化工学报 ›› 2021, Vol. 72 ›› Issue (11): 5751-5760.DOI: 10.11949/0438-1157.20210662
裴俊华1,2( ),杨亮1,2(
),杨亮1,2( ),汪鑫1,2,胡晗1,2,刘道平1,2
),汪鑫1,2,胡晗1,2,刘道平1,2
收稿日期:2021-05-14
修回日期:2021-07-20
出版日期:2021-11-05
发布日期:2021-11-12
通讯作者:
杨亮
作者简介:裴俊华(1996—),男,硕士研究生,基金资助:
Junhua PEI1,2( ),Liang YANG1,2(
),Liang YANG1,2( ),Xin WANG1,2,Han HU1,2,Daoping LIU1,2
),Xin WANG1,2,Han HU1,2,Daoping LIU1,2
Received:2021-05-14
Revised:2021-07-20
Online:2021-11-05
Published:2021-11-12
Contact:
Liang YANG
摘要:
提高水合物生成速率和储气密度对天然气水合物技术应用非常重要。将三种孔密度的泡沫铜(CF)分别浸入十二烷基硫酸钠(SDS)溶液中构建水合储气强化体系,在高压静态反应釜中研究泡沫金属对甲烷水合物生成动力学特性。实验结果表明,泡沫铜骨架能为水合物生成提供充足的结晶点,同时可作为水合物生长过程水合热迁移的“高速公路”。甲烷水合物在SDS/CF体系中可快速生成,最大水合储气速率分布在19.24~21.04 mmol·mol-1·min-1之间,其中添加15 PPI泡沫铜的SDS溶液储气量最高(139 mmol·mol-1),且达到最大储气量90%所用时间最短(10.1 min)。在6.0~8.0 MPa压力下,相比SDS溶液,添加15 PPI泡沫铜的SDS溶液储气量提高了8.8%~35.6%,储气速率提高了4.7%~40.4%;特别在压力为5.0 MPa时,该孔密度SDS/CF体系储气量甚至比SDS溶液增加13倍,储气速率增加16倍。
中图分类号:
裴俊华, 杨亮, 汪鑫, 胡晗, 刘道平. 泡沫铜强化甲烷水合物生成动力学实验研究[J]. 化工学报, 2021, 72(11): 5751-5760.
Junhua PEI, Liang YANG, Xin WANG, Han HU, Daoping LIU. Experimental study on kinetics of methane hydrate formation enhanced by copper foam[J]. CIESC Journal, 2021, 72(11): 5751-5760.
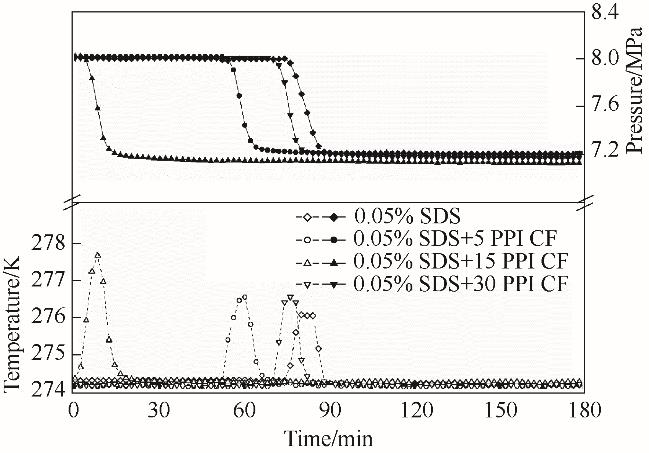
图3 SDS溶液填充泡沫铜体系水合储甲烷过程温压变化(P = 8.0 MPa, T = 274.15 K)
Fig.3 Variation of temperature and pressure during hydration and methane storage of copper foam system with different pore density filled with SDS solution

图4 SDS溶液及其填充泡沫铜体系储气过程中储气量和储气速率变化(P=8.0 MPa, T=274.15 K)
Fig.4 Changes in gas storage capacity and gas storage rate of SDS solution and its filled with copper foam system during gas storage
| Hydrate systems | P/MPa | tin/min | Cs,max/(mmol·mol-1) | Rs,max/(mmol·mol-1·min-1) | t90/min |
|---|---|---|---|---|---|
| 0.05% SDS + 5 PPI CF | 8.0 | 52.1 | 129.1 | 19.24 | 61.4 |
| 0.05% SDS + 15 PPI CF | 8.0 | 1.5 | 139.0 | 20.73 | 10.1 |
| 0.05% SDS + 30 PPI CF | 8.0 | 68.8 | 131.8 | 21.04 | 77.5 |
| 0.05% SDS + 15 PPI CF | 7.0 | 39.5 | 135.7 | 19.57 | 52.1 |
| 0.05% SDS + 15 PPI CF | 6.0 | 49.4 | 133.2 | 15.00 | 74.6 |
| 0.05% SDS + 15 PPI CF | 5.0 | 63.8 | 129.5 | 11.58 | 77.2 |
| 0.05% SDS | 8.0 | 73.5 | 127.8 | 14.77 | 85.3 |
| 0.05% SDS | 7.0 | 58.6 | 122.8 | 15.14 | 81.7 |
| 0.05% SDS | 6.0 | 84.3 | 98.2 | 14.32 | 104.2 |
| 0.05% SDS | 5.0 | — | 9.0 | 0.68 | — |
表1 所有水合储气实验结果
Table 1 Experimental results of all gas storage
| Hydrate systems | P/MPa | tin/min | Cs,max/(mmol·mol-1) | Rs,max/(mmol·mol-1·min-1) | t90/min |
|---|---|---|---|---|---|
| 0.05% SDS + 5 PPI CF | 8.0 | 52.1 | 129.1 | 19.24 | 61.4 |
| 0.05% SDS + 15 PPI CF | 8.0 | 1.5 | 139.0 | 20.73 | 10.1 |
| 0.05% SDS + 30 PPI CF | 8.0 | 68.8 | 131.8 | 21.04 | 77.5 |
| 0.05% SDS + 15 PPI CF | 7.0 | 39.5 | 135.7 | 19.57 | 52.1 |
| 0.05% SDS + 15 PPI CF | 6.0 | 49.4 | 133.2 | 15.00 | 74.6 |
| 0.05% SDS + 15 PPI CF | 5.0 | 63.8 | 129.5 | 11.58 | 77.2 |
| 0.05% SDS | 8.0 | 73.5 | 127.8 | 14.77 | 85.3 |
| 0.05% SDS | 7.0 | 58.6 | 122.8 | 15.14 | 81.7 |
| 0.05% SDS | 6.0 | 84.3 | 98.2 | 14.32 | 104.2 |
| 0.05% SDS | 5.0 | — | 9.0 | 0.68 | — |
| 1 | Koh C A, Sloan E D. Natural gas hydrates: recent advances and challenges in energy and environmental applications[J]. AIChE Journal, 2007, 53(7): 1636-1643. |
| 2 | 陈光进, 孙长宇, 马庆兰. 气体水合物科学与技术[M]. 北京: 化学工业出版社, 2008:6-8. |
| Chen G J, Sun C Y, Ma Q L. Gas Hydrate Science and Technology[M]. Beijing: Chemical Industry Press, 2008:6-8. | |
| 3 | Veluswamy H P, Kumar A, Seo Y, et al. A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates[J]. Applied Energy, 2018, 216: 262-285. |
| 4 | Yang M J, Zhao J, Zheng J N, et al. Hydrate reformation characteristics in natural gas hydrate dissociation process: a review[J]. Applied Energy, 2019, 256: 113878. |
| 5 | Zhong D L, Lu Y Y, Sun D J, et al. Performance evaluation of methane separation from coal mine gas by gas hydrate formation in a stirred reactor and in a fixed bed of silica sand[J]. Fuel, 2015, 143: 586-594. |
| 6 | Xiao P, Yang X M, Sun C Y, et al. Enhancing methane hydrate formation in bulk water using vertical reciprocating impact[J]. Chemical Engineering Journal, 2018, 336: 649-658. |
| 7 | Fujita S, Watanabe K, Mori Y H. Clathrate-hydrate formation by water spraying onto a porous metal plate exuding a hydrophobic liquid coolant[J]. AIChE Journal, 2009, 55(4): 1056-1064. |
| 8 | Yamamura K, Fukuzaki J I, Mori Y H. Clathrate hydrate formation using liquid jets impinging on each other: an observational study using paired water jets or water and methylcyclohexane jets[J]. Chemical Engineering Science, 2011, 66(9): 1844-1858. |
| 9 | Partoon B, Sabil K M, Lau K K, et al. Production of gas hydrate in a semi-batch spray reactor process as a means for separation of carbon dioxide from methane[J]. Chemical Engineering Research and Design, 2018, 138: 168-175. |
| 10 | Li C J, Huang T. Simulation of gas bubbles with gas hydrates rising in deep water[J]. Ocean Engineering, 2016, 112: 16-24. |
| 11 | Fu W Q, Wang Z Y, Sun B J, et al. A mass transfer model for hydrate formation in bubbly flow considering bubble-bubble interactions and bubble-hydrate particle interactions[J]. International Journal of Heat and Mass Transfer, 2018, 127: 611-621. |
| 12 | Zhao J F, Wang B, Sum A K. Dynamics of hydrate formation and deposition under pseudo multiphase flow[J]. AIChE Journal, 2017, 63(9): 4136-4146. |
| 13 | Mali G A, Chapoy A, Tohidi B. Investigation into the effect of subcooling on the kinetics of hydrate formation[J]. The Journal of Chemical Thermodynamics, 2018, 117: 91-96. |
| 14 | 李文昭, 潘振, 马贵阳, 等. 表面活性剂吸附对促进甲烷水合物生成效果的影响[J]. 化工学报, 2017, 68(4): 1542-1549. |
| Li W Z, Pan Z, Ma G Y, et al. Promotion effects of surfactant adsorption on formation of methane hydrates[J]. CIESC Journal, 2017, 68(4): 1542-1549. | |
| 15 | 丁家祥, 史伶俐, 申小冬, 等. SDS对甲烷水合物生成动力学和微观结构的影响[J]. 化工学报, 2017, 68(12): 4802-4808. |
| Ding J X, Shi L L, Shen X D, et al. SDS effect on formation kinetics and microstructure of methane hydrate[J]. CIESC Journal, 2017, 68(12): 4802-4808. | |
| 16 | Naeiji P, Varaminian F. Kinetic study of carbon dioxide hydrate formation by thermal analysis in the presence of two surfactants: sodium dodecyl sulfate (SDS) and lauryl alcohol ethoxylate (LAE)[J]. Journal of Molecular Liquids, 2018, 254: 120-129. |
| 17 | Wang F, Song Y M, Liu G Q, et al. Rapid methane hydrate formation promoted by Ag&SDS-coated nanospheres for energy storage[J]. Applied Energy, 2018, 213: 227-234. |
| 18 | Al-Sowadi A, Roosta H, Dashti A, et al. The effects of SDS, SLES and THF on the growth rate, kinetic behaviors and energy consumption during ethylene hydrate formation process[J]. Journal of Molecular Liquids, 2019, 294: 111608. |
| 19 | Zhong Y, Rogers R E. Surfactant effects on gas hydrate formation[J]. Chemical Engineering Science, 2000, 55(19): 4175-4187. |
| 20 | Ganji H, Manteghian M, Sadaghiani zadeh K, et al. Effect of different surfactants on methane hydrate formation rate, stability and storage capacity[J]. Fuel, 2007, 86(3): 434-441. |
| 21 | Shi C R, Chai F Y, Yang M J, et al. Enhance methane hydrate formation using fungus confining sodium dodecyl sulfate solutions for methane storage[J]. Journal of Molecular Liquids, 2021, 333: 116020. |
| 22 | Okutani K, Kuwabara Y, Mori Y H. Surfactant effects on hydrate formation in an unstirred gas/liquid system: amendments to the previous study using HFC-32 and sodium dodecyl sulfate[J]. Chemical Engineering Science, 2007, 62(14): 3858-3860. |
| 23 | Gupta A, Lachance J, Sloan E D, et al. Measurements of methane hydrate heat of dissociation using high pressure differential scanning calorimetry[J]. Chemical Engineering Science, 2008, 63(24): 5848-5853. |
| 24 | Liu N, Zhu H Q, Zhou J L, et al. Molecular dynamics simulations on formation of CO2 hydrate in the presence of metal particles[J]. Journal of Molecular Liquids, 2021, 331: 115793. |
| 25 | Patel H E, Das S K, Sundararajan T, et al. Thermal conductivities of naked and monolayer protected metal nanoparticle based nanofluids: manifestation of anomalous enhancement and chemical effects[J]. Applied Physics Letters, 2003, 83(14): 2931-2933. |
| 26 | Najibi H, Mirzaee Shayegan M, Heidary H. Experimental investigation of methane hydrate formation in the presence of copper oxide nanoparticles and SDS[J]. Journal of Natural Gas Science and Engineering, 2015, 23: 315-323. |
| 27 | Mohammadi M, Haghtalab A, Fakhroueian Z. Experimental study and thermodynamic modeling of CO2 gas hydrate formation in presence of zinc oxide nanoparticles[J]. The Journal of Chemical Thermodynamics, 2016, 96: 24-33. |
| 28 | Liu G Q, Wang F, Luo S J, et al. Enhanced methane hydrate formation with SDS-coated Fe3O4 nanoparticles as promoters[J]. Journal of Molecular Liquids, 2017, 230: 315-321. |
| 29 | Lu Y Y, Ge B B, Zhong D L. Investigation of using graphite nanofluids to promote methane hydrate formation: application to solidified natural gas storage[J]. Energy, 2020, 199: 117424. |
| 30 | Liu N, Chen L T, Liu C X, et al. Experimental study of carbon dioxide hydrate formation in the presence of graphene oxide[J]. Energy, 2020, 211: 118994. |
| 31 | Xie Y M, Guo K H, Liang D Q, et al. Gas hydrate growth morphology outside of horizontal heat transfer tube[J]. Journal of Crystal Growth, 2005, 276(1/2): 253-264. |
| 32 | Yang L, Liu Z Z, Liu D P, et al. Enhanced natural gas hydrates formation in the suspension with metal particles and fibers[J]. Journal of Molecular Liquids, 2020, 301: 112410. |
| 33 | Lee J, Shin C, Lee Y. Experimental investigation to improve the storage potentials of gas hydrate under the unstirring condition[J]. Energy & Fuels, 2010, 24(2): 1129-1134. |
| 34 | Bhattacharya A, Calmidi V V, Mahajan R L. Thermophysical properties of high porosity metal foams[J]. International Journal of Heat and Mass Transfer, 2002, 45(5): 1017-1031. |
| 35 | Li T X, Wu D L, He F, et al. Experimental investigation on copper foam/hydrated salt composite phase change material for thermal energy storage[J]. International Journal of Heat and Mass Transfer, 2017, 115: 148-157. |
| 36 | Redlich O, Kwong J N S. On the thermodynamics of solutions(Ⅴ): An equation of state. Fugacities of gaseous solutions[J]. Chemical Reviews, 1949, 44(1): 233-244. |
| [1] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [2] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [3] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [4] | 张双星, 刘舫辰, 张义飞, 杜文静. R-134a脉动热管相变蓄放热实验研究[J]. 化工学报, 2023, 74(S1): 165-171. |
| [5] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [6] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [9] | 诸程瑛, 王振雷. 基于改进深度强化学习的乙烯裂解炉操作优化[J]. 化工学报, 2023, 74(8): 3429-3437. |
| [10] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [11] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [12] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [13] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [14] | 张贲, 王松柏, 魏子亚, 郝婷婷, 马学虎, 温荣福. 超亲水多孔金属结构驱动的毛细液膜冷凝及传热强化[J]. 化工学报, 2023, 74(7): 2824-2835. |
| [15] | 王海, 林宏, 王晨, 许浩洁, 左磊, 王军锋. 高压静电场强化多孔介质表面沸腾传热特性研究[J]. 化工学报, 2023, 74(7): 2869-2879. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号