化工学报 ›› 2022, Vol. 73 ›› Issue (11): 5011-5024.DOI: 10.11949/0438-1157.20221062
收稿日期:2022-07-28
修回日期:2022-08-25
出版日期:2022-11-05
发布日期:2022-12-06
通讯作者:
张香兰
作者简介:刘潜(1995—),男,博士研究生,460905289@qq.com
基金资助:
Qian LIU( ), Xianglan ZHANG(
), Xianglan ZHANG( ), Zhiping LI, Zhuoqi LI, Hong YU
), Zhiping LI, Zhuoqi LI, Hong YU
Received:2022-07-28
Revised:2022-08-25
Online:2022-11-05
Published:2022-12-06
Contact:
Xianglan ZHANG
摘要:
离子液体作为萃取溶剂已广泛用于油酚混合物的分离,但由于阴阳离子的可调控性,不同离子液体的性质差异较大,快速筛选得到具有应用前景的离子液体至关重要。针对间甲酚-异丙苯分离体系,采用COSMO-RS模型考察不同阴阳离子结构对离子液体分离性能的影响,并通过分子间相互作用能进行分析。在此基础上,提出一种多尺度的离子液体筛选方法,该方法包含无限稀释热力学性质计算、物性估算、相平衡关系计算和过程性能评价。该多尺度方法从分子尺度到单级平衡尺度,再到多级平衡尺度对离子液体进行逐步筛选。结果表明,1-乙基吡啶硫氰酸盐([C2py][SCN])和1-乙基吡啶双氰胺盐([C2py][DCA])是最终筛选得到的两种更具有油酚分离应用前景的离子液体。
中图分类号:
刘潜, 张香兰, 李志平, 栗卓琦, 喻红. 油酚分离过程离子液体萃取溶剂的多尺度筛选[J]. 化工学报, 2022, 73(11): 5011-5024.
Qian LIU, Xianglan ZHANG, Zhiping LI, Zhuoqi LI, Hong YU. Multiscale screening of ionic liquids as extractive solvents for oil-hydroxybenzene separation[J]. CIESC Journal, 2022, 73(11): 5011-5024.
| Cations | Names | Abbreviations |
|---|---|---|
| [C01] | 1-ethyl-3-methyl-imidazolium | [C2mim]+ |
| [C02] | 1-propyl-3-methyl-imidazolium | [C3mim]+ |
| [C03] | 1-butyl-3-methyl-imidazolium | [C4mim]+ |
| [C04] | 1-pentyl-3-methyl-imidazolium | [C5mim]+ |
| [C05] | 1-hexyl-3-methyl-imidazolium | [C6mim]+ |
| [C06] | 1-ethyl-pyridinium | [C2py]+ |
| [C07] | 1-propyl-pyridinium | [C3py]+ |
| [C08] | 1-butyl-pyridinium | [C4py]+ |
| [C09] | 1-pentyl-pyridinium | [C5py]+ |
| [C10] | 1-hexyl-pyridinium | [C6py]+ |
| [C11] | 1-ethyl-1-methyl-pyrrolidinium | [C2mpyr]+ |
| [C12] | 1-propyl-1-methyl-pyrrolidinium | [C3mpyr]+ |
| [C13] | 1-butyl-1-methyl-pyrrolidinium | [C4mpyr]+ |
| [C14] | 1-pentyl-1-methyl-pyrrolidinium | [C5mpyr]+ |
| [C15] | 1-hexyl-1-methyl-pyrrolidinium | [C6mpyr]+ |
| [C16] | 1-ethyl-1-methyl-piperidinium | [C2mpip]+ |
| [C17] | 1-propyl-1-methyl-piperidinium | [C3mpip]+ |
| [C18] | 1-butyl-1-methyl-piperidinium | [C4mpip]+ |
| [C19] | 1-pentyl-1-methyl-piperidinium | [C5mpip]+ |
| [C20] | 1-hexyl-1-methyl-piperidinium | [C6mpip]+ |
| [C21] | 4-ethyl-4-methyl-morpholinium | [C2mmor]+ |
| [C22] | 4-propyl-4-methyl-morpholinium | [C3mmor]+ |
| [C23] | 4-butyl-4-methyl-morpholinium | [C4mmor]+ |
| [C24] | 4-pentyl-4-methyl-morpholinium | [C5mmor]+ |
| [C25] | 4-hexyl-4-methyl-morpholinium | [C6mmor]+ |
| [C26] | ethyl-trimethyl-ammonium | [N2111]+ |
| [C27] | propyl-trimethyl-ammonium | [N3111]+ |
| [C28] | butyl-trimethyl-ammonium | [N4111]+ |
| [C29] | pentyl-trimethyl-ammonium | [N5111]+ |
| [C30] | hexyl-trimethyl-ammonium | [N6111]+ |
表1 阳离子的名称和缩写
Table 1 Names and abbreviations of cations
| Cations | Names | Abbreviations |
|---|---|---|
| [C01] | 1-ethyl-3-methyl-imidazolium | [C2mim]+ |
| [C02] | 1-propyl-3-methyl-imidazolium | [C3mim]+ |
| [C03] | 1-butyl-3-methyl-imidazolium | [C4mim]+ |
| [C04] | 1-pentyl-3-methyl-imidazolium | [C5mim]+ |
| [C05] | 1-hexyl-3-methyl-imidazolium | [C6mim]+ |
| [C06] | 1-ethyl-pyridinium | [C2py]+ |
| [C07] | 1-propyl-pyridinium | [C3py]+ |
| [C08] | 1-butyl-pyridinium | [C4py]+ |
| [C09] | 1-pentyl-pyridinium | [C5py]+ |
| [C10] | 1-hexyl-pyridinium | [C6py]+ |
| [C11] | 1-ethyl-1-methyl-pyrrolidinium | [C2mpyr]+ |
| [C12] | 1-propyl-1-methyl-pyrrolidinium | [C3mpyr]+ |
| [C13] | 1-butyl-1-methyl-pyrrolidinium | [C4mpyr]+ |
| [C14] | 1-pentyl-1-methyl-pyrrolidinium | [C5mpyr]+ |
| [C15] | 1-hexyl-1-methyl-pyrrolidinium | [C6mpyr]+ |
| [C16] | 1-ethyl-1-methyl-piperidinium | [C2mpip]+ |
| [C17] | 1-propyl-1-methyl-piperidinium | [C3mpip]+ |
| [C18] | 1-butyl-1-methyl-piperidinium | [C4mpip]+ |
| [C19] | 1-pentyl-1-methyl-piperidinium | [C5mpip]+ |
| [C20] | 1-hexyl-1-methyl-piperidinium | [C6mpip]+ |
| [C21] | 4-ethyl-4-methyl-morpholinium | [C2mmor]+ |
| [C22] | 4-propyl-4-methyl-morpholinium | [C3mmor]+ |
| [C23] | 4-butyl-4-methyl-morpholinium | [C4mmor]+ |
| [C24] | 4-pentyl-4-methyl-morpholinium | [C5mmor]+ |
| [C25] | 4-hexyl-4-methyl-morpholinium | [C6mmor]+ |
| [C26] | ethyl-trimethyl-ammonium | [N2111]+ |
| [C27] | propyl-trimethyl-ammonium | [N3111]+ |
| [C28] | butyl-trimethyl-ammonium | [N4111]+ |
| [C29] | pentyl-trimethyl-ammonium | [N5111]+ |
| [C30] | hexyl-trimethyl-ammonium | [N6111]+ |
| Anions | Names | Abbreviations |
|---|---|---|
| [A01] | acetate | [Ac]- |
| [A02] | chloride | [Cl]- |
| [A03] | bromide | [Br]- |
| [A04] | hydrogen sulfate | [HSO4]- |
| [A05] | methyl sulfate | [MeSO4]- |
| [A06] | ethyl sulfate | [EtSO4]- |
| [A07] | dicyanamide | [DCA]- |
| [A08] | thiocyanate | [SCN]- |
| [A09] | tricyanomethane | [C(CN)3]- |
| [A10] | nitrate | [NO3]- |
| [A11] | tetrafluoroborate | [BF4]- |
| [A12] | hexafluorophosphate | [PF6]- |
表2 阴离子的名称和缩写
Table 2 Names and abbreviations of anions
| Anions | Names | Abbreviations |
|---|---|---|
| [A01] | acetate | [Ac]- |
| [A02] | chloride | [Cl]- |
| [A03] | bromide | [Br]- |
| [A04] | hydrogen sulfate | [HSO4]- |
| [A05] | methyl sulfate | [MeSO4]- |
| [A06] | ethyl sulfate | [EtSO4]- |
| [A07] | dicyanamide | [DCA]- |
| [A08] | thiocyanate | [SCN]- |
| [A09] | tricyanomethane | [C(CN)3]- |
| [A10] | nitrate | [NO3]- |
| [A11] | tetrafluoroborate | [BF4]- |
| [A12] | hexafluorophosphate | [PF6]- |
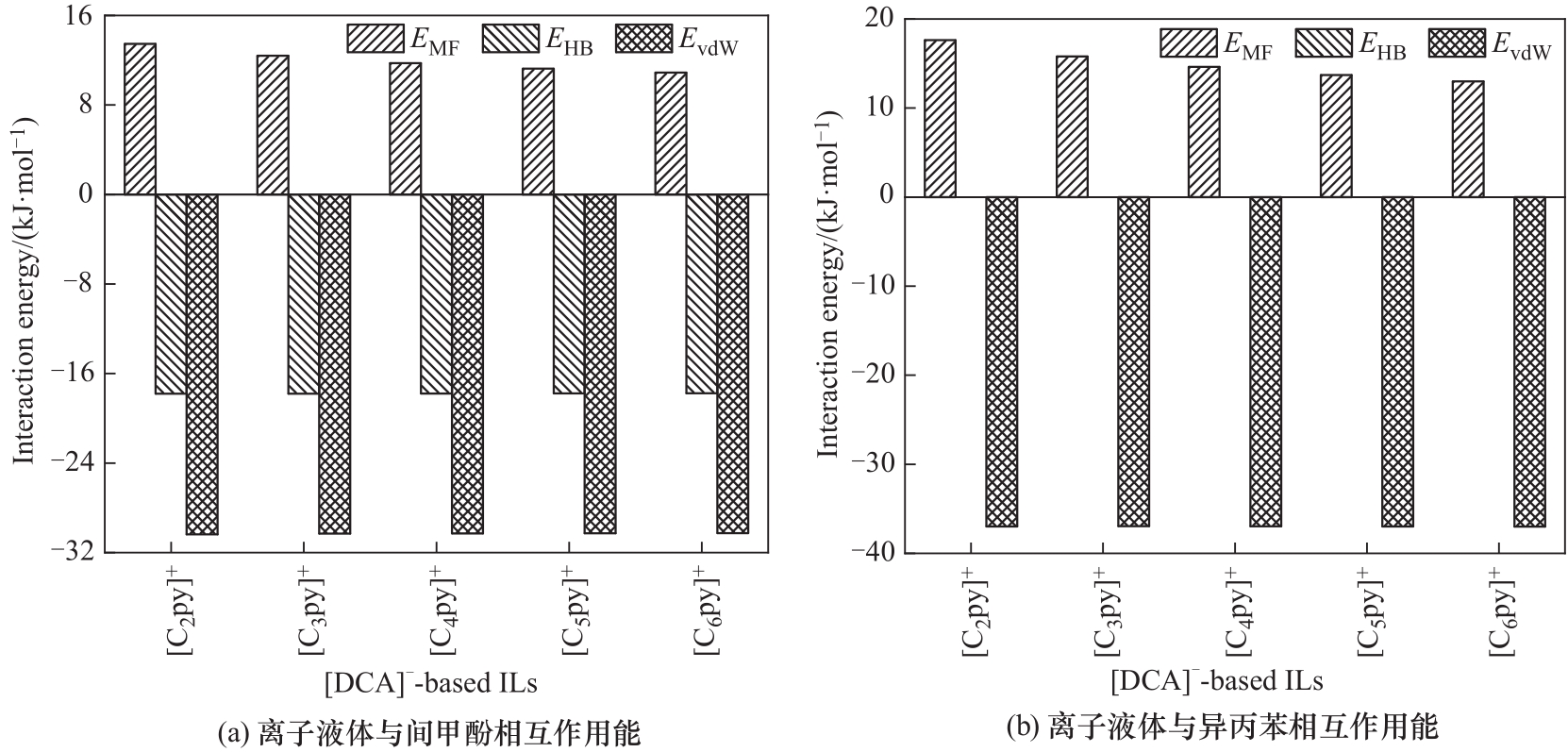
图2 不同烷基含碳数的吡啶离子液体和间甲酚、异丙苯之间的相互作用能
Fig.2 Interaction energy between pyridine ionic liquids with different alkyl carbon numbers and m-cresol, and cumene
| IL | Tm/K | η/cP | ||||
|---|---|---|---|---|---|---|
| [C2mim][DCA] | 19.87 | 728.37 | 0.0281 | 7.66×10-4 | 275.58 | 19.91 |
| [C3mim][DCA] | 19.99 | 584.74 | 0.0375 | 1.32×10-3 | 271.82 | 23.23 |
| [C2py][DCA] | 14.53 | 825.36 | 0.0181 | 5.44×10-4 | 263.55 | 20.47 |
| [C3py][DCA] | 16.46 | 628.81 | 0.0284 | 1.07×10-3 | 259.79 | 23.88 |
| [C3mim][SCN] | 21.66 | 515.33 | 0.0429 | 1.33×10-3 | 297.27 | 52.05 |
| [C2py][SCN] | 21.08 | 686.24 | 0.0285 | 5.57×10-4 | 288.99 | 45.85 |
| [C3py][SCN] | 18.53 | 567.55 | 0.0334 | 1.09×10-3 | 285.24 | 53.51 |
| Glycol | 3.35 | 80.66 | 0.0508 | 1.44×10-3 | 260.25 | 17.33 |
表3 由无限稀释热力学性质和物性约束筛选得到的7种离子液体(以乙二醇为基准溶剂)
Table 3 7 ionic liquids screened after the first two steps by infinite dilution thermodynamic properties and physical property constraints (with glycol as the benchmark)
| IL | Tm/K | η/cP | ||||
|---|---|---|---|---|---|---|
| [C2mim][DCA] | 19.87 | 728.37 | 0.0281 | 7.66×10-4 | 275.58 | 19.91 |
| [C3mim][DCA] | 19.99 | 584.74 | 0.0375 | 1.32×10-3 | 271.82 | 23.23 |
| [C2py][DCA] | 14.53 | 825.36 | 0.0181 | 5.44×10-4 | 263.55 | 20.47 |
| [C3py][DCA] | 16.46 | 628.81 | 0.0284 | 1.07×10-3 | 259.79 | 23.88 |
| [C3mim][SCN] | 21.66 | 515.33 | 0.0429 | 1.33×10-3 | 297.27 | 52.05 |
| [C2py][SCN] | 21.08 | 686.24 | 0.0285 | 5.57×10-4 | 288.99 | 45.85 |
| [C3py][SCN] | 18.53 | 567.55 | 0.0334 | 1.09×10-3 | 285.24 | 53.51 |
| Glycol | 3.35 | 80.66 | 0.0508 | 1.44×10-3 | 260.25 | 17.33 |
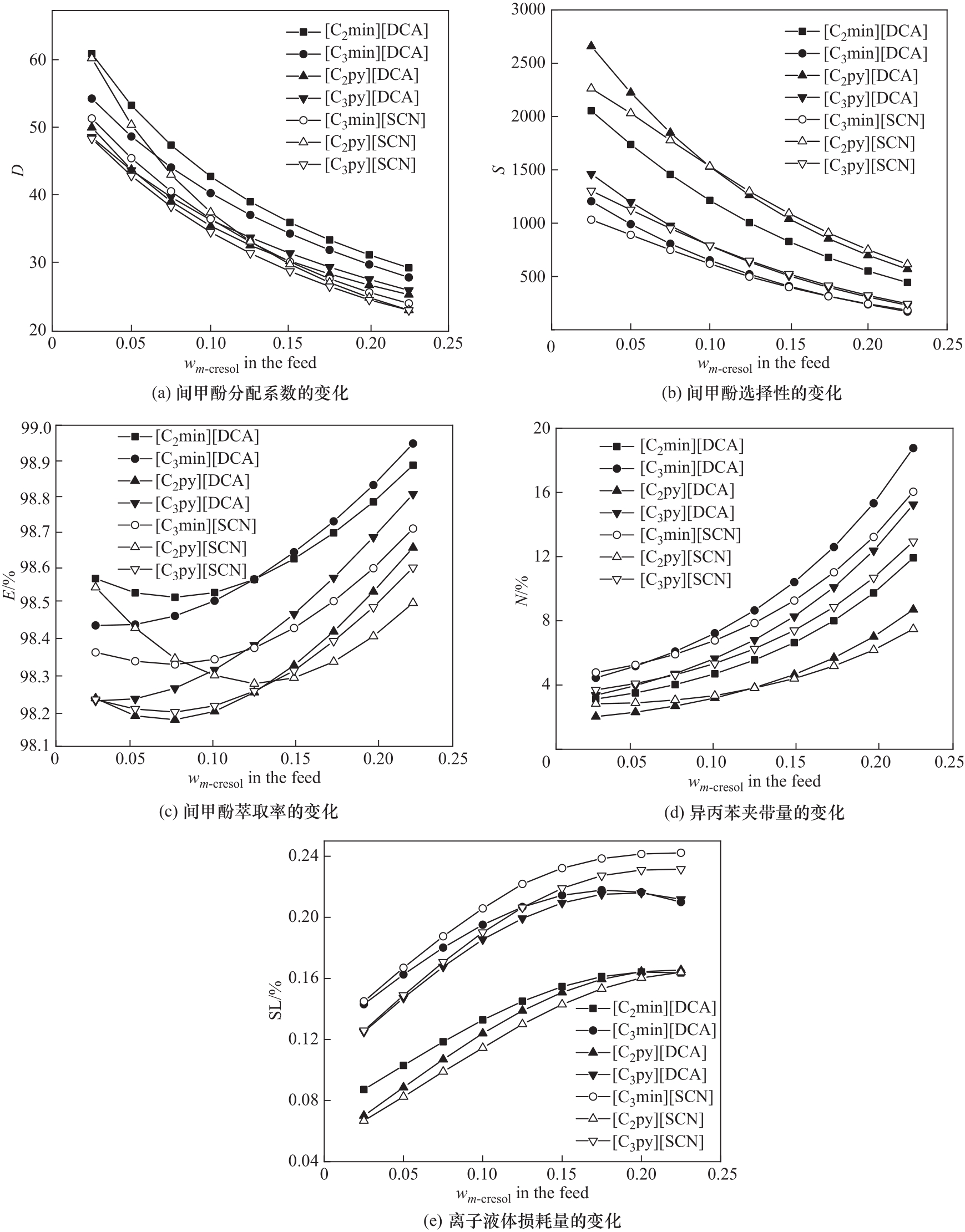
图9 COSMO-RS计算前两步筛选得到的7种离子液体在不同进料组成下的分离性能
Fig.9 COSMO-RS calculated separation performance at different feed compositions of 7 ionic liquids screened after steps 1 and 2
| IL | MW/(g·mol-1) | Tb/K | Tc/K | Pc/bar | Vc/(cm3·mol-1) | ω | ρ/(g·cm-3) |
|---|---|---|---|---|---|---|---|
| [C2mim][DCA] | 177.2 | 670.64 | 998.48 | 35.1 | 562.0 | 0.3548 | 1.0750 |
| [C2py][DCA] | 174.2 | 736.28 | 1123.34 | 32.6 | 571.1 | 0.2336 | 1.0243 |
| [C2py][SCN] | 166.2 | 676.19 | 926.58 | 29.0 | 554.7 | 0.6922 | 1.0970 |
表4 3种离子液体的摩尔质量、沸点、临界参数、偏心因子和密度
Table 4 Molecular weights, normal boiling points, critical properties, acentric factors, and densities of 3 ionic liquids
| IL | MW/(g·mol-1) | Tb/K | Tc/K | Pc/bar | Vc/(cm3·mol-1) | ω | ρ/(g·cm-3) |
|---|---|---|---|---|---|---|---|
| [C2mim][DCA] | 177.2 | 670.64 | 998.48 | 35.1 | 562.0 | 0.3548 | 1.0750 |
| [C2py][DCA] | 174.2 | 736.28 | 1123.34 | 32.6 | 571.1 | 0.2336 | 1.0243 |
| [C2py][SCN] | 166.2 | 676.19 | 926.58 | 29.0 | 554.7 | 0.6922 | 1.0970 |
| IL | A0/(J·mol-1·K-1) | A1/(J·mol-1·K-2) | A2/(J·mol-1·K-3) | A3/(J·mol-1·K-4) |
|---|---|---|---|---|
| [C2mim][DCA] | 13.371 | 0.76460 | -3.728×10-4 | -3.68×10-8 |
| [C2py][DCA] | 20.691 | 0.66048 | -2.091×10-4 | -4.43×10-8 |
| [C2py][SCN] | -22.939 | 0.56668 | -7.310×10-5 | -8.73×10-8 |
表5 3种离子液体的理想气体热容多项式系数
Table 5 Polynomial coefficients of ideal gas heat capacity of 3 ionic liquids
| IL | A0/(J·mol-1·K-1) | A1/(J·mol-1·K-2) | A2/(J·mol-1·K-3) | A3/(J·mol-1·K-4) |
|---|---|---|---|---|
| [C2mim][DCA] | 13.371 | 0.76460 | -3.728×10-4 | -3.68×10-8 |
| [C2py][DCA] | 20.691 | 0.66048 | -2.091×10-4 | -4.43×10-8 |
| [C2py][SCN] | -22.939 | 0.56668 | -7.310×10-5 | -8.73×10-8 |
| Component | NRTL parameters/K | RMSD | ||||||
|---|---|---|---|---|---|---|---|---|
| i-j | aij | aji | bij | bji | cij | |||
| {[C2mim][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 3.57 | -1.77 | 684.03 | -299.00 | 0.3 | 0.0139 | ||
| 1-3 | 2.48 | -3.42 | -108.54 | 2630.06 | 0.2 | |||
| 2-3 | 28.46 | -7.23 | 5067.29 | 2902.47 | 0.3 | |||
| {[C2py][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 0.65 | -1.64 | 1459.52 | -351.76 | 0.3 | 0.0157 | ||
| 1-3 | 2.38 | -2.47 | 39.90 | 2356.43 | 0.2 | |||
| 2-3 | 37.04 | -6.78 | 5271.13 | 2685.83 | 0.3 | |||
| {[C2py][SCN] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 4.18 | -0.87 | 974.17 | -637.33 | 0.3 | 0.0178 | ||
| 1-3 | 4.46 | -1.63 | -513.91 | 2097.21 | 0.2 | |||
| 2-3 | 15.66 | -5.45 | 5657.96 | 2101.41 | 0.3 | |||
表6 3个{离子液体+间甲酚+异丙苯}三元体系的NRTL参数和均方根偏差
Table 6 NRTL parameters and RMSD values of three {ionic liquid + m-cresol + cumene} ternary systems
| Component | NRTL parameters/K | RMSD | ||||||
|---|---|---|---|---|---|---|---|---|
| i-j | aij | aji | bij | bji | cij | |||
| {[C2mim][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 3.57 | -1.77 | 684.03 | -299.00 | 0.3 | 0.0139 | ||
| 1-3 | 2.48 | -3.42 | -108.54 | 2630.06 | 0.2 | |||
| 2-3 | 28.46 | -7.23 | 5067.29 | 2902.47 | 0.3 | |||
| {[C2py][DCA] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 0.65 | -1.64 | 1459.52 | -351.76 | 0.3 | 0.0157 | ||
| 1-3 | 2.38 | -2.47 | 39.90 | 2356.43 | 0.2 | |||
| 2-3 | 37.04 | -6.78 | 5271.13 | 2685.83 | 0.3 | |||
| {[C2py][SCN] (1) + m-cresol (2) + cumene (3)} | ||||||||
| 1-2 | 4.18 | -0.87 | 974.17 | -637.33 | 0.3 | 0.0178 | ||
| 1-3 | 4.46 | -1.63 | -513.91 | 2097.21 | 0.2 | |||
| 2-3 | 15.66 | -5.45 | 5657.96 | 2101.41 | 0.3 | |||
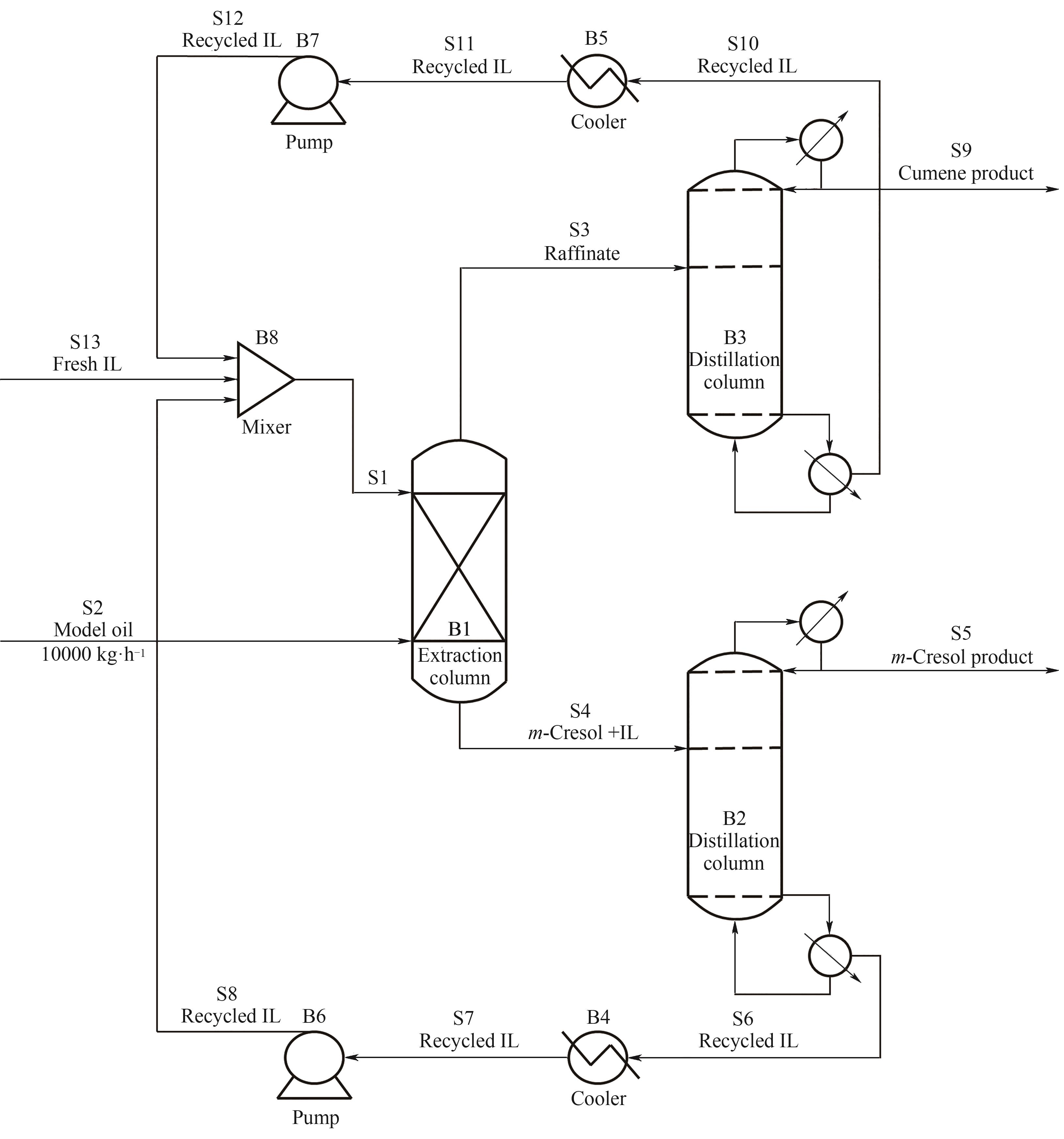
图10 离子液体萃取分离间甲酚和异丙苯的过程模拟流程图
Fig.10 Process simulation flowsheet for the separation of m-cresol and cumene using ionic liquids as the extractive solvent
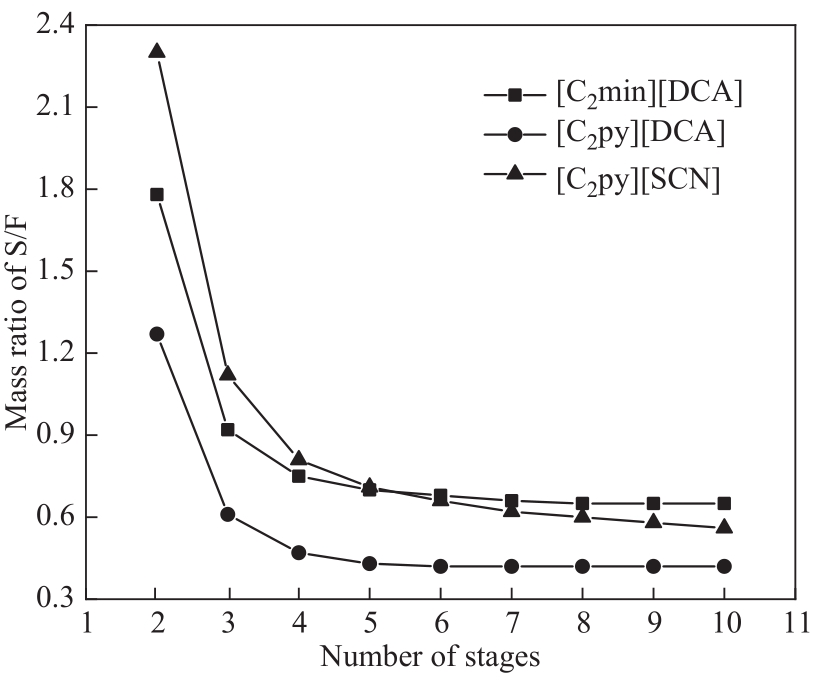
图11 满足间甲酚和异丙苯分离要求萃取塔塔板数和基于质量的溶剂-进料比关系
Fig.11 Mass ratio of solvent-to-feed (S/F) as a function of the number of stages in the extraction column to meet the separation requirements of m-cresol and cumene
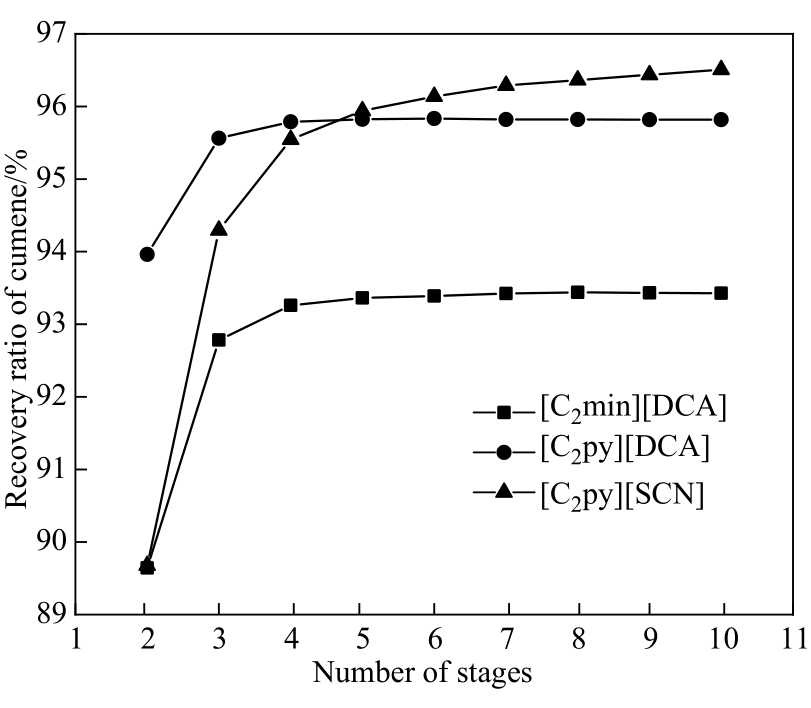
图12 满足间甲酚和异丙苯分离要求时萃取塔塔板数和异丙苯回收率的关系
Fig.12 Recovery ratio of cumene as a function of the number of stages in the extraction column when meeting the separation requirements of m-cresol and cumene
Extraction column (298.15 K, 1 ×105 Pa, 8 stages) | Distillation column (0.005 ×105 Pa) | Cumene product | m-Cresol product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL | IL makeup/ (kg·h-1) | IL in recycle/ (kg·h-1) | Operating conditions | Heat duty/ kW | Recovery ratio/ % | Mass purity/ % | Recovery ratio/ % | Mass purity/ % | ||||
| D∶F | NS | FS | RR | |||||||||
| [C2mim][DCA] | 1.63 | 6498.37 | 0.3479 | 8 | 3 | 0.42 | 1215.23 ① | 93.43 | 99.99 | 99.99 | 86.69 | |
| 0.9981 | 7 | 3 | 0.18 | 769.94 ② | ||||||||
| [C2py][DCA] | 1.17 | 4197.59 | 0.4403 | 8 | 3 | 0.45 | 1126.48 ① | 95.84 | 99.99 | 99.99 | 91.15 | |
| 0.9981 | 6 | 3 | 0.11 | 698.65 ② | ||||||||
| [C2py][SCN] | 0.73 | 5999.27 | 0.3522 | 6 | 3 | 0.25 | 1027.74 ① | 96.37 | 99.99 | 99.99 | 92.16 | |
| 0.9981 | 7 | 3 | 0.17 | 727.01 ② | ||||||||
表7 3种离子液体萃取分离间甲酚和异丙苯过程模拟的主要结果
Table 7 Main results of process simulation for the separation of m-cresol and cumene using 3 ionic liquids
Extraction column (298.15 K, 1 ×105 Pa, 8 stages) | Distillation column (0.005 ×105 Pa) | Cumene product | m-Cresol product | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL | IL makeup/ (kg·h-1) | IL in recycle/ (kg·h-1) | Operating conditions | Heat duty/ kW | Recovery ratio/ % | Mass purity/ % | Recovery ratio/ % | Mass purity/ % | ||||
| D∶F | NS | FS | RR | |||||||||
| [C2mim][DCA] | 1.63 | 6498.37 | 0.3479 | 8 | 3 | 0.42 | 1215.23 ① | 93.43 | 99.99 | 99.99 | 86.69 | |
| 0.9981 | 7 | 3 | 0.18 | 769.94 ② | ||||||||
| [C2py][DCA] | 1.17 | 4197.59 | 0.4403 | 8 | 3 | 0.45 | 1126.48 ① | 95.84 | 99.99 | 99.99 | 91.15 | |
| 0.9981 | 6 | 3 | 0.11 | 698.65 ② | ||||||||
| [C2py][SCN] | 0.73 | 5999.27 | 0.3522 | 6 | 3 | 0.25 | 1027.74 ① | 96.37 | 99.99 | 99.99 | 92.16 | |
| 0.9981 | 7 | 3 | 0.17 | 727.01 ② | ||||||||
| 1 | Ji Y A, Hou Y C, Ren S H, et al. Separation of phenolic compounds from oil mixtures using environmentally benign biological reagents based on Brønsted acid-Lewis base interaction [J]. Fuel, 2019, 239: 926-934. |
| 2 | Yao C F, Hou Y C, Ren S H, et al. Efficient separation of phenol from model oils using environmentally benign quaternary ammonium-based zwitterions via forming deep eutectic solvents [J]. Chemical Engineering Journal, 2017, 326: 620-626. |
| 3 | Yi L, Feng J, Li W Y, et al. High-performance separation of phenolic compounds from coal-based liquid oil by deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(8): 7777-7783. |
| 4 | Jiao T T, Qin X Z, Zhang H W, et al. Separation of phenol and pyridine from coal tar via liquid-liquid extraction using deep eutectic solvents [J]. Chemical Engineering Research and Design, 2019, 145: 112-121. |
| 5 | Gai H J, Qiao L, Zhong C Y, et al. A solvent based separation method for phenolic compounds from low-temperature coal tar [J]. Journal of Cleaner Production, 2019, 223: 1-11. |
| 6 | 侯玉翠, 彭威, 杨春梅, 等. 咪唑基离子液体萃取分离模拟油酚混合物 [J]. 化工学报, 2013, 64(S1): 118-123. |
| Hou Y C, Peng W, Yang C M, et al. Extraction of phenolic compounds from simulated oil with imidazolium based ionic liquids [J]. CIESC Journal, 2013, 64(S1): 118-123. | |
| 7 | Hou Y C, Ren Y H, Peng W, et al. Separation of phenols from oil using imidazolium-based ionic liquids [J]. Industrial & Engineering Chemistry Research, 2013, 52(50): 18071-18075. |
| 8 | Ji Y A, Hou Y C, Ren S H, et al. Highly efficient extraction of phenolic compounds from oil mixtures by trimethylamine-based dicationic ionic liquids via forming deep eutectic solvents [J]. Fuel Processing Technology, 2018, 171: 183-191. |
| 9 | Ji Y A, Hou Y C, Ren S H, et al. Highly efficient separation of phenolic compounds from oil mixtures by imidazolium-based dicationic ionic liquids via forming deep eutectic solvents [J]. Energy & Fuels, 2017, 31(9): 10274-10282. |
| 10 | Yao C F, Hou Y C, Ren S H, et al. Efficient separation of phenolic compounds from model oils by dual-functionalized ionic liquids [J]. Chemical Engineering and Processing - Process Intensification, 2018, 128: 216-222. |
| 11 | Zhu C, Li F F, Zhang J, et al. Performance of functionalized ionic liquid with double chemical sites for separating phenolic compounds: mechanism and liquid-liquid behavior studies [J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106790. |
| 12 | Xu D M, Zhong P, Peng L J, et al. Multiscale evaluation of the efficiently separation of phenols using a designed cationic functionalized ionic liquid based on Brønsted/Lewis coordination [J]. Journal of Molecular Liquids, 2022, 345: 117901. |
| 13 | Xu D M, Wang S X, Zhang T, et al. Extraction and interaction insights for enhanced separation of phenolic compounds from model coal tar using a hydroxyl-functionalized ionic liquid [J]. Chemical Engineering Research and Design, 2022, 178: 567-574. |
| 14 | Song Z, Zhou T, Zhang J N, et al. Screening of ionic liquids for solvent-sensitive extraction -with deep desulfurization as an example [J]. Chemical Engineering Science, 2015, 129: 69-77. |
| 15 | 董一春. 离子液体预测型热力学模型及其在萃取精馏分离甲缩醛和甲醇中的应用[D]. 北京: 北京化工大学, 2020. |
| Dong Y C. Predictive thermodynamics models for ionic liquids and their application in the separation of methylal and methanol mixture by extractive distillation [D]. Beijing: Beijing University of Chemical Technology, 2020. | |
| 16 | Anantharaj R, Banerjee T. Quantum chemical studies on the simultaneous interaction of thiophene and pyridine with ionic liquid [J]. AIChE Journal, 2011, 57(3): 749-764. |
| 17 | Song Z, Zhang C Y, Qi Z W, et al. Computer-aided design of ionic liquids as solvents for extractive desulfurization [J]. AIChE Journal, 2018, 64(3): 1013-1025. |
| 18 | Song Z, Li X X, Chao H, et al. Computer-aided ionic liquid design for alkane/cycloalkane extractive distillation process[J]. Green Energy & Environment, 2019, 4(2): 154-165. |
| 19 | Diedenhofen M, Klamt A. COSMO-RS as a tool for property prediction of IL mixtures— a review [J]. Fluid Phase Equilibria, 2010, 294(1-2): 31-38. |
| 20 | Qin H, Wang Z H, Zhou T, et al. Comprehensive evaluation of COSMO-RS for predicting ternary and binary ionic liquid-containing vapor-liquid equilibria [J]. Industrial & Engineering Chemistry Research, 2021, 60(48): 17761-17777. |
| 21 | Lyu Z X, Zhou T, Chen L F, et al. Simulation based ionic liquid screening for benzene-cyclohexane extractive separation [J]. Chemical Engineering Science, 2014, 113: 45-53. |
| 22 | Gao S R, Chen X C, Abro R, et al. Desulfurization of fuel oil: conductor-like screening model for real solvents study on capacity of ionic liquids for thiophene and dibenzothiophene [J]. Industrial & Engineering Chemistry Research, 2015, 54(38): 9421-9430. |
| 23 | Song Z, Zhang J J, Zeng Q, et al. Effect of cation alkyl chain length on liquid-liquid equilibria of {ionic liquids + thiophene + heptane}: COSMO-RS prediction and experimental verification [J]. Fluid Phase Equilibria, 2016, 425: 244-251. |
| 24 | 张志刚, 张德彪, 张亲亲, 等. 基于COSMO-RS方法筛选离子液体分离乙酸乙酯-乙腈共沸物 [J]. 化工学报, 2019, 70(1): 146-153. |
| Zhang Z G, Zhang D B, Zhang Q Q, et al. Screening of ionic liquids for separation of ethyl acetate-acetonitrile azeotrope based on COSMO-RS [J]. CIESC Journal, 2019, 70(1): 146-153. | |
| 25 | Liu Q, Zhang X L, Li W. Separation of m-cresol from aromatic hydrocarbon and alkane using ionic liquids via hydrogen bond interaction [J]. Chinese Journal of Chemical Engineering, 2019, 27(11): 2675-2686. |
| 26 | 刘潜, 张香兰, 李巍. 基于COSMO-RS模型的分离油酚混合物的离子液体萃取剂筛选 [J]. 化工学报, 2018, 69(12): 5100-5111. |
| Liu Q, Zhang X L, Li W. Screening ionic liquids solvent for separation of oil and hydroxybenzene mixtures based on COSMO-RS model [J]. CIESC Journal, 2018, 69(12): 5100-5111. | |
| 27 | Liu Q, Bi J, Zhang X L. Effect of water on phenol separation from model oil with ionic liquids based on COSMO-RS calculation and experimental study [J]. ACS Omega, 2021, 6(41): 27368-27378. |
| 28 | Liu Q, Zhang X L. Highly efficient separation of phenolic compounds from low-temperature coal tar by composite extractants with low viscosity [J]. Journal of Molecular Liquids, 2022, 360: 119417. |
| 29 | Song Z, Zhou T, Qi Z W, et al. Systematic method for screening ionic liquids as extraction solvents exemplified by an extractive desulfurization process [J]. ACS Sustainable Chemistry & Engineering, 2017, 5(4): 3382-3389. |
| 30 | Kulajanpeng K, Suriyapraphadilok U, Gani R. Systematic screening methodology and energy efficient design of ionic liquid-based separation processes [J]. Journal of Cleaner Production, 2016, 111: 93-107. |
| 31 | Qin Z X, Cheng H Y, Song Z, et al. Selection of deep eutectic solvents for extractive deterpenation of lemon essential oil [J]. Journal of Molecular Liquids, 2022, 350: 118524. |
| 32 | Qin H, Cheng J, Yu H T, et al. Hierarchical ionic liquid screening integrating COSMO-RS and Aspen Plus for selective recovery of hydrofluorocarbons and hydrofluoroolefins from a refrigerant blend [J]. Industrial & Engineering Chemistry Research, 2022, 61(11): 4083-4094. |
| 33 | Jiang C H, Cheng H Y, Qin Z X, et al. COSMO-RS prediction and experimental verification of 1,5-pentanediamine extraction from aqueous solution by ionic liquids [J]. Green Energy & Environment, 2021, 6(3): 422-431. |
| 34 | 吴明尧. 基于季铵盐低共熔溶剂的柑橘精油脱萜过程研究[D]. 上海: 华东理工大学, 2021. |
| Wu M Y. Extractive deterpenation of citrus essential oils using quaternary ammonium-based deep eutectic solvents[D]. Shanghai: East China University of Science and Technology, 2021. | |
| 35 | Song Z, Hu X T, Zhou Y G, et al. Rational design of double salt ionic liquids as extraction solvents: separation of thiophene/n-octane as example [J]. AIChE Journal, 2019, 65(8): e16625. |
| 36 | Lazzús J A. A group contribution method to predict the melting point of ionic liquids [J]. Fluid Phase Equilibria, 2012, 313: 1-6. |
| 37 | Lazzús J A, Pulgar-Villarroel G. A group contribution method to estimate the viscosity of ionic liquids at different temperatures [J]. Journal of Molecular Liquids, 2015, 209: 161-168. |
| 38 | Huang Y, Dong H F, Zhang X P, et al. A new fragment contribution-corresponding states method for physicochemical properties prediction of ionic liquids [J]. AIChE Journal, 2013, 59(4): 1348-1359. |
| 39 | Nancarrow P, Lewis M, AbouChacra L. Group contribution methods for estimation of ionic liquid heat capacities: critical evaluation and extension [J]. Chemical Engineering & Technology, 2015, 38(4): 632-644. |
| 40 | Liu X K, Zhang X L. Solvent screening and liquid-liquid measurement for extraction of phenols from aromatic hydrocarbon mixtures [J]. The Journal of Chemical Thermodynamics, 2019, 129: 12-21. |
| 41 | Li A, Zhu C, Zhang L Z, et al. Efficient extraction and theoretical insights for separating o-, m-, and p-cresol from model coal tar by an ionic liquid [Emim][DCA] [J]. Canadian Journal of Chemical Engineering, 2022, 100: S205-S212. |
| 42 | Li A, Xu X, Zhang L Z, et al. Separation of cresol from coal tar by imidazolium-based ionic liquid [Emim][SCN]: interaction exploration and extraction experiment [J]. Fuel, 2020, 264: 116908. |
| 43 | Sun C G, Tang S K. Guanidinium dicyanamide-based nitrogen-rich energetic salts as additives of hypergolic ionic liquids [J]. Energy & Fuels, 2020, 34(11): 15068-15071. |
| 44 | Liu Q, Zhang X L. Systematic method of screening deep eutectic solvents as extractive solvents for m-cresol/cumene separation [J]. Separation and Purification Technology, 2022, 291: 120853. |
| 45 | Ma X X, Wei J, Guan W, et al. Ionic parachor and its application to pyridinium-based ionic liquids of {[C n py][DCA] (n = 2, 3, 4, 5, 6)} [J]. The Journal of Chemical Thermodynamics, 2015, 89: 51-59. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [5] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [6] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [7] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [8] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [9] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [10] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [11] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [12] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [13] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [14] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [15] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号