化工学报 ›› 2022, Vol. 73 ›› Issue (12): 5314-5323.DOI: 10.11949/0438-1157.20221304
收稿日期:2022-09-27
修回日期:2022-11-07
出版日期:2022-12-05
发布日期:2023-01-17
通讯作者:
李国选
作者简介:高腾飞(1988—),男,博士研究生,20039663@chnenergy.com.cn
基金资助:
Tengfei GAO1( ), Guoxuan LI2(
), Guoxuan LI2( ), Zhigang LEI2
), Zhigang LEI2
Received:2022-09-27
Revised:2022-11-07
Online:2022-12-05
Published:2023-01-17
Contact:
Guoxuan LI
摘要:
针对联苯-正十二烷分离体系,首先采用COSMO-RS模型预测了联苯和正十二烷在不同离子液体中的无限稀释活度系数,通过溶解能力和选择性两个指标对离子液体的分离性能进行了评估和筛选,1-丁基-3-甲基咪唑四氟硼酸盐([BMIM][BF4])被认为是最有潜力的萃取剂。液-液相平衡实验测定了常压、温度为303.2 K条件下,联苯-正十二烷-萃取剂的三元液液相平衡数据。以二甲基亚砜(DMSO)和糠醛为基准萃取剂,评估了[BMIM][BF4]作为萃取剂的分离性能。最后,计算化学理论被用于探究不同萃取剂用于联苯-正十二烷体系的分离机理。C—H…π相互作用、π-π相互作用和氢键作用被认为是萃取剂与联苯之间最主要的相互作用。
中图分类号:
高腾飞, 李国选, 雷志刚. 从催化裂化柴油中分离联苯的溶剂筛选:实验和计算热力学[J]. 化工学报, 2022, 73(12): 5314-5323.
Tengfei GAO, Guoxuan LI, Zhigang LEI. Solvents selection for separation of biphenyl from FCC diesel: experimental and computational thermodynamics[J]. CIESC Journal, 2022, 73(12): 5314-5323.

图1 在303.2 K及无限稀释状态下COSMO-RS模型预测的49种不同离子液体中联苯的溶解能力(a)和正十二烷对联苯的选择性(b)
Fig.1 Solvent capacity of 49 ILs for biphenyl (a) and the selectivity of n-dodecane to biphenyl (b) at infinite dilution at 303.2 K
| 阳离子 | 阴离子 | ||||
|---|---|---|---|---|---|
| 编号 | 名称 | 阳结构式 | 编号 | 名称 | 结构式 |
| C01 | [MMIM]+ |  | A01 | [Ac]- |  |
| C02 | [EMIM]+ |  | A02 | [NO3] - |  |
| C03 | [EEIM]+ |  | A03 | [Tf2N] - |  |
| C04 | [OMIM]+ |  | A04 | [SCN] - |  |
| C05 | [BMIM]+ |  | A05 | [DCA] - |  |
| C06 | [HMIM]+ |  | A06 | [BF4] - |  |
| C07 | [C2OHMIM]+ |  | A07 | [PF6] - |  |
表1 待筛选的49种离子液体
Table 1 49 ionic liquids screened
| 阳离子 | 阴离子 | ||||
|---|---|---|---|---|---|
| 编号 | 名称 | 阳结构式 | 编号 | 名称 | 结构式 |
| C01 | [MMIM]+ |  | A01 | [Ac]- |  |
| C02 | [EMIM]+ |  | A02 | [NO3] - |  |
| C03 | [EEIM]+ |  | A03 | [Tf2N] - |  |
| C04 | [OMIM]+ |  | A04 | [SCN] - |  |
| C05 | [BMIM]+ |  | A05 | [DCA] - |  |
| C06 | [HMIM]+ |  | A06 | [BF4] - |  |
| C07 | [C2OHMIM]+ |  | A07 | [PF6] - |  |
| 萃余相 | 萃取相 | D | S | ||||
|---|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | ||
| 正十二烷 (1) + 联苯 (2) + [BMIM][BF4] (3) | |||||||
| 0.3100 | 0.6781 | 0.0119 | 0.0025 | 0.2537 | 0.7438 | 0.37 | 46.40 |
| 0.4220 | 0.5731 | 0.0049 | 0.0024 | 0.2290 | 0.7686 | 0.40 | 69.09 |
| 0.5133 | 0.4845 | 0.0022 | 0.0023 | 0.2041 | 0.7936 | 0.42 | 93.21 |
| 0.6013 | 0.3975 | 0.0011 | 0.0022 | 0.1783 | 0.8196 | 0.45 | 125.44 |
| 0.6835 | 0.3159 | 0.0006 | 0.0019 | 0.1472 | 0.8509 | 0.47 | 164.14 |
| 0.7892 | 0.2104 | 0.0004 | 0.0017 | 0.1170 | 0.8813 | 0.56 | 253.61 |
| 0.8771 | 0.1227 | 0.0002 | 0.0015 | 0.0810 | 0.9174 | 0.66 | 376.04 |
| 0.9482 | 0.0516 | 0.0001 | 0.0014 | 0.0526 | 0.9460 | 1.02 | 704.84 |
| 正十二烷 (1) + 联苯 (2) + 糠醛 (3) | |||||||
| 0.7779 | 0.1784 | 0.0437 | 0.0425 | 0.4188 | 0.5387 | 2.35 | 42.95 |
| 0.7971 | 0.1605 | 0.0425 | 0.0370 | 0.3831 | 0.5799 | 2.39 | 51.37 |
| 0.8174 | 0.1415 | 0.0412 | 0.0320 | 0.3432 | 0.6249 | 2.43 | 62.01 |
| 0.8389 | 0.1213 | 0.0398 | 0.0273 | 0.2987 | 0.6740 | 2.46 | 75.60 |
| 0.8618 | 0.0999 | 0.0384 | 0.0231 | 0.2494 | 0.7275 | 2.50 | 93.09 |
| 0.8860 | 0.0771 | 0.0369 | 0.0194 | 0.1950 | 0.7857 | 2.53 | 115.87 |
| 0.9118 | 0.0529 | 0.0354 | 0.0160 | 0.1354 | 0.8486 | 2.56 | 145.79 |
| 0.9390 | 0.0272 | 0.0338 | 0.0131 | 0.0704 | 0.9165 | 2.59 | 185.31 |
| 正十二烷 (1) + 联苯 (2) + DMSO (3) | |||||||
| 0.8841 | 0.1054 | 0.0105 | 0.0325 | 0.3892 | 0.5784 | 3.69 | 100.55 |
| 0.9008 | 0.0891 | 0.0101 | 0.0320 | 0.3506 | 0.6174 | 3.94 | 110.91 |
| 0.9175 | 0.0727 | 0.0098 | 0.0320 | 0.3079 | 0.6601 | 4.23 | 121.32 |
| 0.9340 | 0.0566 | 0.0095 | 0.0328 | 0.2605 | 0.7068 | 4.61 | 131.26 |
| 0.9499 | 0.0409 | 0.0092 | 0.0345 | 0.2074 | 0.7581 | 5.08 | 139.85 |
| 0.9651 | 0.0259 | 0.0090 | 0.0376 | 0.1475 | 0.8148 | 5.69 | 145.98 |
| 0.9790 | 0.0122 | 0.0089 | 0.0430 | 0.0791 | 0.8779 | 6.51 | 148.38 |
表2 在P=101.3 kPa和T=303.2 K条件下正十二烷(1)+联苯(2)+[BMIM][BF4]/糠醛/DMSO(3)三元体系的LLE实验数据
Table 2 Experimental LLE data for ternary systems of dodecane(1)+biphenyl(2)+[BMIM][BF4]/furfural/ DMSO (3) at T = 303.2 K and P = 101.3 kPa
| 萃余相 | 萃取相 | D | S | ||||
|---|---|---|---|---|---|---|---|
| w1 | w2 | w3 | w1 | w2 | w3 | ||
| 正十二烷 (1) + 联苯 (2) + [BMIM][BF4] (3) | |||||||
| 0.3100 | 0.6781 | 0.0119 | 0.0025 | 0.2537 | 0.7438 | 0.37 | 46.40 |
| 0.4220 | 0.5731 | 0.0049 | 0.0024 | 0.2290 | 0.7686 | 0.40 | 69.09 |
| 0.5133 | 0.4845 | 0.0022 | 0.0023 | 0.2041 | 0.7936 | 0.42 | 93.21 |
| 0.6013 | 0.3975 | 0.0011 | 0.0022 | 0.1783 | 0.8196 | 0.45 | 125.44 |
| 0.6835 | 0.3159 | 0.0006 | 0.0019 | 0.1472 | 0.8509 | 0.47 | 164.14 |
| 0.7892 | 0.2104 | 0.0004 | 0.0017 | 0.1170 | 0.8813 | 0.56 | 253.61 |
| 0.8771 | 0.1227 | 0.0002 | 0.0015 | 0.0810 | 0.9174 | 0.66 | 376.04 |
| 0.9482 | 0.0516 | 0.0001 | 0.0014 | 0.0526 | 0.9460 | 1.02 | 704.84 |
| 正十二烷 (1) + 联苯 (2) + 糠醛 (3) | |||||||
| 0.7779 | 0.1784 | 0.0437 | 0.0425 | 0.4188 | 0.5387 | 2.35 | 42.95 |
| 0.7971 | 0.1605 | 0.0425 | 0.0370 | 0.3831 | 0.5799 | 2.39 | 51.37 |
| 0.8174 | 0.1415 | 0.0412 | 0.0320 | 0.3432 | 0.6249 | 2.43 | 62.01 |
| 0.8389 | 0.1213 | 0.0398 | 0.0273 | 0.2987 | 0.6740 | 2.46 | 75.60 |
| 0.8618 | 0.0999 | 0.0384 | 0.0231 | 0.2494 | 0.7275 | 2.50 | 93.09 |
| 0.8860 | 0.0771 | 0.0369 | 0.0194 | 0.1950 | 0.7857 | 2.53 | 115.87 |
| 0.9118 | 0.0529 | 0.0354 | 0.0160 | 0.1354 | 0.8486 | 2.56 | 145.79 |
| 0.9390 | 0.0272 | 0.0338 | 0.0131 | 0.0704 | 0.9165 | 2.59 | 185.31 |
| 正十二烷 (1) + 联苯 (2) + DMSO (3) | |||||||
| 0.8841 | 0.1054 | 0.0105 | 0.0325 | 0.3892 | 0.5784 | 3.69 | 100.55 |
| 0.9008 | 0.0891 | 0.0101 | 0.0320 | 0.3506 | 0.6174 | 3.94 | 110.91 |
| 0.9175 | 0.0727 | 0.0098 | 0.0320 | 0.3079 | 0.6601 | 4.23 | 121.32 |
| 0.9340 | 0.0566 | 0.0095 | 0.0328 | 0.2605 | 0.7068 | 4.61 | 131.26 |
| 0.9499 | 0.0409 | 0.0092 | 0.0345 | 0.2074 | 0.7581 | 5.08 | 139.85 |
| 0.9651 | 0.0259 | 0.0090 | 0.0376 | 0.1475 | 0.8148 | 5.69 | 145.98 |
| 0.9790 | 0.0122 | 0.0089 | 0.0430 | 0.0791 | 0.8779 | 6.51 | 148.38 |
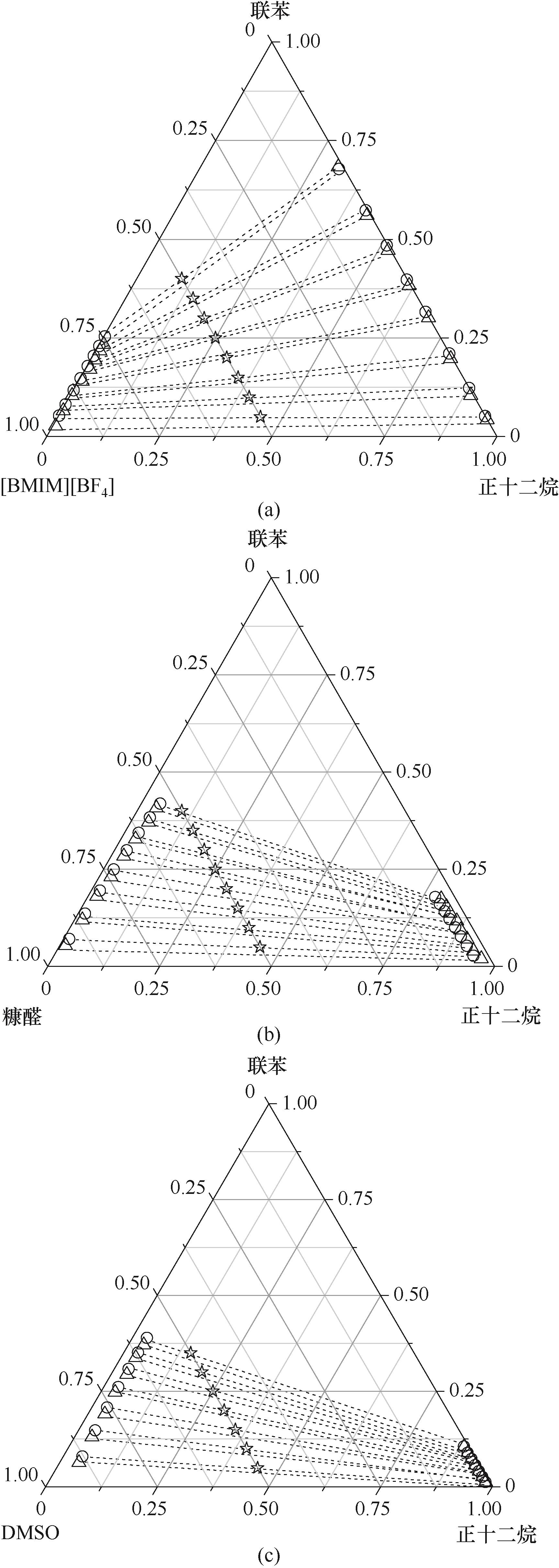
图2 在T=303.2 K和P=101.3 kPa、下正十二烷 (1) + 联苯 (2) + [BMIM][BF4]/糠醛/DMSO三元体系LLE相图(质量分数表示)△ 实验数据; 〇 COSMO-RS模型的计算值; ☆ 进料组成; ┈ 结线
Fig.2 LLE phase diagrams in mass fraction for the dodecane (1) + biphenyl (2) + [BMIM][BF4] / furfural / DMSO (3) systems at T = 303.2 K and P = 101.3 kPa

图3 在T=303.2 K和P=101.3 kPa条件下正十二烷(1)+联苯(2)+[BMIM][BF4]/糠醛/DMSO(3)三元体系的分配系数(a)和选择性系数(b)
Fig.3 Distribution coefficients (a) and selectivity coefficients (b) for the ternary systems of dodecane (1) + biphenyl (2) + [BMIM][BF4] / furfural / DMSO (3) at T = 303.2 K and P = 101.3 kPa
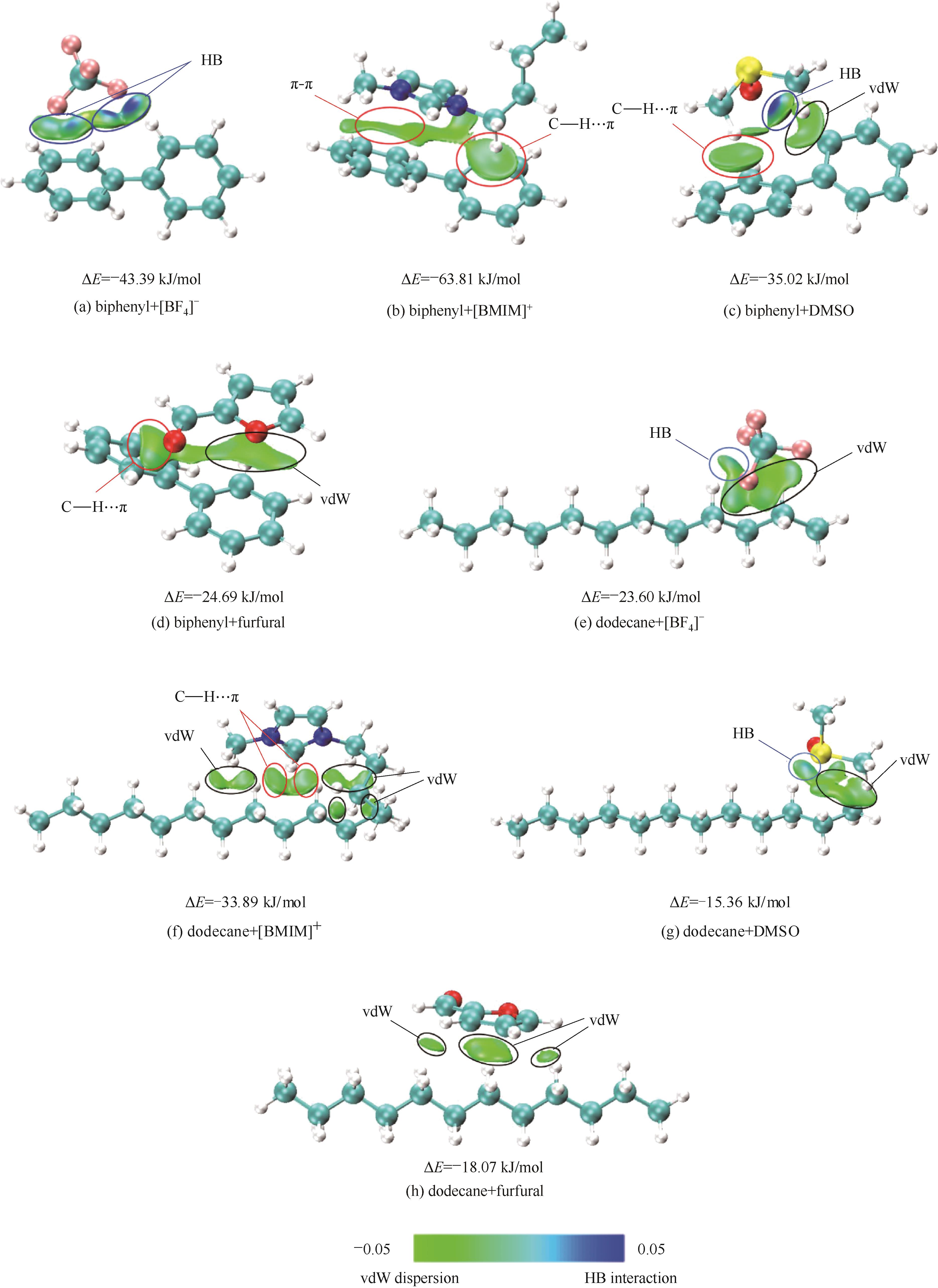
图4 通过使用IGM填色图分析[BF4]-、[BMIM]+、糠醛和DMSO分别与正十二烷或联苯的分子间弱相互作用的影响
Fig.4 Effect of [BF4]-, [BMIM]+, DMSO and furfural on weak intermolecular interactions with dodecane and biphenyl by using IGM maps analysis
| 1 | Che Y J, Yuan M, Qiao Y Y, et al. Fundamental study of hierarchical millisecond gas-phase catalytic cracking process for enhancing the production of light olefins from vacuum residue[J]. Fuel, 2019, 237: 1-9. |
| 2 | Wang Z H, Fan W Y, Xu D M, et al. Liquid-liquid-phase equilibrium for quaternary systems (n-decane + 1-tetradecene + 1-methylnaphthalene+sulfolane/dimethyl sulfoxide) for separation of 1-methylnaphthalene from FCC diesel[J]. Journal of Chemical & Engineering Data, 2021, 66(7): 2803-2811. |
| 3 | Zouad Y, Tarabet L, Khiari K, et al. Effect of heating rate and additives (MgO and Al2O3) on a diesel like-fuel issued from waste engine oil pyrolysis[J]. Petroleum Science and Technology, 2019, 37(10): 1184-1193. |
| 4 | Shi Q, Zhao S Q, Zhou Y S, et al. Development of heavy oil upgrading technologies in China[J]. Reviews in Chemical Engineering, 2019, 36(1): 1-19. |
| 5 | Peng C, Du Y Z, Feng X, et al. Research and development of hydrocracking catalysts and technologies in China[J]. Frontiers of Chemical Science and Engineering, 2018, 12(4): 867-877. |
| 6 | Shin J, Oh Y, Choi Y, et al. Design of selective hydrocracking catalysts for BTX production from diesel-boiling-range polycyclic aromatic hydrocarbons[J]. Applied Catalysis A: General, 2017, 547: 12-21. |
| 7 | Zhang Y H, Wang Y T, Chen F, et al. Research on a dual solvent to separate olefin/aromatic-sulfide from heavy fluid catalytic cracking naphtha[J]. Energy & Fuels, 2018, 32(3): 4057-4064. |
| 8 | 陈博, 廖祖维, 王靖岱, 等. 芳烃抽提过程多目标优化[J]. 化工学报, 2012, 63(3): 851-859. |
| Chen B, Liao Z W, Wang J D, et al. Multi-objective optimization of aromatic extraction process[J]. CIESC Journal, 2012, 63(3): 851-859. | |
| 9 | 王凌燕, 孙晓岩, 李忠杰, 等. 基于回归二元交互参数的芳烃抽提过程模拟[J]. 化工学报, 2011, 62(12): 3452-3457. |
| Wang L Y, Sun X Y, Li Z J, et al. Simulation of aromatic extraction process based on regressed binary interaction parameters[J]. CIESC Journal, 2011, 62(12): 3452-3457. | |
| 10 | Wang Q, Chen J Y, Pan M, et al. A new sulfolane aromatic extractive distillation process and optimization for better energy utilization[J]. Chemical Engineering and Processing-Process Intensification, 2018, 128: 80-95. |
| 11 | 殷梦凡, 唐政, 张睿, 等. 离子液体液液萃取分离正辛烷/邻二甲苯[J]. 化工学报, 2021, 72(12): 6282-6290. |
| Yin M F, Tang Z, Zhang R, et al. Separation of n-octane and o-xylene by liquid-liquid extraction with ionic liquids[J]. CIESC Journal, 2021, 72(12): 6282-6290. | |
| 12 | 姜焱龙, 张妮, 李淡然, 等. 基于COSMO-RS方法筛选离子液体用于焦油脱除[J]. 化工学报, 2022, 73(4): 1704-1713. |
| Jiang Y L, Zhang N, Li D R, et al. Selected ionic liquids by COSMO-RS method for tar removal[J]. CIESC Journal, 2022, 73(4): 1704-1713. | |
| 13 | Meindersma G W, Podt A J, de Haan A B. Selection of ionic liquids for the extraction of aromatic hydrocarbons from aromatic/aliphatic mixtures[J]. Fuel Processing Technology, 2005, 87(1): 59-70. |
| 14 | Pereiro A B, Rodríguez A. An ionic liquid proposed as solvent in aromatic hydrocarbon separation by liquid extraction[J]. AIChE Journal, 2010, 56(2): 381-386. |
| 15 | Larriba M, de Riva J, Navarro P, et al. COSMO-based/Aspen Plus process simulation of the aromatic extraction from pyrolysis gasoline using the { [ 4 e m p y ] [ N T f 2 ] + [ e m i m ] [ D C A ] } ionic liquid mixture[J]. Separation and Purification Technology, 2018, 190: 211-227. |
| 16 | Guo Y C, Shi F M, Shu Q P, et al. Liquid-liquid equilibrium for n-hexane + benzene + sulfolane, + 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM][NTf2]), + 1-ethyl-3-methylimidazolium ethylsulfate ([EMIM][EtSO4]) and + the mixtures of [EMIM][NTf2] and [EMIM][EtSO4][J]. Fluid Phase Equilibria, 2021, 529: 112882. |
| 17 | Shaahmadi F, Anbaz M A. The prediction of liquid-liquid equilibria for benzene/alkane/ionic liquids mixtures using intelligent models[J]. Journal of Molecular Liquids, 2017, 232: 396-407. |
| 18 | García J, Torrecilla J S, Fernández A, et al. (Liquid+liquid) equilibria in the binary systems (aliphatic, or aromatic hydrocarbons+ 1-ethyl-3-methylimidazolium ethylsulfate, or 1-butyl-3-methylimidazolium methylsulfate ionic liquids)[J]. Journal of Chemical Thermodynamics, 2010, 42(1): 144-150. |
| 19 | Klamt A, Jonas V, Bürger T, et al. Refinement and parametrization of COSMO-RS[J]. The Journal of Physical Chemistry A, 1998, 102(26): 5074-5085. |
| 20 | 桂成敏, 朱瑞松, 张傑, 等. 离子液体气体干燥技术的研究进展[J]. 化工学报, 2020, 71(1): 92-105. |
| Gui C M, Zhu R S, Zhang J, et al. Progress on ionic liquids for gas drying[J]. CIESC Journal, 2020, 71(1): 92-105. | |
| 21 | Li G X, Gui C M, Zhu R S, et al. Deep eutectic solvents for efficient capture of cyclohexane in volatile organic compounds: thermodynamic and molecular mechanism[J]. AIChE Journal, 2022, 68(3): e17535. |
| 22 | Li G X, Liu Q H, Gui C M, et al. Thermodynamic and molecular insights into natural gas dehydration using choline chloride-based deep eutectic solvents[J]. AIChE Journal, 2022, 68(7): e17662. |
| 23 | Fu H, Hou Y P, Sang H N, et al. Carbon dioxide capture by new DBU-based DES: the relationship between ionicity and absorptive capacity[J]. AIChE Journal, 2021, 67(7): e17244. |
| 24 | Han J L, Dai C N, Lei Z G, et al. Gas drying with ionic liquids[J]. AIChE Journal, 2018, 64(2): 606-619. |
| 25 | Li G X, Gui C M, Dai C N, et al. Molecular insights into SO2 absorption by [EMIM][Cl]-based deep eutectic solvents[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(41): 13831-13841. |
| 26 | Lu T, Chen F W. Multiwfn: a multifunctional wavefunction analyzer[J]. Journal of Computational Chemistry, 2012, 33(5): 580-592. |
| 27 | Zhang J N, Peng D L, Song Z, et al. COSMO-descriptor based computer-aided ionic liquid design for separation processes(Part Ⅰ): Modified group contribution methodology for predicting surface charge density profile of ionic liquids[J]. Chemical Engineering Science, 2017, 162: 355-363. |
| 28 | Zhang J N, Qin L, Peng D L, et al. COSMO-descriptor based computer-aided ionic liquid design for separation processes(Part Ⅱ): Task-specific design for extraction processes [J]. Chemical Engineering Science, 2017, 162: 364-374. |
| 29 | Li W X, Xu B, Lei Z G, et al. Separation of benzene and cyclohexane by extractive distillation intensified with ionic liquid[J]. Chemical Engineering and Processing-Process Intensification, 2018, 126: 81-89. |
| 30 | Dong Y C, Dai C N, Lei Z G. Separation of the methanol-ethanol-water mixture using ionic liquid[J]. Industrial & Engineering Chemistry Research, 2018, 57(32): 11167-11177. |
| 31 | Ahmady A, Hashim M A, Aroua M K. Density, viscosity, physical solubility and diffusivity of CO2 in aqueous MDEA + [bmim][BF 4]solutions from 303 to 333 K[J]. Chemical Engineering Journal, 2011, 172(2/3): 763-770. |
| 32 | Othmer D, Tobias P. Liquid-liquid extraction data-the line correlation[J]. Industrial & Engineering Chemistry, 1942, 34(6): 693-696. |
| 33 | Hand D B. Dineric distribution[J]. The Journal of Chemical Physics, 1930, 34(9): 1961-2000. |
| 34 | Tamres M. Aromatic compounds as donor molecules in hydrogen bonding[J]. Journal of the American Chemical Society, 1952, 74(13): 3375-3378. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [10] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [11] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [12] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [13] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [14] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [15] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号