化工学报 ›› 2020, Vol. 71 ›› Issue (11): 5067-5079.DOI: 10.11949/0438-1157.20200243
林绪亮1( ),徐帅2(
),徐帅2( ),徐少杰1,袁慢景1,陈金珠2,李宇亮2,秦延林1(
),徐少杰1,袁慢景1,陈金珠2,李宇亮2,秦延林1( )
)
收稿日期:2020-03-10
修回日期:2020-08-01
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
秦延林
作者简介:林绪亮(1988—),男,博士,副教授,基金资助:
Xuliang LIN1( ),Shuai XU2(
),Shuai XU2( ),Shaojie XU1,Manjing YUAN1,Jinzhu CHEN2,Yuliang LI2,Yanlin QIN1(
),Shaojie XU1,Manjing YUAN1,Jinzhu CHEN2,Yuliang LI2,Yanlin QIN1( )
)
Received:2020-03-10
Revised:2020-08-01
Online:2020-11-05
Published:2020-11-05
Contact:
Yanlin QIN
摘要:
测定了离子液体N-乙基吡啶四氟硼酸盐([EPy]BF4)+有机盐[丁二酸钠(CH2COONa)2/柠檬酸铵C6H5O7(NH4)3/柠檬酸钠C6H5O7Na3] +水双水相体系在303.15、308.15和313.15 K下的双节线及系线数据。基于相图中的杠杆定律通过质量法研究双水相体系的系线,并用Othmer-Tobias方程、Bancroft方程等经验方程以及NRTL活度系数模型对数据进行关联。采用有效排除体积和Setschenow-type的拟合参数深入研究三种有机盐的盐析能力。结果表明,双节线的三个经验式拟合效果良好,但较多参数的经验式会导致部分参数失去意义;双水相体系的系线满足经验方程,数据可靠性较好,且均方根偏差表明系线数据很好地符合NRTL模型;低温更有利于该双水相体系的形成;三种盐都能和[EPy]BF4离子液体形成双水相体系,且C6H5O7Na3的盐析能力更优。本文提供的数据为离子液体的回收循环利用提供了理论参考依据。
中图分类号:
林绪亮,徐帅,徐少杰,袁慢景,陈金珠,李宇亮,秦延林. 吡啶基离子液体+有机盐双水相体系相图的测定及盐析性能研究[J]. 化工学报, 2020, 71(11): 5067-5079.
Xuliang LIN,Shuai XU,Shaojie XU,Manjing YUAN,Jinzhu CHEN,Yuliang LI,Yanlin QIN. Phase diagram of pyridyl ionic liquid + organic salt aqueous two-phase system and study of salting-out ability[J]. CIESC Journal, 2020, 71(11): 5067-5079.
| w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS T=303.15 K | |||||||||||
| 77.05 | 0.43 | 59.01 | 3.00 | 47.80 | 5.83 | 37.70 | 9.21 | 26.58 | 13.37 | 15.39 | 19.23 |
| 74.70 | 0.65 | 58.01 | 3.30 | 46.98 | 6.10 | 36.34 | 9.61 | 25.70 | 13.70 | 14.03 | 19.94 |
| 71.64 | 1.06 | 56.92 | 3.52 | 46.10 | 6.32 | 35.16 | 9.99 | 24.88 | 14.14 | 13.24 | 20.51 |
| 70.19 | 1.25 | 55.83 | 3.71 | 45.28 | 6.59 | 33.98 | 10.30 | 24.25 | 14.57 | 12.06 | 21.16 |
| 68.29 | 1.53 | 54.93 | 3.97 | 44.47 | 6.86 | 33.01 | 10.62 | 23.25 | 14.95 | 11.43 | 21.82 |
| 66.84 | 1.77 | 53.93 | 4.14 | 43.56 | 7.19 | 32.38 | 10.98 | 21.98 | 15.61 | 11.07 | 22.33 |
| 65.41 | 1.94 | 52.76 | 4.47 | 42.75 | 7.49 | 31.56 | 11.41 | 21.26 | 16.10 | ||
| 63.78 | 2.20 | 51.69 | 4.74 | 41.84 | 7.79 | 30.56 | 11.85 | 19.74 | 16.88 | ||
| 62.24 | 2.45 | 50.79 | 5.01 | 40.94 | 8.12 | 29.57 | 12.15 | 18.38 | 17.46 | ||
| 61.16 | 2.59 | 49.97 | 5.26 | 39.94 | 8.47 | 28.48 | 12.67 | 17.57 | 18.01 | ||
| 60.43 | 2.78 | 48.79 | 5.53 | 38.87 | 8.80 | 27.67 | 13.02 | 16.66 | 18.57 | ||
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS T=308.15 K | |||||||||||
| 76.24 | 0.70 | 57.85 | 3.45 | 46.69 | 6.50 | 38.44 | 9.47 | 30.54 | 12.75 | 20.51 | 17.21 |
| 72.75 | 1.04 | 56.38 | 3.73 | 45.10 | 6.97 | 37.43 | 9.91 | 29.55 | 13.15 | 19.38 | 17.87 |
| 70.03 | 1.35 | 54.93 | 4.10 | 44.31 | 7.29 | 36.41 | 10.28 | 28.41 | 13.81 | 18.13 | 18.55 |
| 68.02 | 1.70 | 53.68 | 4.41 | 43.29 | 7.60 | 35.52 | 10.63 | 27.17 | 14.40 | 16.68 | 19.15 |
| 66.32 | 1.92 | 52.10 | 4.85 | 42.52 | 7.97 | 34.50 | 11.07 | 25.70 | 14.90 | 15.55 | 19.74 |
| 64.39 | 2.17 | 50.85 | 5.13 | 41.50 | 8.35 | 33.94 | 11.37 | 24.45 | 15.49 | 14.31 | 20.30 |
| 62.04 | 2.60 | 49.52 | 5.63 | 40.60 | 8.69 | 32.58 | 11.94 | 23.11 | 16.06 | 13.29 | 21.08 |
| 59.66 | 2.94 | 47.71 | 6.19 | 39.58 | 9.13 | 31.33 | 12.34 | 21.76 | 16.65 | 12.04 | 21.92 |
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS T=313.15 K | |||||||||||
| 78.05 | 1.06 | 57.28 | 4.47 | 41.93 | 9.24 | 30.93 | 13.62 | 24.16 | 17.10 | 16.75 | 21.25 |
| 75.69 | 1.23 | 55.38 | 4.98 | 40.41 | 9.83 | 30.02 | 13.95 | 23.07 | 17.51 | 15.57 | 21.90 |
| 72.91 | 1.53 | 53.32 | 5.45 | 38.97 | 10.51 | 29.02 | 14.36 | 22.53 | 18.01 | 15.05 | 22.52 |
| 68.92 | 2.18 | 51.51 | 6.02 | 37.24 | 11.14 | 28.14 | 14.79 | 21.46 | 18.36 | 13.79 | 23.13 |
| 66.50 | 2.53 | 49.43 | 6.59 | 36.07 | 11.57 | 27.60 | 15.25 | 20.38 | 18.90 | 12.79 | 23.89 |
| 63.78 | 3.02 | 46.73 | 7.41 | 34.73 | 12.18 | 26.96 | 15.72 | 19.56 | 19.45 | 11.88 | 24.81 |
| 61.52 | 3.46 | 44.83 | 8.12 | 33.19 | 12.64 | 26.06 | 16.15 | 18.74 | 19.97 | ||
| 59.19 | 3.97 | 43.29 | 8.66 | 31.92 | 13.18 | 24.97 | 16.59 | 17.75 | 20.53 | ||
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS T=303.15 K | |||||||||||
| 77.26 | 1.23 | 56.75 | 41.21 | 9.18 | 47.80 | 28.60 | 14.69 | 17.93 | 21.77 | 9.27 | 31.12 |
| 75.19 | 1.47 | 55.27 | 40.34 | 9.57 | 46.98 | 27.89 | 15.15 | 16.88 | 22.71 | 8.25 | 32.22 |
| 72.85 | 1.86 | 53.95 | 39.45 | 9.97 | 46.10 | 26.56 | 15.78 | 16.02 | 23.57 | 7.81 | 33.25 |
| 70.38 | 2.10 | 52.34 | 38.00 | 10.52 | 45.28 | 25.54 | 16.34 | 15.12 | 24.43 | 6.79 | 34.43 |
| 68.16 | 2.41 | 50.43 | 36.68 | 11.23 | 44.47 | 24.22 | 17.20 | 14.26 | 25.14 | 6.21 | 35.77 |
| 66.25 | 2.81 | 49.13 | 35.20 | 11.94 | 43.56 | 23.20 | 17.99 | 13.24 | 26.09 | 5.90 | 36.94 |
| 64.06 | 3.20 | 47.07 | 34.03 | 12.57 | 42.75 | 21.88 | 18.77 | 12.78 | 26.88 | 5.16 | 38.28 |
| 62.30 | 3.60 | 45.62 | 32.42 | 12.95 | 41.84 | 20.70 | 19.48 | 11.61 | 27.89 | 4.43 | 39.77 |
| 60.54 | 3.98 | 44.45 | 31.10 | 13.43 | 40.94 | 19.81 | 20.11 | 10.90 | 28.68 | 3.99 | 41.43 |
| 58.94 | 4.30 | 42.69 | 30.08 | 14.14 | 39.94 | 18.95 | 21.14 | 10.16 | 29.86 | 3.56 | 43.39 |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS T=308.15 K | |||||||||||
| 75.24 | 1.63 | 53.34 | 6.25 | 36.78 | 11.49 | 23.38 | 18.46 | 13.42 | 26.09 | 6.41 | 36.54 |
| 72.52 | 2.00 | 51.30 | 6.63 | 35.35 | 12.09 | 22.77 | 19.06 | 13.01 | 27.18 | 6.21 | 37.69 |
| 70.17 | 2.38 | 49.67 | 7.13 | 34.13 | 12.64 | 21.85 | 19.50 | 12.20 | 28.04 | 5.39 | 38.72 |
| 67.93 | 2.82 | 48.24 | 7.40 | 32.60 | 13.39 | 20.83 | 20.26 | 11.58 | 29.03 | 4.88 | 40.25 |
| 65.89 | 3.15 | 46.84 | 7.84 | 31.40 | 14.21 | 20.02 | 21.07 | 10.77 | 29.85 | 4.27 | 42.16 |
| 64.39 | 3.53 | 45.31 | 8.49 | 29.77 | 15.14 | 19.10 | 21.62 | 10.28 | 30.72 | 3.56 | 44.01 |
| 62.25 | 3.86 | 43.99 | 9.15 | 28.04 | 16.07 | 17.88 | 22.38 | 9.37 | 31.64 | 3.05 | 45.92 |
| 60.62 | 4.24 | 42.25 | 9.41 | 26.61 | 16.39 | 17.19 | 23.15 | 8.35 | 32.57 | 2.36 | 48.10 |
| 58.38 | 4.84 | 40.62 | 9.85 | 25.70 | 16.82 | 16.47 | 23.97 | 8.04 | 33.28 | 1.95 | 49.72 |
| 56.77 | 5.12 | 39.91 | 10.28 | 24.80 | 17.26 | 15.35 | 24.61 | 7.53 | 34.43 | ||
| 54.74 | 5.81 | 38.41 | 11.05 | 24.40 | 17.91 | 14.54 | 25.43 | 6.92 | 35.67 | ||
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS T=313.15 K | |||||||||||
| 77.41 | 1.47 | 52.93 | 6.20 | 35.78 | 11.77 | 23.48 | 18.14 | 13.37 | 26.80 | 7.38 | 35.60 |
| 74.76 | 1.86 | 51.17 | 6.58 | 34.77 | 12.17 | 22.61 | 18.77 | 12.93 | 27.58 | 6.64 | 36.54 |
| 71.24 | 2.26 | 49.57 | 7.14 | 33.75 | 12.64 | 21.88 | 19.40 | 12.20 | 28.21 | 5.90 | 37.65 |
| 69.05 | 2.66 | 47.96 | 7.37 | 32.57 | 13.35 | 20.86 | 20.11 | 11.91 | 28.83 | 5.62 | 38.99 |
| 66.99 | 2.97 | 46.79 | 7.77 | 31.53 | 13.89 | 20.27 | 20.66 | 11.61 | 29.71 | 5.01 | 40.09 |
| 65.23 | 3.28 | 45.31 | 7.92 | 30.23 | 14.52 | 19.38 | 21.37 | 10.90 | 30.42 | 4.43 | 41.58 |
| 63.32 | 3.60 | 43.99 | 8.63 | 28.91 | 15.23 | 18.36 | 22.31 | 10.44 | 31.20 | 4.30 | 43.23 |
| 61.44 | 4.06 | 42.38 | 9.18 | 27.89 | 15.78 | 17.77 | 23.02 | 9.72 | 31.91 | 3.99 | 44.49 |
| 59.68 | 4.38 | 40.78 | 9.65 | 27.15 | 16.41 | 16.88 | 23.80 | 9.27 | 32.62 | 3.84 | 45.91 |
| 58.20 | 4.94 | 39.45 | 10.28 | 25.98 | 16.72 | 15.71 | 24.68 | 8.83 | 33.32 | ||
| 56.60 | 5.09 | 38.13 | 10.75 | 24.80 | 17.37 | 15.00 | 25.46 | 8.40 | 34.11 | ||
| 54.41 | 5.72 | 36.80 | 11.23 | 24.07 | 17.75 | 14.11 | 26.17 | 7.97 | 34.82 | ||
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS T=303.15 K | |||||||||||
| 59.83 | 1.59 | 45.16 | 5.19 | 34.79 | 8.54 | 26.08 | 11.85 | 18.33 | 16.10 | 12.51 | 21.35 |
| 57.07 | 2.11 | 44.20 | 5.60 | 33.83 | 8.90 | 25.09 | 12.26 | 17.78 | 16.74 | 11.55 | 21.99 |
| 54.71 | 2.65 | 42.82 | 5.90 | 32.84 | 9.20 | 24.42 | 12.74 | 16.79 | 17.39 | 10.71 | 22.82 |
| 52.79 | 3.06 | 41.57 | 6.18 | 31.73 | 9.55 | 23.43 | 13.08 | 16.12 | 17.92 | 9.89 | 23.71 |
| 51.25 | 3.48 | 40.47 | 6.66 | 31.06 | 9.97 | 22.62 | 13.62 | 15.57 | 18.40 | 9.19 | 24.59 |
| 49.73 | 3.95 | 39.07 | 7.13 | 30.07 | 10.20 | 21.36 | 14.09 | 15.01 | 19.05 | ||
| 48.48 | 4.36 | 38.11 | 7.49 | 29.11 | 10.61 | 20.67 | 14.62 | 14.03 | 19.57 | ||
| 46.96 | 4.72 | 37.15 | 7.83 | 27.74 | 11.09 | 19.99 | 15.03 | 13.62 | 20.11 | ||
| 46.14 | 5.01 | 35.90 | 8.26 | 27.04 | 11.43 | 19.15 | 15.50 | 13.35 | 20.70 | ||
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS T=308.15 K | |||||||||||
| 60.65 | 1.83 | 46.55 | 5.13 | 35.90 | 8.49 | 26.20 | 12.14 | 17.64 | 17.10 | 11.26 | 23.17 |
| 58.17 | 2.30 | 45.59 | 5.43 | 34.64 | 8.96 | 25.38 | 12.50 | 17.23 | 17.69 | 10.71 | 23.77 |
| 55.55 | 2.82 | 44.61 | 5.71 | 33.68 | 9.25 | 24.42 | 12.91 | 16.12 | 18.40 | 9.89 | 24.48 |
| 53.89 | 3.12 | 43.38 | 6.01 | 32.72 | 9.61 | 23.58 | 13.50 | 15.42 | 18.99 | 9.60 | 25.01 |
| 52.79 | 3.42 | 42.39 | 6.31 | 31.73 | 10.02 | 22.88 | 13.91 | 14.87 | 19.57 | 9.19 | 25.72 |
| 51.53 | 3.76 | 41.29 | 6.60 | 30.92 | 10.44 | 21.92 | 14.45 | 14.17 | 20.11 | ||
| 50.57 | 4.06 | 40.32 | 6.95 | 30.07 | 10.67 | 20.96 | 15.03 | 13.76 | 20.75 | ||
| 49.47 | 4.42 | 39.22 | 7.25 | 28.97 | 10.96 | 19.70 | 15.50 | 12.92 | 21.29 | ||
| 48.48 | 4.72 | 38.11 | 7.66 | 28.00 | 11.38 | 19.01 | 16.04 | 12.37 | 21.88 | ||
| 47.52 | 4.95 | 36.86 | 8.19 | 27.19 | 11.73 | 18.45 | 16.57 | 11.96 | 22.53 | ||
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS T=313.15 K | |||||||||||
| 62.60 | 1.88 | 47.93 | 5.07 | 37.96 | 8.07 | 28.70 | 11.32 | 20.81 | 15.45 | 13.35 | 21.58 |
| 60.39 | 2.24 | 46.82 | 5.36 | 37.00 | 8.54 | 27.74 | 11.73 | 19.85 | 15.86 | 12.10 | 23.00 |
| 58.17 | 2.65 | 45.86 | 5.71 | 35.90 | 8.84 | 27.04 | 12.20 | 19.15 | 16.57 | 10.85 | 24.41 |
| 56.11 | 3.01 | 44.48 | 6.13 | 34.79 | 9.20 | 25.94 | 12.61 | 18.33 | 17.10 | 9.74 | 26.00 |
| 54.71 | 3.35 | 43.23 | 6.42 | 33.95 | 9.61 | 25.09 | 13.08 | 17.35 | 17.75 | 8.64 | 27.37 |
| 53.34 | 3.71 | 41.84 | 6.78 | 32.84 | 9.97 | 23.99 | 13.56 | 16.53 | 18.33 | ||
| 52.23 | 3.95 | 40.73 | 7.13 | 31.47 | 10.25 | 22.88 | 13.98 | 16.12 | 18.93 | ||
| 50.98 | 4.30 | 40.06 | 7.49 | 30.63 | 10.67 | 22.06 | 14.45 | 15.28 | 19.76 | ||
| 49.59 | 4.60 | 39.22 | 7.78 | 29.66 | 11.02 | 21.36 | 15.03 | 14.32 | 20.58 | ||
表1 [EPy]BF4(w1)+有机盐(w2)+水双水相体系(ATPS)在不同温度和常压下的双节线数据
Table 1 Binodal data of [EPy]BF4(w1)+ salts(w2)+ water ATPS at different temperaturesand atmospheric pressure
| w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS T=303.15 K | |||||||||||
| 77.05 | 0.43 | 59.01 | 3.00 | 47.80 | 5.83 | 37.70 | 9.21 | 26.58 | 13.37 | 15.39 | 19.23 |
| 74.70 | 0.65 | 58.01 | 3.30 | 46.98 | 6.10 | 36.34 | 9.61 | 25.70 | 13.70 | 14.03 | 19.94 |
| 71.64 | 1.06 | 56.92 | 3.52 | 46.10 | 6.32 | 35.16 | 9.99 | 24.88 | 14.14 | 13.24 | 20.51 |
| 70.19 | 1.25 | 55.83 | 3.71 | 45.28 | 6.59 | 33.98 | 10.30 | 24.25 | 14.57 | 12.06 | 21.16 |
| 68.29 | 1.53 | 54.93 | 3.97 | 44.47 | 6.86 | 33.01 | 10.62 | 23.25 | 14.95 | 11.43 | 21.82 |
| 66.84 | 1.77 | 53.93 | 4.14 | 43.56 | 7.19 | 32.38 | 10.98 | 21.98 | 15.61 | 11.07 | 22.33 |
| 65.41 | 1.94 | 52.76 | 4.47 | 42.75 | 7.49 | 31.56 | 11.41 | 21.26 | 16.10 | ||
| 63.78 | 2.20 | 51.69 | 4.74 | 41.84 | 7.79 | 30.56 | 11.85 | 19.74 | 16.88 | ||
| 62.24 | 2.45 | 50.79 | 5.01 | 40.94 | 8.12 | 29.57 | 12.15 | 18.38 | 17.46 | ||
| 61.16 | 2.59 | 49.97 | 5.26 | 39.94 | 8.47 | 28.48 | 12.67 | 17.57 | 18.01 | ||
| 60.43 | 2.78 | 48.79 | 5.53 | 38.87 | 8.80 | 27.67 | 13.02 | 16.66 | 18.57 | ||
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS T=308.15 K | |||||||||||
| 76.24 | 0.70 | 57.85 | 3.45 | 46.69 | 6.50 | 38.44 | 9.47 | 30.54 | 12.75 | 20.51 | 17.21 |
| 72.75 | 1.04 | 56.38 | 3.73 | 45.10 | 6.97 | 37.43 | 9.91 | 29.55 | 13.15 | 19.38 | 17.87 |
| 70.03 | 1.35 | 54.93 | 4.10 | 44.31 | 7.29 | 36.41 | 10.28 | 28.41 | 13.81 | 18.13 | 18.55 |
| 68.02 | 1.70 | 53.68 | 4.41 | 43.29 | 7.60 | 35.52 | 10.63 | 27.17 | 14.40 | 16.68 | 19.15 |
| 66.32 | 1.92 | 52.10 | 4.85 | 42.52 | 7.97 | 34.50 | 11.07 | 25.70 | 14.90 | 15.55 | 19.74 |
| 64.39 | 2.17 | 50.85 | 5.13 | 41.50 | 8.35 | 33.94 | 11.37 | 24.45 | 15.49 | 14.31 | 20.30 |
| 62.04 | 2.60 | 49.52 | 5.63 | 40.60 | 8.69 | 32.58 | 11.94 | 23.11 | 16.06 | 13.29 | 21.08 |
| 59.66 | 2.94 | 47.71 | 6.19 | 39.58 | 9.13 | 31.33 | 12.34 | 21.76 | 16.65 | 12.04 | 21.92 |
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS T=313.15 K | |||||||||||
| 78.05 | 1.06 | 57.28 | 4.47 | 41.93 | 9.24 | 30.93 | 13.62 | 24.16 | 17.10 | 16.75 | 21.25 |
| 75.69 | 1.23 | 55.38 | 4.98 | 40.41 | 9.83 | 30.02 | 13.95 | 23.07 | 17.51 | 15.57 | 21.90 |
| 72.91 | 1.53 | 53.32 | 5.45 | 38.97 | 10.51 | 29.02 | 14.36 | 22.53 | 18.01 | 15.05 | 22.52 |
| 68.92 | 2.18 | 51.51 | 6.02 | 37.24 | 11.14 | 28.14 | 14.79 | 21.46 | 18.36 | 13.79 | 23.13 |
| 66.50 | 2.53 | 49.43 | 6.59 | 36.07 | 11.57 | 27.60 | 15.25 | 20.38 | 18.90 | 12.79 | 23.89 |
| 63.78 | 3.02 | 46.73 | 7.41 | 34.73 | 12.18 | 26.96 | 15.72 | 19.56 | 19.45 | 11.88 | 24.81 |
| 61.52 | 3.46 | 44.83 | 8.12 | 33.19 | 12.64 | 26.06 | 16.15 | 18.74 | 19.97 | ||
| 59.19 | 3.97 | 43.29 | 8.66 | 31.92 | 13.18 | 24.97 | 16.59 | 17.75 | 20.53 | ||
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS T=303.15 K | |||||||||||
| 77.26 | 1.23 | 56.75 | 41.21 | 9.18 | 47.80 | 28.60 | 14.69 | 17.93 | 21.77 | 9.27 | 31.12 |
| 75.19 | 1.47 | 55.27 | 40.34 | 9.57 | 46.98 | 27.89 | 15.15 | 16.88 | 22.71 | 8.25 | 32.22 |
| 72.85 | 1.86 | 53.95 | 39.45 | 9.97 | 46.10 | 26.56 | 15.78 | 16.02 | 23.57 | 7.81 | 33.25 |
| 70.38 | 2.10 | 52.34 | 38.00 | 10.52 | 45.28 | 25.54 | 16.34 | 15.12 | 24.43 | 6.79 | 34.43 |
| 68.16 | 2.41 | 50.43 | 36.68 | 11.23 | 44.47 | 24.22 | 17.20 | 14.26 | 25.14 | 6.21 | 35.77 |
| 66.25 | 2.81 | 49.13 | 35.20 | 11.94 | 43.56 | 23.20 | 17.99 | 13.24 | 26.09 | 5.90 | 36.94 |
| 64.06 | 3.20 | 47.07 | 34.03 | 12.57 | 42.75 | 21.88 | 18.77 | 12.78 | 26.88 | 5.16 | 38.28 |
| 62.30 | 3.60 | 45.62 | 32.42 | 12.95 | 41.84 | 20.70 | 19.48 | 11.61 | 27.89 | 4.43 | 39.77 |
| 60.54 | 3.98 | 44.45 | 31.10 | 13.43 | 40.94 | 19.81 | 20.11 | 10.90 | 28.68 | 3.99 | 41.43 |
| 58.94 | 4.30 | 42.69 | 30.08 | 14.14 | 39.94 | 18.95 | 21.14 | 10.16 | 29.86 | 3.56 | 43.39 |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS T=308.15 K | |||||||||||
| 75.24 | 1.63 | 53.34 | 6.25 | 36.78 | 11.49 | 23.38 | 18.46 | 13.42 | 26.09 | 6.41 | 36.54 |
| 72.52 | 2.00 | 51.30 | 6.63 | 35.35 | 12.09 | 22.77 | 19.06 | 13.01 | 27.18 | 6.21 | 37.69 |
| 70.17 | 2.38 | 49.67 | 7.13 | 34.13 | 12.64 | 21.85 | 19.50 | 12.20 | 28.04 | 5.39 | 38.72 |
| 67.93 | 2.82 | 48.24 | 7.40 | 32.60 | 13.39 | 20.83 | 20.26 | 11.58 | 29.03 | 4.88 | 40.25 |
| 65.89 | 3.15 | 46.84 | 7.84 | 31.40 | 14.21 | 20.02 | 21.07 | 10.77 | 29.85 | 4.27 | 42.16 |
| 64.39 | 3.53 | 45.31 | 8.49 | 29.77 | 15.14 | 19.10 | 21.62 | 10.28 | 30.72 | 3.56 | 44.01 |
| 62.25 | 3.86 | 43.99 | 9.15 | 28.04 | 16.07 | 17.88 | 22.38 | 9.37 | 31.64 | 3.05 | 45.92 |
| 60.62 | 4.24 | 42.25 | 9.41 | 26.61 | 16.39 | 17.19 | 23.15 | 8.35 | 32.57 | 2.36 | 48.10 |
| 58.38 | 4.84 | 40.62 | 9.85 | 25.70 | 16.82 | 16.47 | 23.97 | 8.04 | 33.28 | 1.95 | 49.72 |
| 56.77 | 5.12 | 39.91 | 10.28 | 24.80 | 17.26 | 15.35 | 24.61 | 7.53 | 34.43 | ||
| 54.74 | 5.81 | 38.41 | 11.05 | 24.40 | 17.91 | 14.54 | 25.43 | 6.92 | 35.67 | ||
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS T=313.15 K | |||||||||||
| 77.41 | 1.47 | 52.93 | 6.20 | 35.78 | 11.77 | 23.48 | 18.14 | 13.37 | 26.80 | 7.38 | 35.60 |
| 74.76 | 1.86 | 51.17 | 6.58 | 34.77 | 12.17 | 22.61 | 18.77 | 12.93 | 27.58 | 6.64 | 36.54 |
| 71.24 | 2.26 | 49.57 | 7.14 | 33.75 | 12.64 | 21.88 | 19.40 | 12.20 | 28.21 | 5.90 | 37.65 |
| 69.05 | 2.66 | 47.96 | 7.37 | 32.57 | 13.35 | 20.86 | 20.11 | 11.91 | 28.83 | 5.62 | 38.99 |
| 66.99 | 2.97 | 46.79 | 7.77 | 31.53 | 13.89 | 20.27 | 20.66 | 11.61 | 29.71 | 5.01 | 40.09 |
| 65.23 | 3.28 | 45.31 | 7.92 | 30.23 | 14.52 | 19.38 | 21.37 | 10.90 | 30.42 | 4.43 | 41.58 |
| 63.32 | 3.60 | 43.99 | 8.63 | 28.91 | 15.23 | 18.36 | 22.31 | 10.44 | 31.20 | 4.30 | 43.23 |
| 61.44 | 4.06 | 42.38 | 9.18 | 27.89 | 15.78 | 17.77 | 23.02 | 9.72 | 31.91 | 3.99 | 44.49 |
| 59.68 | 4.38 | 40.78 | 9.65 | 27.15 | 16.41 | 16.88 | 23.80 | 9.27 | 32.62 | 3.84 | 45.91 |
| 58.20 | 4.94 | 39.45 | 10.28 | 25.98 | 16.72 | 15.71 | 24.68 | 8.83 | 33.32 | ||
| 56.60 | 5.09 | 38.13 | 10.75 | 24.80 | 17.37 | 15.00 | 25.46 | 8.40 | 34.11 | ||
| 54.41 | 5.72 | 36.80 | 11.23 | 24.07 | 17.75 | 14.11 | 26.17 | 7.97 | 34.82 | ||
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS T=303.15 K | |||||||||||
| 59.83 | 1.59 | 45.16 | 5.19 | 34.79 | 8.54 | 26.08 | 11.85 | 18.33 | 16.10 | 12.51 | 21.35 |
| 57.07 | 2.11 | 44.20 | 5.60 | 33.83 | 8.90 | 25.09 | 12.26 | 17.78 | 16.74 | 11.55 | 21.99 |
| 54.71 | 2.65 | 42.82 | 5.90 | 32.84 | 9.20 | 24.42 | 12.74 | 16.79 | 17.39 | 10.71 | 22.82 |
| 52.79 | 3.06 | 41.57 | 6.18 | 31.73 | 9.55 | 23.43 | 13.08 | 16.12 | 17.92 | 9.89 | 23.71 |
| 51.25 | 3.48 | 40.47 | 6.66 | 31.06 | 9.97 | 22.62 | 13.62 | 15.57 | 18.40 | 9.19 | 24.59 |
| 49.73 | 3.95 | 39.07 | 7.13 | 30.07 | 10.20 | 21.36 | 14.09 | 15.01 | 19.05 | ||
| 48.48 | 4.36 | 38.11 | 7.49 | 29.11 | 10.61 | 20.67 | 14.62 | 14.03 | 19.57 | ||
| 46.96 | 4.72 | 37.15 | 7.83 | 27.74 | 11.09 | 19.99 | 15.03 | 13.62 | 20.11 | ||
| 46.14 | 5.01 | 35.90 | 8.26 | 27.04 | 11.43 | 19.15 | 15.50 | 13.35 | 20.70 | ||
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS T=308.15 K | |||||||||||
| 60.65 | 1.83 | 46.55 | 5.13 | 35.90 | 8.49 | 26.20 | 12.14 | 17.64 | 17.10 | 11.26 | 23.17 |
| 58.17 | 2.30 | 45.59 | 5.43 | 34.64 | 8.96 | 25.38 | 12.50 | 17.23 | 17.69 | 10.71 | 23.77 |
| 55.55 | 2.82 | 44.61 | 5.71 | 33.68 | 9.25 | 24.42 | 12.91 | 16.12 | 18.40 | 9.89 | 24.48 |
| 53.89 | 3.12 | 43.38 | 6.01 | 32.72 | 9.61 | 23.58 | 13.50 | 15.42 | 18.99 | 9.60 | 25.01 |
| 52.79 | 3.42 | 42.39 | 6.31 | 31.73 | 10.02 | 22.88 | 13.91 | 14.87 | 19.57 | 9.19 | 25.72 |
| 51.53 | 3.76 | 41.29 | 6.60 | 30.92 | 10.44 | 21.92 | 14.45 | 14.17 | 20.11 | ||
| 50.57 | 4.06 | 40.32 | 6.95 | 30.07 | 10.67 | 20.96 | 15.03 | 13.76 | 20.75 | ||
| 49.47 | 4.42 | 39.22 | 7.25 | 28.97 | 10.96 | 19.70 | 15.50 | 12.92 | 21.29 | ||
| 48.48 | 4.72 | 38.11 | 7.66 | 28.00 | 11.38 | 19.01 | 16.04 | 12.37 | 21.88 | ||
| 47.52 | 4.95 | 36.86 | 8.19 | 27.19 | 11.73 | 18.45 | 16.57 | 11.96 | 22.53 | ||
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS T=313.15 K | |||||||||||
| 62.60 | 1.88 | 47.93 | 5.07 | 37.96 | 8.07 | 28.70 | 11.32 | 20.81 | 15.45 | 13.35 | 21.58 |
| 60.39 | 2.24 | 46.82 | 5.36 | 37.00 | 8.54 | 27.74 | 11.73 | 19.85 | 15.86 | 12.10 | 23.00 |
| 58.17 | 2.65 | 45.86 | 5.71 | 35.90 | 8.84 | 27.04 | 12.20 | 19.15 | 16.57 | 10.85 | 24.41 |
| 56.11 | 3.01 | 44.48 | 6.13 | 34.79 | 9.20 | 25.94 | 12.61 | 18.33 | 17.10 | 9.74 | 26.00 |
| 54.71 | 3.35 | 43.23 | 6.42 | 33.95 | 9.61 | 25.09 | 13.08 | 17.35 | 17.75 | 8.64 | 27.37 |
| 53.34 | 3.71 | 41.84 | 6.78 | 32.84 | 9.97 | 23.99 | 13.56 | 16.53 | 18.33 | ||
| 52.23 | 3.95 | 40.73 | 7.13 | 31.47 | 10.25 | 22.88 | 13.98 | 16.12 | 18.93 | ||
| 50.98 | 4.30 | 40.06 | 7.49 | 30.63 | 10.67 | 22.06 | 14.45 | 15.28 | 19.76 | ||
| 49.59 | 4.60 | 39.22 | 7.78 | 29.66 | 11.02 | 21.36 | 15.03 | 14.32 | 20.58 | ||
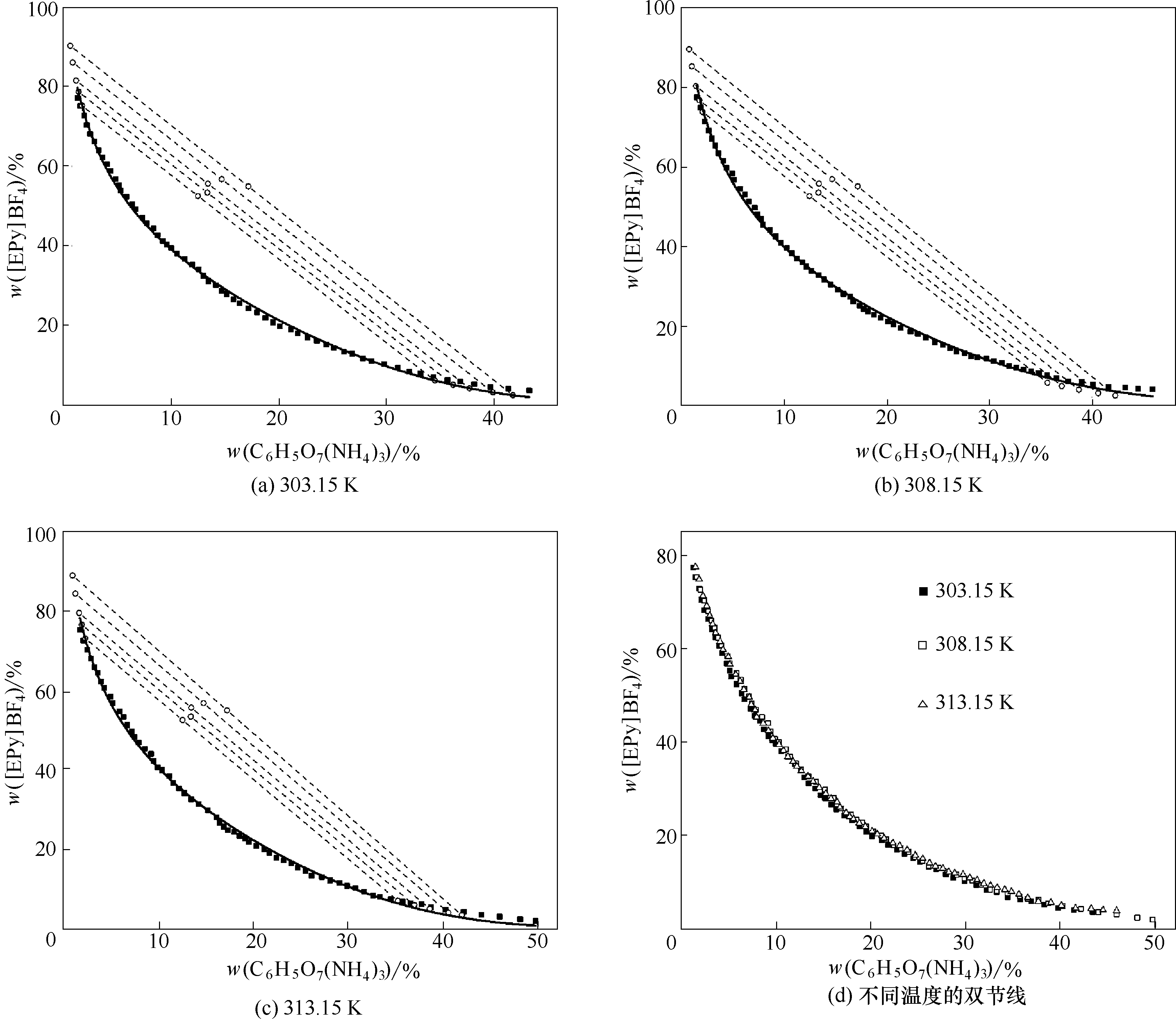
图2 [EPy]BF4 + C6H5O7(NH4)3 + H2O体系在不同温度下的双节线和系线图
Fig.2 Binodal curve and tie-line data of [EPy]BF4+ C6H5O7(NH4)3 + H2O system at different temperatures
| System | T/K | a | b | c | R2 | SD① |
|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 303.15 | 96.41 | -0.2858 | 8.6916×10-5 | 0.9982 | 0.27 |
| 308.15 | 97.27 | -0.2807 | 7.4187×10-5 | 0.9997 | 0.20 | |
| 313.15 | 105.8 | -0.2923 | 5.3099×10-5 | 0.9995 | 0.34 | |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O | 303.15 | 116.1 | -0.3373 | 5.3415×10-5 | 0.9979 | 0.94 |
| 308.15 | 120.8 | -0.3408 | 2.107×10-5 | 0.9974 | 0.95 | |
| 313.15 | 123.3 | -0.3525 | 1.7644×10-5 | 0.9979 | 0.85 | |
| [EPy]BF4 + C6H5O7Na3+ H2O | 303.15 | 96.19 | -0.3395 | 5.736×10-5 | 0.9942 | 0.98 |
| 308.15 | 102.7 | -0.3571 | 4.624×10-5 | 0.9955 | 0.97 | |
| 313.15 | 104.0 | -0.3527 | 4.636×10-5 | 0.9937 | 0.99 |
表2 [EPy]BF4+有机盐+水双水相体系拟合式(1)的参数结果、相关系数和标准偏差
Table 2 Values of the parameters of Eq.(1) for [EPy]BF4 + organic salt + H2O ATPSs
| System | T/K | a | b | c | R2 | SD① |
|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 303.15 | 96.41 | -0.2858 | 8.6916×10-5 | 0.9982 | 0.27 |
| 308.15 | 97.27 | -0.2807 | 7.4187×10-5 | 0.9997 | 0.20 | |
| 313.15 | 105.8 | -0.2923 | 5.3099×10-5 | 0.9995 | 0.34 | |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O | 303.15 | 116.1 | -0.3373 | 5.3415×10-5 | 0.9979 | 0.94 |
| 308.15 | 120.8 | -0.3408 | 2.107×10-5 | 0.9974 | 0.95 | |
| 313.15 | 123.3 | -0.3525 | 1.7644×10-5 | 0.9979 | 0.85 | |
| [EPy]BF4 + C6H5O7Na3+ H2O | 303.15 | 96.19 | -0.3395 | 5.736×10-5 | 0.9942 | 0.98 |
| 308.15 | 102.7 | -0.3571 | 4.624×10-5 | 0.9955 | 0.97 | |
| 313.15 | 104.0 | -0.3527 | 4.636×10-5 | 0.9937 | 0.99 |
| T/K | a | b | c | d | R2 | SD① |
|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS | ||||||
| 303.15 | 4.501 | -0.2218 | -0.00534 | -0.00187 | 0.9997 | 0.35 |
| 308.15 | 4.615 | -0.3567 | 0.03889 | -0.00262 | 0.9997 | 0.32 |
| 313.15 | 4.666 | -0.3261 | 0.02409 | -0.0019 | 0.9983 | 0.42 |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS | ||||||
| 303.15 | 4.602 | -0.2015 | -0.02387 | -5.398×10-4 | 0.9998 | 0.26 |
| 308.15 | 4.570 | -0.1526 | -0.03351 | -4.163×10-4 | 0.9998 | 0.29 |
| 313.15 | 4.627 | -0.1929 | -0.02857 | -3.551×10-4 | 0.9998 | 0.33 |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS | ||||||
| 303.15 | 4.119 | 0.1004 | -0.1046 | 3.217×10-4 | 0.9995 | 0.45 |
| 308.15 | 4.180 | 0.06926 | -0.09801 | 3.119×10-4 | 0.9997 | 0.34 |
| 313.15 | 4.181 | 0.09774 | -0.1067 | 5.177×10-4 | 0.9995 | 0.36 |
表3 [EPy]BF4+有机盐+水双水相体系拟合式(2)的参数结果、相关系数和标准偏差
Table 3 Values of the parameters of Eq. (2) for [EPy]BF4 + organic salt + H2O ATPSs
| T/K | a | b | c | d | R2 | SD① |
|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS | ||||||
| 303.15 | 4.501 | -0.2218 | -0.00534 | -0.00187 | 0.9997 | 0.35 |
| 308.15 | 4.615 | -0.3567 | 0.03889 | -0.00262 | 0.9997 | 0.32 |
| 313.15 | 4.666 | -0.3261 | 0.02409 | -0.0019 | 0.9983 | 0.42 |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS | ||||||
| 303.15 | 4.602 | -0.2015 | -0.02387 | -5.398×10-4 | 0.9998 | 0.26 |
| 308.15 | 4.570 | -0.1526 | -0.03351 | -4.163×10-4 | 0.9998 | 0.29 |
| 313.15 | 4.627 | -0.1929 | -0.02857 | -3.551×10-4 | 0.9998 | 0.33 |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS | ||||||
| 303.15 | 4.119 | 0.1004 | -0.1046 | 3.217×10-4 | 0.9995 | 0.45 |
| 308.15 | 4.180 | 0.06926 | -0.09801 | 3.119×10-4 | 0.9997 | 0.34 |
| 313.15 | 4.181 | 0.09774 | -0.1067 | 5.177×10-4 | 0.9995 | 0.36 |
| T/K | a1 | b1 | a2 | b2 | c | R2 | SD① |
|---|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS | |||||||
| 303.15 | 3.814 | 4.372×1027 | 79.80 | 13.49 | -7.280 | 0.9975 | 0.83 |
| 308.15 | 3.279 | 4.372×1027 | 80.58 | 14.78 | -7.816 | 0.9953 | 1.2 |
| 313.15 | 4.152 | 4.372×1027 | 83.06 | 14.84 | -6.942 | 0.9970 | 0.96 |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS | |||||||
| 303.15 | 6.039 | 4.372×1027 | 80.41 | 13.64 | -5.056 | 0.9984 | 0.68 |
| 308.15 | 5.490 | 4.372×1027 | 81.98 | 14.61 | -5.605 | 0.9993 | 0.56 |
| 313.15 | 6.360 | 4.372×1027 | 81.18 | 13.71 | -4.735 | 0.9985 | 0.65 |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS | |||||||
| 303.15 | 5.092 | 4.372×1027 | 69.03 | 12.79 | -6.003 | 0.9999 | 0.32 |
| 308.15 | 5.803 | 4.372×1027 | 69.93 | 12.27 | -5.292 | 0.9999 | 0.28 |
| 313.15 | 6.121 | 4.372×1027 | 71.13 | 12.11 | -4.974 | 0.9999 | 0.35 |
表4 [EPy]BF4+有机盐+水双水相体系拟合式(3)的参数结果、相关系数和标准偏差
Table 4 Values of the parameters of Eq. (3) for [EPy]BF4 + organic salt + H2O ATPSs
| T/K | a1 | b1 | a2 | b2 | c | R2 | SD① |
|---|---|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS | |||||||
| 303.15 | 3.814 | 4.372×1027 | 79.80 | 13.49 | -7.280 | 0.9975 | 0.83 |
| 308.15 | 3.279 | 4.372×1027 | 80.58 | 14.78 | -7.816 | 0.9953 | 1.2 |
| 313.15 | 4.152 | 4.372×1027 | 83.06 | 14.84 | -6.942 | 0.9970 | 0.96 |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS | |||||||
| 303.15 | 6.039 | 4.372×1027 | 80.41 | 13.64 | -5.056 | 0.9984 | 0.68 |
| 308.15 | 5.490 | 4.372×1027 | 81.98 | 14.61 | -5.605 | 0.9993 | 0.56 |
| 313.15 | 6.360 | 4.372×1027 | 81.18 | 13.71 | -4.735 | 0.9985 | 0.65 |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS | |||||||
| 303.15 | 5.092 | 4.372×1027 | 69.03 | 12.79 | -6.003 | 0.9999 | 0.32 |
| 308.15 | 5.803 | 4.372×1027 | 69.93 | 12.27 | -5.292 | 0.9999 | 0.28 |
| 313.15 | 6.121 | 4.372×1027 | 71.13 | 12.11 | -4.974 | 0.9999 | 0.35 |
| T/K | Total system | Top phase | Bottom phase | TLL | S | |||
|---|---|---|---|---|---|---|---|---|
| w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | |||
| [EPy]BF4 + (CH2COONa)2 + H2O | ||||||||
| 303.15 | 37.81 | 14.44 | 88.49 | 0.09 | 9.21 | 22.52 | 82.40 | -3.53 |
| 37.56 | 13.82 | 85.40 | 0.18 | 10.97 | 21.40 | 77.40 | -3.51 | |
| 37.29 | 12.89 | 81.03 | 0.37 | 14.44 | 19.44 | 69.26 | -3.49 | |
| 36.88 | 12.24 | 77.41 | 0.59 | 17.89 | 17.70 | 61.93 | -3.48 | |
| 36.52 | 11.50 | 73.07 | 0.94 | 22.58 | 15.53 | 52.56 | -3.46 | |
| 308.15 | 61.26 | 7.67 | 86.93 | 0.14 | 10.72 | 22.45 | 79.41 | -3.42 |
| 60.94 | 7.21 | 84.65 | 0.22 | 12.18 | 21.55 | 75.55 | -3.40 | |
| 58.26 | 7.05 | 80.86 | 0.40 | 15.57 | 19.64 | 68.06 | -3.39 | |
| 57.24 | 6.68 | 77.71 | 0.60 | 18.32 | 18.23 | 61.95 | -3.37 | |
| 56.37 | 6.06 | 73.58 | 0.94 | 22.98 | 16.00 | 52.79 | -3.36 | |
| 313.15 | 41.07 | 15.32 | 89.87 | 0.31 | 11.53 | 24.41 | 81.97 | -3.25 |
| 40.55 | 14.75 | 86.37 | 0.48 | 13.24 | 23.26 | 76.59 | -3.21 | |
| 40.12 | 14.18 | 83.40 | 0.66 | 15.40 | 21.91 | 71.24 | -3.20 | |
| 39.26 | 13.64 | 79.77 | 0.93 | 18.39 | 20.18 | 64.33 | -3.19 | |
| 38.67 | 12.94 | 75.67 | 1.31 | 22.24 | 18.11 | 56.02 | -3.18 | |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS | ||||||||
| 303.15 | 54.96 | 17.17 | 90.39 | 0.55 | 2.35 | 41.87 | 97.26 | -2.13 |
| 56.77 | 14.68 | 86.18 | 0.78 | 3.07 | 40.01 | 91.90 | -2.12 | |
| 55.65 | 13.39 | 81.62 | 1.09 | 4.12 | 37.81 | 85.76 | -2.11 | |
| 53.38 | 13.34 | 78.78 | 1.32 | 4.98 | 36.28 | 81.67 | -2.11 | |
| 52.54 | 12.46 | 75.26 | 1.65 | 6.06 | 34.59 | 76.64 | -2.10 | |
| 308.15 | 54.96 | 17.17 | 89.52 | 0.74 | 2.27 | 42.26 | 96.63 | -2.10 |
| 56.77 | 14.68 | 85.21 | 1.01 | 2.89 | 40.58 | 91.34 | -2.08 | |
| 55.65 | 13.39 | 80.21 | 1.40 | 3.74 | 38.71 | 85.08 | -2.05 | |
| 53.38 | 13.34 | 76.68 | 1.73 | 4.63 | 37.05 | 80.24 | -2.04 | |
| 52.54 | 12.46 | 73.70 | 2.05 | 5.49 | 35.64 | 76.03 | -2.03 | |
| 313.15 | 54.96 | 17.17 | 88.91 | 0.86 | 3.43 | 41.94 | 94.84 | -2.08 |
| 56.77 | 14.68 | 84.34 | 1.16 | 4.06 | 40.52 | 89.42 | -2.04 | |
| 55.65 | 13.39 | 79.49 | 1.55 | 5.03 | 38.57 | 83.16 | -2.01 | |
| 53.38 | 13.34 | 76.52 | 1.83 | 5.97 | 36.93 | 78.81 | -2.01 | |
| 52.54 | 12.46 | 73.08 | 2.20 | 7.08 | 35.19 | 73.79 | -2.00 | |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS | ||||||||
| 303.15 | 32.99 | 16.94 | 76.59 | 0.45 | 4.83 | 27.61 | 76.74 | -2.64 |
| 32.30 | 16.89 | 75.30 | 0.52 | 5.14 | 27.22 | 75.07 | -2.63 | |
| 31.96 | 16.30 | 73.00 | 0.66 | 6.03 | 26.20 | 71.68 | -2.62 | |
| 31.72 | 16.21 | 71.96 | 0.73 | 6.18 | 26.03 | 70.48 | -2.60 | |
| 31.38 | 15.36 | 68.73 | 0.98 | 7.77 | 24.44 | 65.32 | -2.60 | |
| 308.15 | 32.99 | 16.94 | 73.63 | 0.87 | 5.93 | 27.63 | 72.80 | -2.53 |
| 32.30 | 16.89 | 72.41 | 0.96 | 6.29 | 27.21 | 71.14 | -2.52 | |
| 31.96 | 16.30 | 69.93 | 1.16 | 7.24 | 26.15 | 67.49 | -2.51 | |
| 31.72 | 16.21 | 69.14 | 1.23 | 7.47 | 25.91 | 66.42 | -2.50 | |
| 31.38 | 15.36 | 65.57 | 1.58 | 9.03 | 24.37 | 60.96 | -2.48 | |
| 313.15 | 32.99 | 16.94 | 72.35 | 1.06 | 5.94 | 27.85 | 71.61 | -2.48 |
| 32.30 | 16.89 | 70.80 | 1.19 | 6.20 | 27.54 | 69.77 | -2.45 | |
| 31.96 | 16.30 | 68.02 | 1.45 | 7.12 | 26.52 | 65.86 | -2.43 | |
| 31.72 | 16.21 | 67.24 | 1.53 | 7.35 | 26.28 | 64.80 | -2.42 | |
| 31.38 | 15.36 | 63.55 | 1.95 | 8.96 | 24.70 | 59.14 | -2.40 | |
表5 [EPy]BF4(w1)+有机盐(w2)+水双水相体系在不同温度和大气压下的系线数据
Table 5 Tie-lines data of [EPy]BF4(w1) + salts(w2) + water ATPS at different temperatures and atmospheric pressure
| T/K | Total system | Top phase | Bottom phase | TLL | S | |||
|---|---|---|---|---|---|---|---|---|
| w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | w1/%(mass) | w2/%(mass) | |||
| [EPy]BF4 + (CH2COONa)2 + H2O | ||||||||
| 303.15 | 37.81 | 14.44 | 88.49 | 0.09 | 9.21 | 22.52 | 82.40 | -3.53 |
| 37.56 | 13.82 | 85.40 | 0.18 | 10.97 | 21.40 | 77.40 | -3.51 | |
| 37.29 | 12.89 | 81.03 | 0.37 | 14.44 | 19.44 | 69.26 | -3.49 | |
| 36.88 | 12.24 | 77.41 | 0.59 | 17.89 | 17.70 | 61.93 | -3.48 | |
| 36.52 | 11.50 | 73.07 | 0.94 | 22.58 | 15.53 | 52.56 | -3.46 | |
| 308.15 | 61.26 | 7.67 | 86.93 | 0.14 | 10.72 | 22.45 | 79.41 | -3.42 |
| 60.94 | 7.21 | 84.65 | 0.22 | 12.18 | 21.55 | 75.55 | -3.40 | |
| 58.26 | 7.05 | 80.86 | 0.40 | 15.57 | 19.64 | 68.06 | -3.39 | |
| 57.24 | 6.68 | 77.71 | 0.60 | 18.32 | 18.23 | 61.95 | -3.37 | |
| 56.37 | 6.06 | 73.58 | 0.94 | 22.98 | 16.00 | 52.79 | -3.36 | |
| 313.15 | 41.07 | 15.32 | 89.87 | 0.31 | 11.53 | 24.41 | 81.97 | -3.25 |
| 40.55 | 14.75 | 86.37 | 0.48 | 13.24 | 23.26 | 76.59 | -3.21 | |
| 40.12 | 14.18 | 83.40 | 0.66 | 15.40 | 21.91 | 71.24 | -3.20 | |
| 39.26 | 13.64 | 79.77 | 0.93 | 18.39 | 20.18 | 64.33 | -3.19 | |
| 38.67 | 12.94 | 75.67 | 1.31 | 22.24 | 18.11 | 56.02 | -3.18 | |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS | ||||||||
| 303.15 | 54.96 | 17.17 | 90.39 | 0.55 | 2.35 | 41.87 | 97.26 | -2.13 |
| 56.77 | 14.68 | 86.18 | 0.78 | 3.07 | 40.01 | 91.90 | -2.12 | |
| 55.65 | 13.39 | 81.62 | 1.09 | 4.12 | 37.81 | 85.76 | -2.11 | |
| 53.38 | 13.34 | 78.78 | 1.32 | 4.98 | 36.28 | 81.67 | -2.11 | |
| 52.54 | 12.46 | 75.26 | 1.65 | 6.06 | 34.59 | 76.64 | -2.10 | |
| 308.15 | 54.96 | 17.17 | 89.52 | 0.74 | 2.27 | 42.26 | 96.63 | -2.10 |
| 56.77 | 14.68 | 85.21 | 1.01 | 2.89 | 40.58 | 91.34 | -2.08 | |
| 55.65 | 13.39 | 80.21 | 1.40 | 3.74 | 38.71 | 85.08 | -2.05 | |
| 53.38 | 13.34 | 76.68 | 1.73 | 4.63 | 37.05 | 80.24 | -2.04 | |
| 52.54 | 12.46 | 73.70 | 2.05 | 5.49 | 35.64 | 76.03 | -2.03 | |
| 313.15 | 54.96 | 17.17 | 88.91 | 0.86 | 3.43 | 41.94 | 94.84 | -2.08 |
| 56.77 | 14.68 | 84.34 | 1.16 | 4.06 | 40.52 | 89.42 | -2.04 | |
| 55.65 | 13.39 | 79.49 | 1.55 | 5.03 | 38.57 | 83.16 | -2.01 | |
| 53.38 | 13.34 | 76.52 | 1.83 | 5.97 | 36.93 | 78.81 | -2.01 | |
| 52.54 | 12.46 | 73.08 | 2.20 | 7.08 | 35.19 | 73.79 | -2.00 | |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS | ||||||||
| 303.15 | 32.99 | 16.94 | 76.59 | 0.45 | 4.83 | 27.61 | 76.74 | -2.64 |
| 32.30 | 16.89 | 75.30 | 0.52 | 5.14 | 27.22 | 75.07 | -2.63 | |
| 31.96 | 16.30 | 73.00 | 0.66 | 6.03 | 26.20 | 71.68 | -2.62 | |
| 31.72 | 16.21 | 71.96 | 0.73 | 6.18 | 26.03 | 70.48 | -2.60 | |
| 31.38 | 15.36 | 68.73 | 0.98 | 7.77 | 24.44 | 65.32 | -2.60 | |
| 308.15 | 32.99 | 16.94 | 73.63 | 0.87 | 5.93 | 27.63 | 72.80 | -2.53 |
| 32.30 | 16.89 | 72.41 | 0.96 | 6.29 | 27.21 | 71.14 | -2.52 | |
| 31.96 | 16.30 | 69.93 | 1.16 | 7.24 | 26.15 | 67.49 | -2.51 | |
| 31.72 | 16.21 | 69.14 | 1.23 | 7.47 | 25.91 | 66.42 | -2.50 | |
| 31.38 | 15.36 | 65.57 | 1.58 | 9.03 | 24.37 | 60.96 | -2.48 | |
| 313.15 | 32.99 | 16.94 | 72.35 | 1.06 | 5.94 | 27.85 | 71.61 | -2.48 |
| 32.30 | 16.89 | 70.80 | 1.19 | 6.20 | 27.54 | 69.77 | -2.45 | |
| 31.96 | 16.30 | 68.02 | 1.45 | 7.12 | 26.52 | 65.86 | -2.43 | |
| 31.72 | 16.21 | 67.24 | 1.53 | 7.35 | 26.28 | 64.80 | -2.42 | |
| 31.38 | 15.36 | 63.55 | 1.95 | 8.96 | 24.70 | 59.14 | -2.40 | |
| System | T/K | k1 | n | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 303.15 | 0.0126 | 2.010 | 0.9630 | 8.3×10-3 |
| 308.15 | 0.0154 | 1.914 | 0.9722 | 6.0×10-3 | |
| 313.15 | 0.00901 | 2.388 | 0.9572 | 8.2×10-3 | |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 303.15 | 0.04153 | 3.284 | 0.9785 | 6.3×10-3 |
| 308.15 | 0.04408 | 3.590 | 0.9707 | 7.2×10-3 | |
| 313.15 | 0.05206 | 3.257 | 0.9521 | 9.4×10-3 | |
| [EPy]BF4 + C6H5O7Na3+ H2O | 303.15 | 0.03308 | 2.331 | 0.9784 | 2.7×10-3 |
| 308.15 | 0.04323 | 2.210 | 0.9946 | 1.4×10-3 | |
| 313.15 | 0.04205 | 2.354 | 0.9770 | 3.2×10-3 |
表6 [EPy]BF4+有机盐+水双水相体系拟合式(11)的参数结果、相关系数和标准偏差
Table 6 Values of the parameters of Eq. (11) for [EPy]BF4 + organic salt + H2O ATPSs
| System | T/K | k1 | n | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 303.15 | 0.0126 | 2.010 | 0.9630 | 8.3×10-3 |
| 308.15 | 0.0154 | 1.914 | 0.9722 | 6.0×10-3 | |
| 313.15 | 0.00901 | 2.388 | 0.9572 | 8.2×10-3 | |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 303.15 | 0.04153 | 3.284 | 0.9785 | 6.3×10-3 |
| 308.15 | 0.04408 | 3.590 | 0.9707 | 7.2×10-3 | |
| 313.15 | 0.05206 | 3.257 | 0.9521 | 9.4×10-3 | |
| [EPy]BF4 + C6H5O7Na3+ H2O | 303.15 | 0.03308 | 2.331 | 0.9784 | 2.7×10-3 |
| 308.15 | 0.04323 | 2.210 | 0.9946 | 1.4×10-3 | |
| 313.15 | 0.04205 | 2.354 | 0.9770 | 3.2×10-3 |
| System | T/K | k2 | r | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 303.15 | 5.200 | 0.2748 | 0.9605 | 6.8×10-3 |
| 308.15 | 5.146 | 0.2960 | 0.9722 | 4.9×10-3 | |
| 313.15 | 4.246 | 0.2277 | 0.9454 | 7.0×10-3 | |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 303.15 | 2.225 | 0.2300 | 0.9762 | 5.2×10-3 |
| 308.15 | 2.054 | 0.2084 | 0.9706 | 6.2×10-3 | |
| 313.15 | 2.039 | 0.2174 | 0.9426 | 8.4×10-3 | |
| [EPy]BF4 + C6H5O7Na3+ H2O | 303.15 | 3.603 | 0.3256 | 0.9801 | 2.4×10-3 |
| 308.15 | 3.470 | 0.3492 | 0.9952 | 1.2×10-3 | |
| 313.15 | 3.244 | 0.3187 | 0.9766 | 2.8×10-3 |
表7 [EPy]BF4+有机盐+水双水相体系拟合式(12)的参数结果、相关系数和标准偏差
Table 7 Values of the parameters of Eq.(12) for [EPy]BF4 + organic salt + H2O ATPSs
| System | T/K | k2 | r | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 303.15 | 5.200 | 0.2748 | 0.9605 | 6.8×10-3 |
| 308.15 | 5.146 | 0.2960 | 0.9722 | 4.9×10-3 | |
| 313.15 | 4.246 | 0.2277 | 0.9454 | 7.0×10-3 | |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 303.15 | 2.225 | 0.2300 | 0.9762 | 5.2×10-3 |
| 308.15 | 2.054 | 0.2084 | 0.9706 | 6.2×10-3 | |
| 313.15 | 2.039 | 0.2174 | 0.9426 | 8.4×10-3 | |
| [EPy]BF4 + C6H5O7Na3+ H2O | 303.15 | 3.603 | 0.3256 | 0.9801 | 2.4×10-3 |
| 308.15 | 3.470 | 0.3492 | 0.9952 | 1.2×10-3 | |
| 313.15 | 3.244 | 0.3187 | 0.9766 | 2.8×10-3 |
| i-j | ?gij/(J·mol-1) | ?gji/(J·mol-1) | α | 100RMSD |
|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS at 308.15 K | ||||
| 1-2 | 33832.2 | 288097 | 0.01 | 0.57 |
| 1-3 | 10600.8 | 6855.21 | 0.37 | |
| 2-3 | -131468 | 201527 | 0.01 | |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS at 308.15 K | ||||
| 1-2 | -14636.3 | 160854 | 0.02 | 0.08 |
| 1-3 | 8517.21 | 21576.4 | 0.25 | |
| 2-3 | 140479 | 94027.1 | 0.06 | |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS at 308.15 K | ||||
| 1-2 | -66136.2 | 273227 | 0.01 | 0.43 |
| 1-3 | 17898.4 | 8660.73 | 0.38 | |
| 2-3 | 69459.4 | 63221.9 | 0.01 | |
表8 [EPy]BF4(1)+有机盐(2)+水(3)双水相体系拟合NRTL模型的参数结果和均方根偏差
Table 8 Values of the parameters of NRTL model for [EPy]BF4 (1)+ organic salt(2) + H2O(3)ATPS
| i-j | ?gij/(J·mol-1) | ?gji/(J·mol-1) | α | 100RMSD |
|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O ATPS at 308.15 K | ||||
| 1-2 | 33832.2 | 288097 | 0.01 | 0.57 |
| 1-3 | 10600.8 | 6855.21 | 0.37 | |
| 2-3 | -131468 | 201527 | 0.01 | |
| [EPy]BF4 + C6H5O7(NH4)3+ H2O ATPS at 308.15 K | ||||
| 1-2 | -14636.3 | 160854 | 0.02 | 0.08 |
| 1-3 | 8517.21 | 21576.4 | 0.25 | |
| 2-3 | 140479 | 94027.1 | 0.06 | |
| [EPy]BF4 + C6H5O7Na3+ H2O ATPS at 308.15 K | ||||
| 1-2 | -66136.2 | 273227 | 0.01 | 0.43 |
| 1-3 | 17898.4 | 8660.73 | 0.38 | |
| 2-3 | 69459.4 | 63221.9 | 0.01 | |
| System | T/K | V*213 | f213 | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 308.15 | 4.284 | 0.1822 | 0.9975 | 0.61 |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 308.15 | 5.365 | 0.09533 | 0.9872 | 1.1 |
| [EPy]BF4 + C6H5O7Na3 + H2O | 308.15 | 6.884 | 0.06212 | 0.9974 | 0.75 |
表9 [EPy]BF4+有机盐+水双水相体系拟合式(15)的参数结果、相关系数和标准偏差
Table 9 Values of the parameters of Eq.(15) for [EPy]BF4 + organic salt + H2O ATPSs
| System | T/K | V*213 | f213 | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 308.15 | 4.284 | 0.1822 | 0.9975 | 0.61 |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 308.15 | 5.365 | 0.09533 | 0.9872 | 1.1 |
| [EPy]BF4 + C6H5O7Na3 + H2O | 308.15 | 6.884 | 0.06212 | 0.9974 | 0.75 |
| System | T/K | ks | β | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 308.15 | 2078 | -0.7934 | 0.9939 | 4.9×10-3 |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 308.15 | 3310 | -1.993 | 0.9978 | 7.8×10-3 |
| [EPy]BF4 + C6H5O7Na3 + H2O | 308.15 | 3483 | -1.099 | 0.9989 | 2.4×10-3 |
表10 [EPy]BF4+有机盐+水双水相体系拟合式(16)的参数结果、相关系数和标准偏差
Table 10 Values of the parameters of Eq. (16) for [EPy]BF4 + organic salt + H2O ATPSs
| System | T/K | ks | β | R2 | SD |
|---|---|---|---|---|---|
| [EPy]BF4 + (CH2COONa)2 + H2O | 308.15 | 2078 | -0.7934 | 0.9939 | 4.9×10-3 |
| [EPy]BF4 + C6H5O7(NH4)3 + H2O | 308.15 | 3310 | -1.993 | 0.9978 | 7.8×10-3 |
| [EPy]BF4 + C6H5O7Na3 + H2O | 308.15 | 3483 | -1.099 | 0.9989 | 2.4×10-3 |
| 1 | Iqbal M, Tao Y, Xie S, et al. Aqueous two-phase system (ATPS): an overview and advances in its applications[J]. Biological Procedures Online, 2016, 18(1): 18. |
| 2 | 周红航, 王维香. 聚乙二醇/硫酸铵双水相体系萃取猪胰蛋白酶[J]. 化工进展, 2009, 28(2): 305-308. |
| Zhou H H, Wang W X. Extraction of porcine trypsin from a polyethylene glycol/ammonium sulfate two-phase system[J]. Chemical Industry and Engineering Progress, 2009, 28(2): 305-308. | |
| 3 | Dupont J, de Souza R F, Suarez P A Z. Ionic liquid (molten salt) phase organometallic catalysis[J]. Chemical Reviews, 2002, 102(10): 3667-3692. |
| 4 | Gutowski K E, Broker G A, Willauer H D, et al. Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations[J]. J. Am. Chem. Soc., 2003, 125: 6632-6633. |
| 5 | Freire M G, Claudio A F M, Araujo J M M, et al. Aqueous biphasic systems: a boost brought about by using ionic liquids[J]. Chemical Society Reviews, 2012, 41(14): 4966-4995. |
| 6 | 徐晓冬, 丹媛媛, 李欣欣, 等. 离子液体双水相体系及其在生物分离中的应用[J]. 生物技术通报, 2008, (5): 88-91. |
| Xu X D, Dan Y Y, Li X X, et al. Ionic liquid two-aqueous system and its application in biological separation[J]. Biotechnology Bulletin, 2008, (5): 88-91. | |
| 7 | 刘丙艳, 刘培元, 王国平, 等. [Bmim]BF4-H2O-Na2CO3离子液体双水相体系液液相平衡数据的测定与关联[J]. 化工学报, 2007, 58(8): 1885-1890. |
| Liu B Y, Liu P Y, Wang G P, et al. Measurement and correlation of liquid-liquid equilibrium data for ionic liquid-based aqueous two-phase system of [Bmim]BF4-H2O-Na2CO3[J]. Journal of Chemical Industry and Engineering (China), 2007, 58(8): 1885-1890. | |
| 8 | 赵晓军, 田刚, 李鑫垚, 等. [Bmim]BF4/EA-(NH4)2SO4-H2O双水相体系萃取黄连中盐酸小檗碱[J]. 山东化工, 2017, 46(18): 14-17. |
| Zhao X J, Tian G, Li X Y, et al. Extraction berberine hydrochloride from coptis by [Bmim]BF4/EA-(NH4)2SO4-H2O aqueous two-phase system[J]. Shandong Chemical Industry, 2017, 46(18): 14-17. | |
| 9 | 蔡涛, 张海德, 董安华, 等. 木瓜蛋白酶在[Cnmim]BF4-NaH2PO4双水相体系中的分配行为[J].化工学报, 2015, 66(6): 2017-2022. |
| Cai T, Zhang H D, Dong A H, et al. Partition behavior of papain in [Cnmim]BF4-NaH2PO4 aqueous two-phase system[J]. CIESC Journal, 2015, 66(6): 2017-2022. | |
| 10 | 余垒, 朱新儒, 曾颖, 等. [N2,2,2,2]BF4的合成及其对双水相体系成相行为的影响[J]. 无机盐工业, 2019, 51(10): 22-27. |
| Yu L, Zhu X R, Zeng Y, et al. Synthesis of [N2,2,2,2]BF4 and its effect on phase formation behavior of aqueous two-phase system[J]. Inorganic Chemicals Industry, 2019, 51(10): 22-27. | |
| 11 | 朱新儒, 张海德, 余垒. Box-Behnken响应面法优化[CnPy]Cl (n=2, 4, 6)-K2HPO4双水相体系萃取木瓜蛋白酶[J].食品科技, 2018, 43(9): 290-297. |
| Zhu X R, Zhang H D, Yu L. Optimization of [CnPy]Cl (n=2, 4, 6)-K2HPO4 ionic liquid aqueous two-phase system extraction of papain using response surface methodology with Box-Behnken design[J]. Food Science and Technology, 2018, 43(9): 290-297. | |
| 12 | Li Y, Lu X, He W, et al. Influence of the salting-out ability and temperature on the liquid–liquid equilibria of aqueous two-phase systems based on ionic liquid–organic salts–water[J]. Journal of Chemical & Engineering Data, 2016, 61(1): 475-486. |
| 13 | Oppermann S, Stein F, Kragl U. Ionic liquids for two-phase systems and their application for purification,extraction and biocatalysis[J]. Applied microbiology and Biotechnology, 2011, 89(3): 493-499. |
| 14 | Gómez E, Macedo E A. Partitioning of DNP-amino acids in ionic liquid/citrate salt based aqueous two-phase system[J]. Fluid Phase Equilibria, 2019, 484: 82-87. |
| 15 | 邓凡政, 郭东方. 离子液体双水相体系萃取分离牛血清白蛋白[J]. 分析化学, 2006, 34(10): 1451-1453. |
| Deng F Z, Guo D F. Extraction and separation of bovine serum albumin in ionic liquid two-aqueous system[J]. Chinese Journal of Analytical Chemistry, 2006, 34(10): 1451-1453. | |
| 16 | Guo J, Xu S, Qin Y, et al. The temperature influence on the phase behavior of ionic liquid based aqueous two-phase systems and its extraction efficiency of 2-chlorophenol[J]. Fluid Phase Equilibria, 2020, 506: 112394. |
| 17 | Petkovic M, Seddon K R, Rebelo L P N, et al. Ionic liquids: a pathway to environmental acceptability[J]. Chemical Society Reviews, 2011, 40(3): 1383-1403. |
| 18 | Garcia M T, Gathergood N, Scammells P J. Biodegradable ionic liquids (Ⅱ): Effect of the anion and toxicology[J]. Green Chemistry, 2005, 7(1): 9-14. |
| 19 | Petkovic M, Seddon K R, Rebelo L P, et al. Ionic liquids: a pathway to environmental acceptability[J]. Chemical Society Reviews, 2011, 40(3): 1383-1403. |
| 20 | 高鹏. 不同碳链长度的离子液体对蛋白核小球藻的毒性影响研究[J]. 安徽化工, 2017, 43(4): 44-46. |
| Gao P. Toxicity effects of ionic liquids with different carbon chain length on Chlorella proteus[J]. Anhui Chemical Industry, 2017, 43(4): 44-46. | |
| 21 | 王全泽, 袁堂丰, 瞿利民, 等. 微胶囊双水相法提取罗汉松挥发油及成分分析[J]. 精细化工, 2019, 36(4): 684-690. |
| Wang Q Z, Yuan T F, Qu L M, et al. Extraction of volatile oil from podocarpus macrophyllus by microcapsule aqueous two-phase system and its composition analysis[J]. Fine Chemicals, 2019, 36(4): 684-690. | |
| 22 | 董彩文, 王浩瑾, 张明俊, 等. 双水相技术提取黄秋葵黄酮的工艺条件研究[J]. 粮食与油脂, 2019, 32(10): 85-88. |
| Ding C W, Wang H J, Zhang M J,et al. Study on extraction of flavonoids from okra by aqueous two-phase technology[J]. Cereals & Oils, 2019, 32(10): 85-88. | |
| 23 | 王丽, 费春芳. 两种双水相体系提取枇杷叶中黄酮的工艺研究[J]. 化学与生物工程, 2014, 31(4): 55-59. |
| Wang L, Fei C F. Study on extraction technology of elavonoids from loquat leaves by two kinds of aqueous two-phase systems [J]. Chemistry & Bioengineering, 2014, 31(4): 55-59. | |
| 24 | 李青云, 袁凤梅, 吴金英. 聚乙二醇/硫酸钠双水相萃取赖氨酸[J]. 食品与发酵工业, 2014, 40(4): 221-226. |
| Li Q Y, Yuan F M, Wu J Y. Investigation on extraction of L-lysine by a polyethylene glycol /sodium sulfate aqueous two-phase system [J]. Food and Fermentation Industries, 2014, 40(4): 221-226. | |
| 25 | Xu S, Zhu Q, Luo Q, et al. Influence of ions and temperature on aqueous biphasic systems containing ionic liquid and ammonium sulfate[J]. Journal of Chemical & Engineering Data, 2019, 64(7): 3139-3147. |
| 26 | 李宇亮, 杨思语, 张文杉. 温敏性离子液体氯化N-丁基吡啶双水相体系相图的测定及关联[J]. 高校化学工程学报, 2016, 30(6): 1445-1450. |
| Li Y L, Yang S Y, Zhang W S. Determination and correlation of phase diagrams of thermosensitive ionic liquid chlorinated N-butylpyridine aqueous two phase system[J]. Journal of Chemical Engineering of Chinese Universities, 2016, 30(6): 1445-1450. | |
| 27 | Merchuk J C, Andrews B A, Asenjo J A. Aqueous two-phase systems for protein separation: studies on phase inversion[J]. Journal of Chromatography B: Biomedical Sciences and Applications, 1998, 711(1/2): 285-293. |
| 28 | Guan Y, Lilley T H, Treffry T E. A new excluded volume theory and its application to the coexistence curves of aqueous polymer two-phase systems[J]. Macromolecules, 1993, 26(15): 3971-3979. |
| 29 | Hey M J, Jackson D P, Yan H. The salting-out effect and phase separation in aqueous solutions of electrolytes and poly (ethylene glycol)[J]. Polymer, 2005, 46(8): 2567-2572. |
| 30 | Li Y, Xu Z, Luo Q, et al. Phase diagram of ionic liquid aqueous two-phase systems with N-butylpyridinium tetrafluoroborate, ammonium Citrate/Sodium acetate, and water from 308.15 K to 328.15 K[J]. Thermochimica Acta, 2016, 632: 72-78. |
| 31 | Xu S, Zhu Q, Luo Q, et al. Influence of ions and temperature on aqueous biphasic systems containing ionic liquid and ammonium sulfate[J]. Journal of Chemical & Engineering Data, 2019, 64(7): 3139-3147. |
| 32 | Alvarez-Guerra E, Ventura S P M, Alvarez-Guerra M, et al. Modeling of the binodal curve of ionic liquid/salt aqueous systems[J]. Fluid Phase Equilibria, 2016, 426: 10-16. |
| 33 | Wang Y, Yan Y, Hu S, et al. Phase diagrams of ammonium sulfate+ethanol/1-propanol/2-propanol + water aqueous two-phase systems at 298.15 K and correlation[J]. Journal of Chemical & Engineering Data, 2010, 55(2): 876-881. |
| 34 | Othmer D F, Tobias P E. Liquid-liquid extraction data-toluene and acetaldehyde systems[J]. Industrial & Engineering Chemistry, 1942, 34(6): 690-692. |
| 35 | Bancroft W D, Hubard S S. A new method for determining dineric distribution [J]. Journal of the American Chemical Society, 1942, 64(2): 347-353. |
| 36 | Rodriguez-Escontrela I, Arce A, Soto A, et al. Correlation of three-liquid-phase equilibria involving ionic liquids[J]. Physical Chemistry Chemical Physics, 2016, 18(31): 21610-21617. |
| 37 | Marcus Y. Thermodynamics of solvation of ions(Part 5): Gibbs free energy of hydration at 298.15 K[J]. Journal of the Chemical Society, Faraday Transactions, 1991, 87(18): 2995-2999. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [5] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [6] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [7] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [8] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [9] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [10] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [11] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [12] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [13] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [14] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [15] | 邢雷, 苗春雨, 蒋明虎, 赵立新, 李新亚. 井下微型气液旋流分离器优化设计与性能分析[J]. 化工学报, 2023, 74(8): 3394-3406. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号