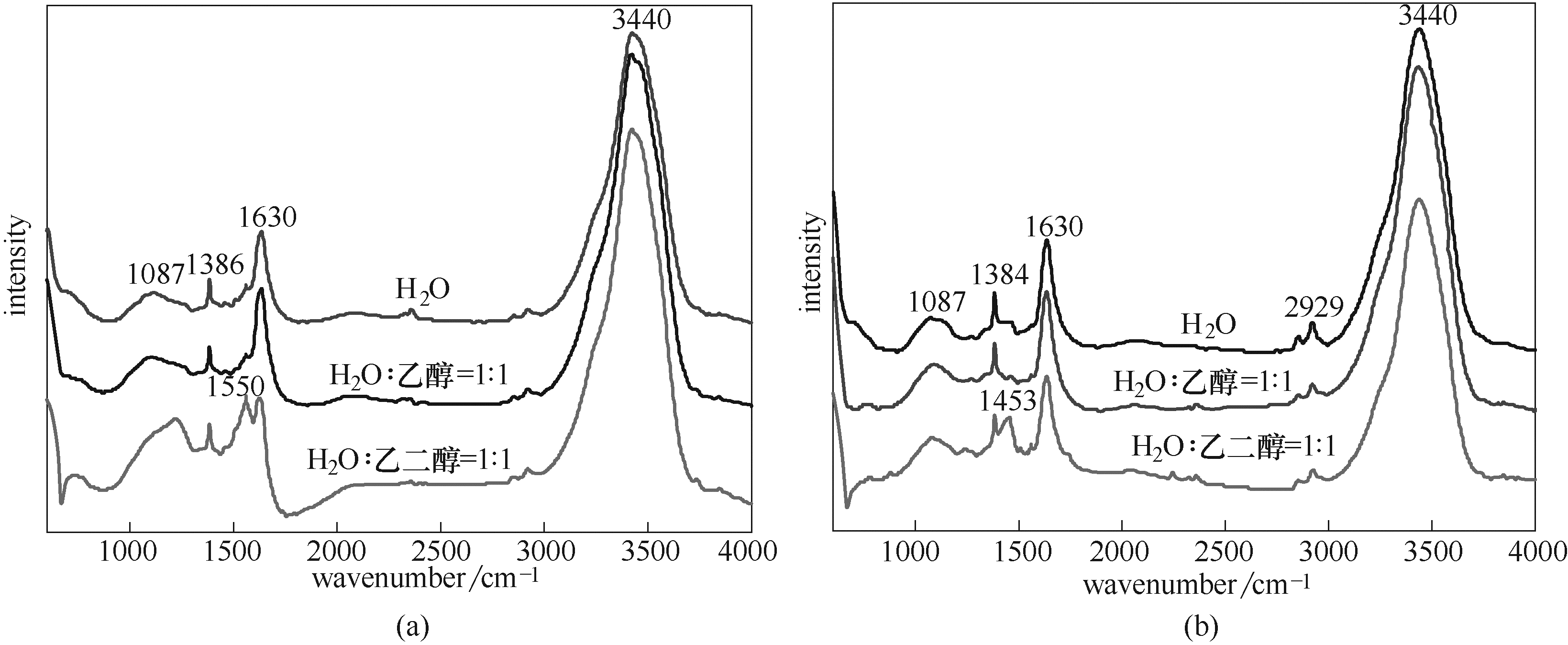
图7 不同溶剂条件下催化剂在水热后(a)与N2焙烧后(b)的FT-IR谱图
Fig.7 FT-IR spectra of the catalysts after hydrothermal (a) and N2 calcined (b) under different solvent conditions
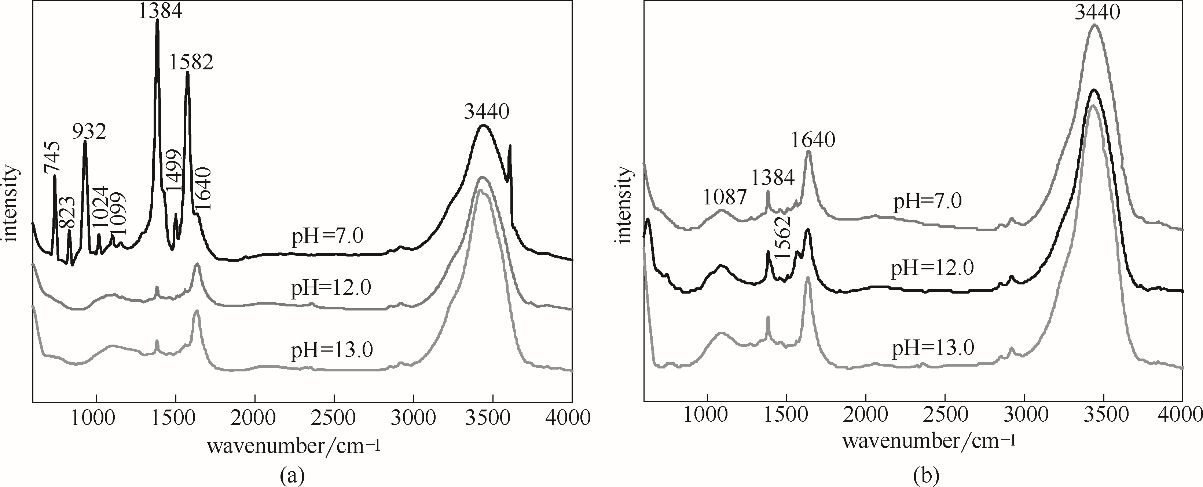
图8 不同沉淀pH条件下催化剂在水热后(a)与N2焙烧后(b)的FT-IR谱图
Fig.8 FT-IR spectra of the catalysts after hydrothermal (a) and N2 calcined (b) with different precipitation pH

图10 GO(插图为GO的XRD图)(a)、水热后CuO/rGO(b)和还原后的Cu/rGO的SEM图(c);Cu/rGO的TEM图(d)
Fig.10 SEM images of GO(inset: XRD pattern of GO)(a), hydrothermal products CuO/rGO (b) and Cu/rGO after reduction (c); TEM image of Cu/rGO (d)
| 序号 | 催化剂制备条件 | 反应时间/h | 初次排气温度/℃ | IDA 收率/% |
|---|---|---|---|---|
| 1 | Cu/rGO(无TPA) | 5.4 | 163.4 | 68.82 |
| 2 | 水 | 4.2 | 158.0 | 76.89 |
| 3 | V(乙二醇)∶V(水)=1∶1 | 3.7 | 145.5 | 82.24 |
| 4 | V(乙醇)∶V(水)=1∶1 | 3.1 | 129.6 | 86.55 |
| 5 | pH= 12.0 | 3.9 | 156.1 | 78.21 |
| 6 | pH= 11.0 | 4.5 | 162.3 | 70.96 |
| 7 | pH= 7.0 | 6.5 | 167.2 | 29.37 |
| 8 | 水热2 h | 5.9 | 162.1 | 35.26 |
| 9 | 水热6 h | 4.4 | 159.9 | 68.19 |
| 10 | GO | 3.0 | — | — |
表1 催化剂用于催化二乙醇胺脱氢制亚氨基二乙酸的性能测试结果
Table 1 Catalytic performance of samples in dehydrogenation of diethanolamine
| 序号 | 催化剂制备条件 | 反应时间/h | 初次排气温度/℃ | IDA 收率/% |
|---|---|---|---|---|
| 1 | Cu/rGO(无TPA) | 5.4 | 163.4 | 68.82 |
| 2 | 水 | 4.2 | 158.0 | 76.89 |
| 3 | V(乙二醇)∶V(水)=1∶1 | 3.7 | 145.5 | 82.24 |
| 4 | V(乙醇)∶V(水)=1∶1 | 3.1 | 129.6 | 86.55 |
| 5 | pH= 12.0 | 3.9 | 156.1 | 78.21 |
| 6 | pH= 11.0 | 4.5 | 162.3 | 70.96 |
| 7 | pH= 7.0 | 6.5 | 167.2 | 29.37 |
| 8 | 水热2 h | 5.9 | 162.1 | 35.26 |
| 9 | 水热6 h | 4.4 | 159.9 | 68.19 |
| 10 | GO | 3.0 | — | — |
| 1 | Daniel A H, Katherine M, James W R. A continuous diethanolamine dehydrogenation fixed bed catalyst and reactor system[J]. Chemical Engineering Journal, 2016, 278(15): 447-453. |
| 2 | Wang Y, Zhu H, Duan Z, et al. Study on the structure of Cu/ZrO2 catalyst and the formation mechanism of disodium iminodiacetate and sodium glycine[J]. Catalysis Letters, 2019, 150(4): 1111-1120. |
| 3 | Samson K, Sliwa M, Socha R, et al.Influence of ZrO2 structure and copper electronic state on activity of Cu/ZrO2 catalysts in methanol synthesis from CO2[J]. ACS Catalysis, 2016, 4(10): 3730-3741. |
| 4 | Zhu Y, Kong X, Li X, et al. Cu Nanoparticles inlaid mesoporous Al2O3 as a high-performance bifunctional catalyst for ethanol synthesis via dimethyl oxalate hydrogenation[J]. ACS Catalysis, 2014, 4(10): 3612-3620. |
| 5 | 王永胜, 赵云鹭, 赵珍珍, 等. 氮掺杂碳包覆Cu-ZrO2催化剂的制备及其催化脱氢性能研究[J]. 化学学报, 2019, 77: 661-668. |
| Wang Y S, Zhao Y L, Zhao Z Z, et al. Study on preparation of Cu-ZrO2 catalyst coated by nitrogen-doped carbon and catalytic dehydrogenation performance[J]. Acta Chim. Sinica, 2019, 77: 661-668. | |
| 6 | Lan X, Duan Z, Wang Y, et al. Advance in synthesizing Cu-based catalysts applying to dehydrogenation process[J]. Perroleum Chemistry, 2019, 59: 609-617. |
| 7 | Gawande M B, Goswami A, Felpin F X, et al. Cu and Cu-based nanoparticles: synthesis and applications in catalysis[J]. Chemical Reviews, 2016, 116: 3722-3811 |
| 8 | Wang D, Yang G, Ma Q, et al. Confinement effect of carbon nanotubes: copper nanoparticles filled carbon nanotubes for hydrogenation of methyl acetate[J]. ACS Catalysis, 2012, 2: 1958-1966. |
| 9 | Morales M V, Nieto E A, Baeza B B, et al. Bioethanol dehydrogenation over copper supported on functionalized graphene materials and a high surface area graphite[J]. Carbon, 2016, 102: 426-436. |
| 10 | Dvaid A M, Juan P A H. Catalyst for dehydrogenating primary alcohols: US8298985[P]. 2010-10-30. |
| 11 | Wang L, Wang L, Meng X, et al. New strategies for the preparation of sinter-resistant metal-nanoparticle-based catalysts[J]. Advanced Materials, 2019, 32: 1901-1905. |
| 12 | 兰小林, 段正康, 王永胜, 等. 不同晶相结构ZrO2负载铜基催化剂用于二乙醇胺脱氢反应[J]. 精细化工, 2019, 36(12): 2438-2446. |
| Lan X L, Duan Z K, Wang Y S, et al. ZrO2 with different crystal structure supported Cu catalysts for the dehydrogenation of diethanolamine[J]. Fine Chemicals, 2019, 36(12): 2438-2446. | |
| 13 | 兰小林, 段正康, 徐金霞, 等. 基于Cu基催化剂的二乙醇胺脱氢工艺研究进展[J]. 精细化工, 2019, 36(7): 1286-1293. |
| Lan X L, Duan Z K, Xu J X, et al. Research advance in dehydrogenation process of diethanolamine based on Cu-based catalysts[J]. Fine Chemicals, 2019, 36(7): 1286-1293. | |
| 14 | Cai X, Luo Y, Liu B, et al. Preparation of 2D material dispersions and their applications[J]. Chemical Society Reviews, 2018, 47: 6224-6266. |
| 15 | 贺新福, 龙雪颖, 吴红菊, 等. 氮掺杂石墨烯/多孔碳复合材料的制备及其氧还原催化性能[J]. 化工学报, 2019, 70(6): 2308-2315. |
| He X F, Long X Y, Wu H J, et al. Synthesis of N-doped graphene/porous carbon composite and its electrocatalytic performance on oxygen reduction reaction[J]. CIESC Journal, 2019, 70(6): 2308-2315. | |
| 16 | Wu W, Yang Y, Zhou H, et al. Highly efficient removal of Cu(II) from aqueous solution by using graphene oxide[J]. Water Air Soil Pollution, 2013, 224(1): 1372-1379. |
| 17 | Du Y, Cao N, Yang L, et al. One-step synthesis of magnetically recyclable rGO supported Cu@Co core-shell nanoparticles: highly efficient catalysts for hydrolytic dehydrogenation of ammonia borane and methylamine borane[J]. New Journal of Chemistry, 2013, 37(1): 335-342. |
| 18 | Ş Betül, Esma H A, Ş Aysun, et al. A novel thiocarbamide functionalized graphene oxide supported bimetallic monodisperse Rh-Pt nanoparticles (RhPt/TC@GO NPs) for knoevenagel condensation of aryl aldehydes together with malononitrile[J]. Applied Catalysis B: Environmental, 2018, 225: 148-153. |
| 19 | Zhang P, Wang Q, Li W, et al. A highly porous graphitic-N rich carbon stabilized copper nanocatalysts for efficient ethanol dehydrogenation[J]. ChemCatChem, 2017, 9: 1-7. |
| 20 | Wang D, Niu W, Tan M, et al. Pt nanocatalysts supported on reduced graphene oxide for selective conversion of cellulose or cellobiose to sorbitol[J]. ChemSusChem, 2014, 7: 1398-1406. |
| 21 | Wang J, Qin Y, Liu X, et al. In situ synthesis of magnetically recyclable graphene-supported Pd@Co core-shell nanoparticles as efficient catalysts for hydrolytic dehydrogenation of ammonia borane[J]. Journal of Materials Chemistry, 2012, 22(25): 12468-12470. |
| 22 | Pietrzak K, Strojny-Nędza A, Olesińska W, et al. Cu-rGO subsurface layer creation on copper substrate and its resistance to oxidation[J]. Applied Surface Science, 2017, 421: 228-233. |
| 23 | Wang Y S, Zhao Z Z, Zhao Y L, et al. A ZrO2-RGO composite as support enhanced performance for Cu-based catalyst in dehydrogenation of diethanolamine[J]. RSC Advances, 2019, 9: 30439-30447. |
| 24 | Moussa S, Siamaki A R, Gupton B F, et al. Pd-partially reduced graphene oxide catalysts (Pd/PRGO): laser synthesis of Pd nanoparticles supported on PRGO nanosheets for carbon-carbon cross coupling reactions[J]. ACS Catalysis, 2011, 2(1): 145-154. |
| 25 | Huang J, Zhang L, Chen B, et al. Nanocomposites of size-controlled gold nanoparticles and graphene oxide: formation and applications in SERS and catalysis[J]. Nanoscale, 2010, 2(12): 2733-2738. |
| 26 | Liu S, Zhao B, Jiang L. Core-shell Cu@rGO hybrids filled in epoxy composites with high thermal conduction[J]. Journal of Materials Chemistry C, 2017, 6(2): 257-265. |
| 27 | 龚水水, 光善仪, 柯福佑, 等. 红外光谱法氧化石墨烯羧基官能团含量的测定[J]. 中国测试, 2016, 42(4): 38-44. |
| Gong S S, Guang S Y, Ke F Y, et al. Determination of the content of carboxyl functional groups of graphene oxide by infrared spectroscopy[J]. China Measurement & Test, 2016, 42(4): 38-44. | |
| 28 | Jia Z, Chen T, Wang J, et al. Synthesis characterization and tribological properties of Cu/reduced graphene oxide composites[J]. Tribology International, 2015, 88: 17-24. |
| 29 | Pham T A, Kim J S. One-step reduction of graphene oxide with L-glutathione[J]. Colloids and Surfaces A: Physicochemical and Engineering, 2011, 384(1): 543-548. |
| 30 | Gupta R K, Alahmed Z A, Yakuphanoglu F. Graphene oxide based low cost battery[J]. Materials Letters, 2013, 112(12): 75-77. |
| 31 | Wang Y, Zhu S, Tsubaki N, et al. Highly dispersed Mo2C anchored on N, P-codoped graphene as efficient electrocatalyst for hydrogen evolution reaction[J]. ChemCatChem, 2018, 10(10): 2300-2304. |
| 32 | Kim S. Charge transfer transition accompanying X-ray photoionization in transition-metal compounds[J]. Journal of Electron Spectroscopy and Related Phenom, 1974, 3: 217-226. |
| 33 | Severino F, Brito J L, Laine J, et al. Nature of copper active sites in the carbon monoxide oxidation on CuAl2O4 and CuCr2O4 spinel type catalysts[J]. Journal of Catalysis, 1998, 177: 82-95. |
| 34 | Acharyya S S, Ghosh S, Bal R. Catalytic oxidation of aniline to azoxybenzene over CuCr2O4 spinel nanoparticle catalyst[J]. ACS Sustainable Chemistry & Engineering, 2014, 2(4): 584-589. |
| 35 | Maiti S, Llorca J, Dominguez M, et al. Combustion synthesized copper-ion substituted FeAl2O4 (Cu0.1Fe0.9Al2O4): a superior catalyst for methanol steam reforming compared to its impregnated analogue[J]. Journal of Power Sources, 2016, 304: 319-331. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [3] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [4] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [5] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [6] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [7] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [11] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [12] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [13] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [14] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [15] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号