化工学报 ›› 2021, Vol. 72 ›› Issue (8): 4155-4165.DOI: 10.11949/0438-1157.20201652
收稿日期:2020-11-16
修回日期:2021-01-20
出版日期:2021-08-05
发布日期:2021-08-05
通讯作者:
崔现宝
作者简介:宋振兴(1996—),男,硕士研究生,基金资助:
Zhenxing SONG( ),Xianbao CUI(
),Xianbao CUI( ),Ying ZHANG,Xuemei ZHANG,Jie HE,Tianyang FENG,Jixiao WANG
),Ying ZHANG,Xuemei ZHANG,Jie HE,Tianyang FENG,Jixiao WANG
Received:2020-11-16
Revised:2021-01-20
Online:2021-08-05
Published:2021-08-05
Contact:
Xianbao CUI
摘要:
离子液体催化反应精馏是提高酯交换平衡反应转化率的一种绿色有效方法。以离子液体1-丙基磺酸-3-甲基咪唑三氟甲烷磺酸盐([PSO3HMIm][OTf])和离子液体1-辛基-2,3-二甲基咪唑双(三氟甲烷磺酰)亚胺盐([OMMIm][Tf2N])的混合物作为乙酸甲酯和正己醇进行酯交换反应合成乙酸正己酯的催化剂,测定了酯交换反应动力学。探讨了混合比、反应温度、反应物初始摩尔比、催化剂浓度对反应速率和乙酸甲酯转化率的影响,考察了催化剂的回收性能。利用实验数据回归得出混合离子液体催化酯交换反应动力学方程。在反应动力学的基础上进行了乙酸甲酯和正己醇的酯交换反应精馏流程模拟,分析了理论板数、回流比、进料位置及反应段塔板数、催化剂用量、持液量等参数对反应精馏塔的影响。在优化的操作条件下,获得纯度为0.9993的乙酸正己酯产品。
中图分类号:
宋振兴, 崔现宝, 张缨, 张雪梅, 何杰, 冯天扬, 王纪孝. 混合离子液体催化反应精馏合成乙酸正己酯[J]. 化工学报, 2021, 72(8): 4155-4165.
Zhenxing SONG, Xianbao CUI, Ying ZHANG, Xuemei ZHANG, Jie HE, Tianyang FENG, Jixiao WANG. Synthesis of n-hexyl acetate via reactive distillation catalyzed by mixed ionic liquids[J]. CIESC Journal, 2021, 72(8): 4155-4165.
| 试剂名称 | 纯度/% | 供应商 |
|---|---|---|
| 乙酸甲酯(MeOAC) | 99.0 | 上海阿拉丁生化科技股份有限公司 |
| 甲醇(MeOH) | 99.8 | 凯玛特化工科技有限公司 |
| 正己醇(HeOH) | 99.8 | 凯玛特化工科技有限公司 |
| 乙酸正己酯(HeAC) | 99.0 | 上海麦克林生化科技有限公司 |
| 1-丙基磺酸-3-甲基咪唑三氟甲烷磺酸盐([PSO3HMIm][OTf]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-丁基磺酸-3-甲基咪唑硫酸氢盐([BSO3HMIm][HSO4]) | 99.0 | 兰州奥力科化工有限公司 |
| N-丁基磺酸吡啶三氟甲烷磺酸盐([BSO3HPy][OTf]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-丁基磺酸-3-甲基咪唑三氟甲烷磺酸盐([BSO3HMIm][OTf]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-丁基磺酸-3-甲基咪唑盐酸盐([BSO3HMIm][Cl]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-辛基-2,3-二甲基咪唑双(三氟甲烷磺酰)亚胺盐([OMMIm][Tf2N]) | 99.0 | 兰州奥力科化工有限公司 |
表1 实验试剂
Table 1 The reagents used in experiment
| 试剂名称 | 纯度/% | 供应商 |
|---|---|---|
| 乙酸甲酯(MeOAC) | 99.0 | 上海阿拉丁生化科技股份有限公司 |
| 甲醇(MeOH) | 99.8 | 凯玛特化工科技有限公司 |
| 正己醇(HeOH) | 99.8 | 凯玛特化工科技有限公司 |
| 乙酸正己酯(HeAC) | 99.0 | 上海麦克林生化科技有限公司 |
| 1-丙基磺酸-3-甲基咪唑三氟甲烷磺酸盐([PSO3HMIm][OTf]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-丁基磺酸-3-甲基咪唑硫酸氢盐([BSO3HMIm][HSO4]) | 99.0 | 兰州奥力科化工有限公司 |
| N-丁基磺酸吡啶三氟甲烷磺酸盐([BSO3HPy][OTf]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-丁基磺酸-3-甲基咪唑三氟甲烷磺酸盐([BSO3HMIm][OTf]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-丁基磺酸-3-甲基咪唑盐酸盐([BSO3HMIm][Cl]) | 99.0 | 兰州奥力科化工有限公司 |
| 1-辛基-2,3-二甲基咪唑双(三氟甲烷磺酰)亚胺盐([OMMIm][Tf2N]) | 99.0 | 兰州奥力科化工有限公司 |
| i | j | Bij/K | Bji/K | αij |
|---|---|---|---|---|
| 乙酸甲酯 | 甲醇 | 214.419 | 139.516 | 0.3 |
| 乙酸甲酯 | 正己醇 | 522.257 | -222.8145 | 0.3 |
| 乙酸甲酯 | 乙酸正己酯 | 499 | -336.127 | 0.3 |
| 甲醇 | 正己醇 | 516.202 | -301.081 | 0.3364 |
| 甲醇 | 乙酸正己酯 | 101.869 | 180.573 | 0.3 |
| 正己醇 | 乙酸正己酯 | -144.421 | 354.82 | 0.3 |
| 乙酸甲酯 | [PSO3HMIm] [OTf] | 5360.95 | -3043.333 | 0.2 |
| 乙酸甲酯 | [OMMIm][Tf2N] | 9079.14 | -536.9 | 0.3 |
| 甲醇 | [PSO3HMIm] [OTf] | 1676.0488 | -2365.70732 | 0.3 |
| 甲醇 | [OMMIm][Tf2N] | -301.5 | 6230.09 | 0.3 |
| 正己醇 | [PSO3HMIm] [OTf] | -855.274 | -1808.009 | 0.2 |
| 正己醇 | [OMMIm][Tf2N] | -4.3407 | -1432.65 | 0.3 |
| 乙酸正己酯 | [PSO3HMIm] [OTf] | 1723.06632 | -1685.6435 | 0.2 |
| 乙酸正己酯 | [OMMIm][Tf2N] | 610.14752 | -1299.8232 | 0.3 |
| [PSO3HMIm] [OTf] | [OMMIm][Tf2N] | 2256.214 | 1710.3584 | 0.3 |
表2 NRTL模型参数
Table 2 NRTL model parameters
| i | j | Bij/K | Bji/K | αij |
|---|---|---|---|---|
| 乙酸甲酯 | 甲醇 | 214.419 | 139.516 | 0.3 |
| 乙酸甲酯 | 正己醇 | 522.257 | -222.8145 | 0.3 |
| 乙酸甲酯 | 乙酸正己酯 | 499 | -336.127 | 0.3 |
| 甲醇 | 正己醇 | 516.202 | -301.081 | 0.3364 |
| 甲醇 | 乙酸正己酯 | 101.869 | 180.573 | 0.3 |
| 正己醇 | 乙酸正己酯 | -144.421 | 354.82 | 0.3 |
| 乙酸甲酯 | [PSO3HMIm] [OTf] | 5360.95 | -3043.333 | 0.2 |
| 乙酸甲酯 | [OMMIm][Tf2N] | 9079.14 | -536.9 | 0.3 |
| 甲醇 | [PSO3HMIm] [OTf] | 1676.0488 | -2365.70732 | 0.3 |
| 甲醇 | [OMMIm][Tf2N] | -301.5 | 6230.09 | 0.3 |
| 正己醇 | [PSO3HMIm] [OTf] | -855.274 | -1808.009 | 0.2 |
| 正己醇 | [OMMIm][Tf2N] | -4.3407 | -1432.65 | 0.3 |
| 乙酸正己酯 | [PSO3HMIm] [OTf] | 1723.06632 | -1685.6435 | 0.2 |
| 乙酸正己酯 | [OMMIm][Tf2N] | 610.14752 | -1299.8232 | 0.3 |
| [PSO3HMIm] [OTf] | [OMMIm][Tf2N] | 2256.214 | 1710.3584 | 0.3 |
| 组分 | ΔfH0/(kJ/mol) | Δr | Δr |
|---|---|---|---|
| 乙酸甲酯 | -411.9 | 5.2 | 7.67 |
| 正己醇 | -320.5 | ||
| 甲醇 | -200.9 | ||
| 乙酸正己酯 | -526.3 |
表3 标准反应焓计算结果
Table 3 Calculation results of standard reaction enthalpy
| 组分 | ΔfH0/(kJ/mol) | Δr | Δr |
|---|---|---|---|
| 乙酸甲酯 | -411.9 | 5.2 | 7.67 |
| 正己醇 | -320.5 | ||
| 甲醇 | -200.9 | ||
| 乙酸正己酯 | -526.3 |
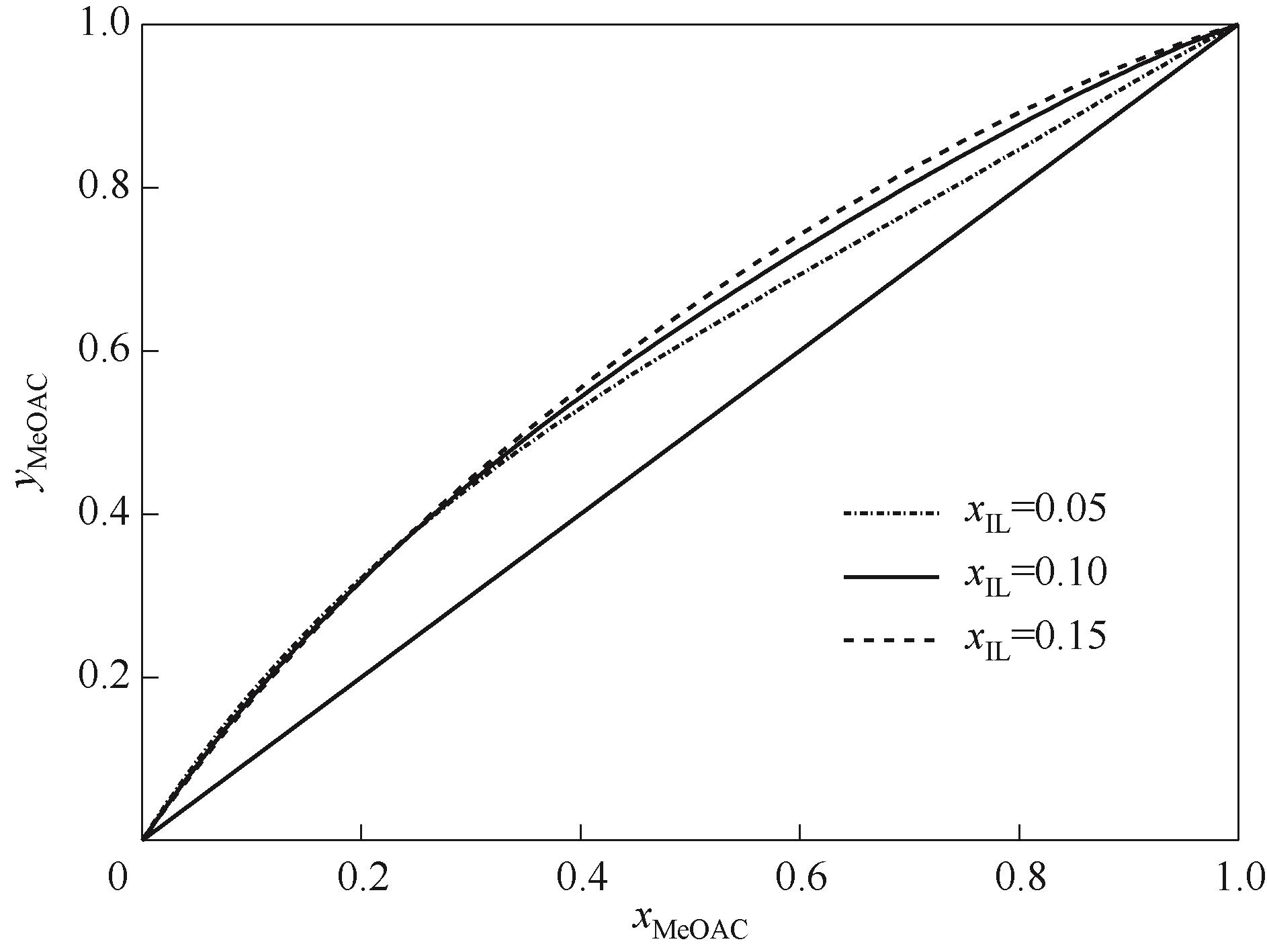
图11 混合离子液体催化剂不同浓度下乙酸甲酯-甲醇体系的y-x相图
Fig.11 The y-x phase diagram of methyl acetate methanol system with different concentrations of mixed ionic liquid catalyst
| 离子液体进料位置 | 正己醇 进料位置 | 乙酸甲酯混合物进料位置 | 反应段进料间隔板数 | 收率 | 乙酸正己酯纯度 |
|---|---|---|---|---|---|
| 5 | 30 | 50 | 20 | 0.9180 | 0.9242 |
| 5 | 24 | 50 | 26 | 0.9454 | 0.9497 |
| 5 | 20 | 50 | 30 | 0.9617 | 0.9648 |
| 5 | 15 | 50 | 35 | 0.9672 | 0.9711 |
| 15 | 15 | 50 | 35 | 0.9669 | 0.9702 |
| 5 | 10 | 50 | 40 | 0.9783 | 0.9809 |
| 10 | 10 | 50 | 40 | 0.9788 | 0.9811 |
| 5 | 6 | 46 | 40 | 0.9636 | 0.9688 |
| 5 | 8 | 48 | 40 | 0.9710 | 0.9746 |
| 5 | 10 | 50 | 40 | 0.9783 | 0.9809 |
| 5 | 12 | 52 | 40 | 0.9619 | 0.9650 |
表4 混合离子液体和反应物的进料位置及间隔板数的影响
Table 4 The effect of feeding position and number of spacer plates
| 离子液体进料位置 | 正己醇 进料位置 | 乙酸甲酯混合物进料位置 | 反应段进料间隔板数 | 收率 | 乙酸正己酯纯度 |
|---|---|---|---|---|---|
| 5 | 30 | 50 | 20 | 0.9180 | 0.9242 |
| 5 | 24 | 50 | 26 | 0.9454 | 0.9497 |
| 5 | 20 | 50 | 30 | 0.9617 | 0.9648 |
| 5 | 15 | 50 | 35 | 0.9672 | 0.9711 |
| 15 | 15 | 50 | 35 | 0.9669 | 0.9702 |
| 5 | 10 | 50 | 40 | 0.9783 | 0.9809 |
| 10 | 10 | 50 | 40 | 0.9788 | 0.9811 |
| 5 | 6 | 46 | 40 | 0.9636 | 0.9688 |
| 5 | 8 | 48 | 40 | 0.9710 | 0.9746 |
| 5 | 10 | 50 | 40 | 0.9783 | 0.9809 |
| 5 | 12 | 52 | 40 | 0.9619 | 0.9650 |
| 1 | 揭会民, 崔现宝, 彭艳枚, 等. 离子液体反应萃取精馏合成乙酸乙酯[J]. 化工学报, 2016, 67(2): 606-613. |
| Jie H M, Cui X B, Peng Y M, et al. Synthesis of ethyl acetate via reactive and extractive distillation column using ionic liquids as catalyst and entrainer[J]. CIESC Journal, 2016, 67(2): 606-613. | |
| 2 | 袁骏. 乙酸甲酯水解的工艺[J]. 化工进展, 2012, 31(S2): 265-270. |
| Yuan J. Advances in hydrolysis of methyl acetate[J]. Chemical Industry and Engineering Progress, 2012, 31(S2): 265-270. | |
| 3 | 李柏春, 耿春霞, 张文林, 等. 酯交换法制备乙酸正丁酯动力学研究[J]. 化学工程, 2016, 44(4): 59-63. |
| Li B C, Geng C X, Zhang W L, et al. Kinetics of n-butyl acetate prepared by trans-esterification[J]. Chemical Engineering (China), 2016, 44(4): 59-63. | |
| 4 | Liu Y, Wei M, Gao L, et al. Kinetics of transesterification of methyl acetate and n-octanol catalyzed by cation exchange resins[J]. Korean Journal of Chemical Engineering, 2013, 30(5): 1039-1042. |
| 5 | Sert E, Atalay F S. Determination of adsorption and kinetic parameters for transesterification of methyl acetate with hexanol catalyzed by ion exchange resin[J]. Industrial & Engineering Chemistry Research, 2012, 51(18): 6350-6355. |
| 6 | Kozhevnikov I V. Catalysis by heteropoly acids and multicomponent polyoxometalates in liquid-phase reactions[J]. Chemical Reviews, 1998, 98(1): 171-198. |
| 7 | Bożek-Winkler E, Gmehling J. Transesterification of methyl acetate and n-butanol catalyzed by amberlyst 15[J]. Industrial & Engineering Chemistry Research, 2006, 45(20): 6648-6654. |
| 8 | Jiménez L, Garvín A, Costa-López J. The production of butyl acetate and methanol via reactive and extractive distillation(Ⅰ): Chemical equilibrium, kinetics, and mass-transfer issues[J]. Industrial & Engineering Chemistry Research, 2002, 41(26): 6663-6669. |
| 9 | Jermy B R, Pandurangan A. A highly efficient catalyst for the esterification of acetic acid using n-butyl alcohol[J]. Journal of Molecular Catalysis A: Chemical, 2005, 237(1/2): 146-154. |
| 10 | Pappu V K S, Yanez A J, Peereboom L, et al. A kinetic model of the Amberlyst-15 catalyzed transesterification of methyl stearate with n-butanol[J]. Bioresource Technology, 2011, 102(5): 4270-4272. |
| 11 | Xiao Y, Cai W F, Sun H L, et al. Kinetics study and process simulation of transesterification of ethylene glycol with methyl acetate for ethylene glycol diacetate[J]. The Canadian Journal of Chemical Engineering, 2018, 96(3): 722-730. |
| 12 | Yang Z, Cui X B, Jie H M, et al. Kinetic study and process simulation of transesterification of methyl acetate and isoamyl alcohol catalyzed by ionic liquid[J]. Industrial & Engineering Chemistry Research, 2015, 54(4): 1204-1215. |
| 13 | 梁晓通, 李国兵, 沈京华, 等. 离子液体一步法催化合成4-乙酰胺基苯亚磺酸[J]. 化学工业与工程, 2020, 37(4): 7-14. |
| Liang X T, Li G B, Shen J H, et al. Catalytic synthesis of 4-acetamidobenzenesulfinic acid by ionic liquids in one step[J]. Chemical Industry and Engineering, 2020, 37(4): 7-14. | |
| 14 | Zhang P B, Liu H, Fan M M, et al. A review on biodiesel production by transesterification catalyzed by ionic liquid catalysts[J]. Current Organic Chemistry, 2016, 20(7): 752-760. |
| 15 | Deshmukh K M, Qureshi Z S, Dhake K P, et al. Transesterification of dimethyl carbonate with phenol using Brønsted and Lewis acidic ionic liquids[J]. Catalysis Communications, 2010, 12(3): 207-211. |
| 16 | Niedermeyer H, Hallett J P, Villar-Garcia I J, et al. Mixtures of ionic liquids[J]. Chemical Society Reviews, 2012, 41(23): 7780-7802. |
| 17 | Chatel G, Pereira J F B, Debbeti V, et al. Mixing ionic liquids — “simple mixtures” or “double salts”?[J]. Green Chemistry, 2014, 16(4): 2051-2083. |
| 18 | Annat G, Forsyth M, MacFarlane D R. Ionic liquid mixtures—variations in physical properties and their origins in molecular structure[J]. The Journal of Physical Chemistry B, 2012, 116(28): 8251-8258. |
| 19 | Baltazar Q Q, Leininger S K, Anderson J L. Binary ionic liquid mixtures as gas chromatography stationary phases for improving the separation selectivity of alcohols and aromatic compounds[J]. Journal of Chromatography A, 2008, 1182(1): 119-127. |
| 20 | Clough M T, Crick C R, Gräsvik J, et al. A physicochemical investigation of ionic liquid mixtures[J]. Chemical Science, 2015, 6(2): 1101-1114. |
| 21 | D'Anna F, Marullo S, Vitale P, et al. Binary mixtures of ionic liquids: a joint approach to investigate their properties and catalytic ability[J]. Chem Phys Chem, 2012, 13(7): 1877-1884. |
| 22 | Fox E T, Weaver J E F, Henderson W A. Tuning binary ionic liquid mixtures: linking alkyl chain length to phase behavior and ionic conductivity[J]. The Journal of Physical Chemistry C, 2012, 116(8): 5270-5274. |
| 23 | Larriba M, de Riva J, Navarro P, et al. COSMO-based/Aspen Plus process simulation of the aromatic extraction from pyrolysis gasoline using the {[4empy][NTf2] + [emim][DCA]} ionic liquid mixture[J]. Separation and Purification Technology, 2018, 190: 211-227. |
| 24 | Taige M, Hilbert D, Schubert T J S. Mixtures of ionic liquids as possible electrolytes for lithium ion batteries[J]. Zeitschrift Für Physikalische Chemie, 2012, 226(2): 129-139. |
| 25 | Yang Z, Cui X B, Yu X F, et al. Transesterification of methyl acetate with n-butanol catalyzed by single and mixed ionic liquids[J]. Catalysis Letters, 2015, 145(6): 1281-1289. |
| 26 | Peng X, Wang L K. Design and control of ionic liquid-catalyzed reactive distillation for n-butyl acetate production[J]. Chemical Engineering & Technology, 2015, 38(2): 223-234. |
| 27 | Zhang Z S, Wang C, Guang C, et al. Cost-saving and control investigation for isopentyl acetate ionic liquid catalyzed synthesis through conventional and dividing-wall reactive distillation[J]. Process Safety and Environmental Protection, 2019, 129: 89-102. |
| 28 | Yang J B, Cai D R, Zeng T, et al. Application of Brönsted acid ionic liquids as green catalyst in the synthesis of 2-propanol with reactive distillation[J]. Chinese Journal of Chemical Engineering, 2016, 24(11): 1561-1569. |
| 29 | Amarasekara A S. Acidic ionic liquids[J]. Chemical Reviews, 2016, 116(10): 6133-6183. |
| 30 | Sarma P, Dutta A K, Borah R. Design and exploration of —SO3H group functionalized Brønsted acidic ionic liquids (BAILs) as task-specific catalytic systems for organic reactions: a review of literature[J]. Catalysis Surveys from Asia, 2017, 21(2): 70-93. |
| 31 | Zhang Q Q, Cui X B, Feng T Y, et al. Hydrolysis of methyl acetate using ionic liquids as catalyst and solvent[J]. Molecular Catalysis, 2020, 484: 110785. |
| 32 | Ni L L, Xin J Y, Jiang K, et al. One-step conversion of biomass-derived furanics into aromatics by Brønsted acid ionic liquids at room temperature[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(2): 2541-2551. |
| 33 | Cui X, Zhang S, Shi F, et al. The influence of the acidity of ionic liquids on catalysis[J]. Chem Sus Chem, 2010, 3(9): 1043-1047. |
| 34 | He W S, Li L L, Huang Q J, et al. Highly efficient synthesis of phytosterol linolenate in the presence of Bronsted acidic ionic liquid[J]. Food Chemistry, 2018, 263: 1-7. |
| 35 | Krasovskiy V G, Chernikova E A, Glukhov L M, et al. Effect of hydroxyl groups in a cation structure on the properties of ionic liquids[J]. Russian Journal of Physical Chemistry A, 2018, 92(12): 2379-2385. |
| 36 | He R N, Zou Y, Dong Y B, et al. Kinetic study and process simulation of esterification of acetic acid and ethanol catalyzed by [HSO3-bmim][HSO4][J]. Chemical Engineering Research and Design, 2018, 137: 235-245. |
| 37 | Amarasekara A S, Owereh O S. Thermal properties of sulfonic acid group functionalized Brönsted acidic ionic liquids[J]. Journal of Thermal Analysis and Calorimetry, 2011, 103(3): 1027-1030. |
| 38 | Shang W Y, Cui X B, Yu X F, et al. Isobaric vapor-liquid equilibrium for methanol + methyl acetate with ionic liquids [OMMIM][Tf2N] and [OMIM][Tf2N] as entrainers at 101.3 kPa[J]. Fluid Phase Equilibria, 2018, 473: 90-97. |
| 39 | Athès V, Paricaud P, Ellaite M, et al. Vapour-liquid equilibria of aroma compounds in hydroalcoholic solutions: measurements with a recirculation method and modelling with the NRTL and COSMO-SAC approaches[J]. Fluid Phase Equilibria, 2008, 265(1/2): 139-154. |
| 40 | Dell'Era C, Pokki J P, Uusi-Kyyny P, et al. Vapour-liquid equilibrium for the systems diethyl sulphide + 1-butene, +cis-2-butene, +2-methylpropane, +2-methylpropene, +n-butane, +trans-2-butene[J]. Fluid Phase Equilibria, 2010, 291(2): 180-187. |
| 41 | Fang J, Zhao R, Su W Y, et al. A molecular design method based on the COSMO-SAC model for solvent selection in ionic liquid extractive distillation[J]. AIChE Journal, 2016, 62(8): 2853-2869. |
| 42 | Wang H X, Wu C M, Bu X W, et al. A benign preparation of sec-butanol via transesterification from sec-butyl acetate using the acidic imidazolium ionic liquids as catalysts[J]. Chemical Engineering Journal, 2014, 246: 366-372. |
| 43 | Peng Y M, Cui X B, Zhang Y, et al. Kinetics of transesterification of methyl acetate and ethanol catalyzed by ionic liquid[J]. International Journal of Chemical Kinetics, 2014, 46(2): 116-125. |
| 44 | Suo X M, Ye Q, Li R, et al. Investigation about energy saving for synthesis of isobutyl acetate in the reactive dividing-wall column[J]. Industrial & Engineering Chemistry Research, 2017, 56(19): 5607-5617. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [3] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [4] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [5] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [6] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [7] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [8] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [11] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [12] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [13] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [14] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [15] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号