化工学报 ›› 2021, Vol. 72 ›› Issue (5): 2493-2505.DOI: 10.11949/0438-1157.20201412
收稿日期:2020-10-09
修回日期:2021-01-14
出版日期:2021-05-05
发布日期:2021-05-05
通讯作者:
高静
作者简介:张莉莉(1996—),女,硕士研究生,基金资助:
ZHANG Lili1,2( ),LI Yan1,2,GAO Jing1(
),LI Yan1,2,GAO Jing1( )
)
Received:2020-10-09
Revised:2021-01-14
Online:2021-05-05
Published:2021-05-05
Contact:
GAO Jing
摘要:
与聚合物双水相体系相比,离子液体双水相体系具有相分离效率高和选择性强的特点,在萃取领域得到了广泛关注。然而,离子液体双水相体系中常见的kosmotropic组分为无机盐或有机盐,溶液环境通常呈现强碱性,不利于保持萃取分子的稳定性和生物活性。以低共熔溶剂替代盐类作为kosmotropic组分、以离子液体为chaotropic组分构建新型离子液体-低共熔溶剂双水相体系,考察了不同温度下的相行为规律,筛选出具有高临界共熔温度(UCST)和低临界共熔温度(LCST)两种截然相反的热可逆相转变行为的萃取体系,从宏观角度探索了离子液体-低共熔溶剂-水三元体系的黏度、密度、电导率以及pH等理化特性随温度变化的规律,利用量子化学计算从微观角度分析离子液体、低共熔溶剂与水分子之间的相互作用。研究旨在揭示热可逆离子液体-低共熔溶剂双水相体系的成相机理,为萃取温敏性生物分子设计新的理想萃取体系提供基础数据。
中图分类号:
张莉莉, 李艳, 高静. 热可逆离子液体-低共熔溶剂双水相体系的相行为及理化特性研究[J]. 化工学报, 2021, 72(5): 2493-2505.
ZHANG Lili, LI Yan, GAO Jing. Phase behavior and physicochemical properties of thermoreversible aqueous biphasic systems composed of ionic liquids and deep eutectic solvents[J]. CIESC Journal, 2021, 72(5): 2493-2505.
| ILs/DESs | Wc/% | η×10-3/(mPa·s)(298.15 K) | α (298.15 K) | β (298.15 K) | π* (298.15 K) | (298.15 K) | Ti/K | Tpeak/K | Tf/K | Wlost/% | W973.15/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [C4mim]BF4 | 0.52±0.01 | 0.10±0.00 | 0.69±0.02 | 0.32±0.01 | 1.05±0.03 | 0.70±0.02 | 644.45 | 691.05 | 701.75 | 370.71 | 1.12 |
| [C4mim]Br | 0.27±0.02 | ND | ND | ND | ND | ND | 556.75 | 587.15 | 595.25 | 362.91 | 3.61 |
| [C4mim]Cl | 0.13±0.02 | ND | ND | ND | ND | ND | 538.95 | 560.75 | 569.85 | 360.51 | 4.33 |
| [C4mim]CF3COO | 1.49±0.02 | 0.08±0.00 | 0.61±0.03 | 0.71±0.03 | 0.96±0.02 | 0.64±0.01 | 454.05 | 471.45/551.45 | 519.25 | 353.05 | 7.16 |
| [P4444]BF4 | 0.29±0.01 | ND | ND | ND | ND | ND | 719.45 | 741.35 | 753.45 | 364.74 | 3.19 |
| [P4444]Br | 0.52±0.02 | ND | ND | ND | ND | ND | 645.55 | 675.45 | 691.05 | 369.57 | 1.88 |
| [P4444]Cl | 0.02±0.01 | ND | ND | ND | ND | ND | 640.25 | 669.95 | 685.65 | 364.47 | 3.72 |
| [P4444]CF3COO | 0.15±0.01 | ND | ND | ND | ND | ND | 462.15 | 476.65/499.15/518.35 | 512.05 | 350.60 | 3.91 |
| [P4448]BF4 | 1.25±0.45 | 0.89±0.00 | ND | 0.68±0.01 | 0.87±0.02 | ND | 703.95 | 743.95 | 751.35 | 366.02 | 5.31 |
| [P4448]Br | 2.69±0.30 | ND | ND | ND | ND | ND | 639.85 | 665.45 | 685.05 | 364.95 | 6.31 |
| [P4448]Cl | 3.11±0.11 | 1.26±0.02 | ND | 1.30±0.01 | 0.04±0.01 | ND | 635.25 | 664.55 | 680.65 | 357.40 | 5.80 |
| [P4448]CF3COO | 0.27±0.01 | 0.24±0.00 | ND | 1.07±0.04 | 0.87±0.02 | ND | 480.95 | 472.85/548.55 | 569.85 | 359.71 | 6.17 |
| [N4444]BF4 | 0.94±0.06 | ND | ND | ND | ND | ND | 469.75 | 492.25/664.75 | 676.65 | 367.82 | 3.65 |
| [N4444]Br | 0.94±0.00 | ND | ND | ND | ND | ND | 473.35 | 492.85/536.15 | 506.35 | 349.57 | 4.14 |
| [N4444]Cl | 8.51±0.08 | ND | ND | ND | ND | ND | 464.95 | 485.25 | 497.35 | 361.95 | 4.76 |
| [N4444]CF3COO | 0.16±0.03 | ND | ND | ND | ND | ND | 480.25 | 504.25 | 513.75 | 365.62 | 6.35 |
| [ChCl][Rib] | 0.06±0.04 | 23.40±0.25 | ND | ND | ND | ND | 520.55 | 545.05 | 557.75 | 342.81 | 18.29 |
| [ChCl][Glu] | 0.01±0.00 | 654.05±39.65 | ND | ND | ND | ND | 494.35 | 520.85 | 563.25 | 341.91 | 19.06 |
| [ChCl][Fru] | 12.93±0.12 | 54.06±0.48 | ND | ND | ND | ND | 432.75 | 448.85/514.55/564.35 | 563.75 | 347.21 | 16.17 |
| [ChCl][Sur] | 7.87±0.08 | >2201.00±83.00 | ND | ND | ND | ND | 509.05 | 543.85 | 559.65 | 338.53 | 20.83 |
表1 离子液体及低共熔溶剂的基本性质
Table 1 Properties of ionic liquids and deep eutectic solvents
| ILs/DESs | Wc/% | η×10-3/(mPa·s)(298.15 K) | α (298.15 K) | β (298.15 K) | π* (298.15 K) | (298.15 K) | Ti/K | Tpeak/K | Tf/K | Wlost/% | W973.15/% |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [C4mim]BF4 | 0.52±0.01 | 0.10±0.00 | 0.69±0.02 | 0.32±0.01 | 1.05±0.03 | 0.70±0.02 | 644.45 | 691.05 | 701.75 | 370.71 | 1.12 |
| [C4mim]Br | 0.27±0.02 | ND | ND | ND | ND | ND | 556.75 | 587.15 | 595.25 | 362.91 | 3.61 |
| [C4mim]Cl | 0.13±0.02 | ND | ND | ND | ND | ND | 538.95 | 560.75 | 569.85 | 360.51 | 4.33 |
| [C4mim]CF3COO | 1.49±0.02 | 0.08±0.00 | 0.61±0.03 | 0.71±0.03 | 0.96±0.02 | 0.64±0.01 | 454.05 | 471.45/551.45 | 519.25 | 353.05 | 7.16 |
| [P4444]BF4 | 0.29±0.01 | ND | ND | ND | ND | ND | 719.45 | 741.35 | 753.45 | 364.74 | 3.19 |
| [P4444]Br | 0.52±0.02 | ND | ND | ND | ND | ND | 645.55 | 675.45 | 691.05 | 369.57 | 1.88 |
| [P4444]Cl | 0.02±0.01 | ND | ND | ND | ND | ND | 640.25 | 669.95 | 685.65 | 364.47 | 3.72 |
| [P4444]CF3COO | 0.15±0.01 | ND | ND | ND | ND | ND | 462.15 | 476.65/499.15/518.35 | 512.05 | 350.60 | 3.91 |
| [P4448]BF4 | 1.25±0.45 | 0.89±0.00 | ND | 0.68±0.01 | 0.87±0.02 | ND | 703.95 | 743.95 | 751.35 | 366.02 | 5.31 |
| [P4448]Br | 2.69±0.30 | ND | ND | ND | ND | ND | 639.85 | 665.45 | 685.05 | 364.95 | 6.31 |
| [P4448]Cl | 3.11±0.11 | 1.26±0.02 | ND | 1.30±0.01 | 0.04±0.01 | ND | 635.25 | 664.55 | 680.65 | 357.40 | 5.80 |
| [P4448]CF3COO | 0.27±0.01 | 0.24±0.00 | ND | 1.07±0.04 | 0.87±0.02 | ND | 480.95 | 472.85/548.55 | 569.85 | 359.71 | 6.17 |
| [N4444]BF4 | 0.94±0.06 | ND | ND | ND | ND | ND | 469.75 | 492.25/664.75 | 676.65 | 367.82 | 3.65 |
| [N4444]Br | 0.94±0.00 | ND | ND | ND | ND | ND | 473.35 | 492.85/536.15 | 506.35 | 349.57 | 4.14 |
| [N4444]Cl | 8.51±0.08 | ND | ND | ND | ND | ND | 464.95 | 485.25 | 497.35 | 361.95 | 4.76 |
| [N4444]CF3COO | 0.16±0.03 | ND | ND | ND | ND | ND | 480.25 | 504.25 | 513.75 | 365.62 | 6.35 |
| [ChCl][Rib] | 0.06±0.04 | 23.40±0.25 | ND | ND | ND | ND | 520.55 | 545.05 | 557.75 | 342.81 | 18.29 |
| [ChCl][Glu] | 0.01±0.00 | 654.05±39.65 | ND | ND | ND | ND | 494.35 | 520.85 | 563.25 | 341.91 | 19.06 |
| [ChCl][Fru] | 12.93±0.12 | 54.06±0.48 | ND | ND | ND | ND | 432.75 | 448.85/514.55/564.35 | 563.75 | 347.21 | 16.17 |
| [ChCl][Sur] | 7.87±0.08 | >2201.00±83.00 | ND | ND | ND | ND | 509.05 | 543.85 | 559.65 | 338.53 | 20.83 |
| ILs | [ChCl][Rib] | [ChCl][Glu] | [ChCl][Fru] | [ChCl][Sur] |
|---|---|---|---|---|
| [C4mim]BF4 | ? | UCST | UCST | UCST |
| [C4mim]Br | ? | ? | ? | ? |
| [C4mim]Cl | ? | ? | ? | ? |
| [C4mim]CF3COO | ? | ? | ? | ? |
| [P4444]BF4 | × | × | × | × |
| [P4444]Br | LCST | LCST | LCST | LCST |
| [P4444]Cl | LCST | ? | LCST | LCST |
| [P4444]CF3COO | ? | ? | ? | ? |
| [P4448]BF4 | × | × | × | × |
| [P4448]Br | LCST | LCST | LCST | LCST |
| [P4448]Cl | LCST | LCST | LCST | LCST |
| [P4448]CF3COO | × | × | × | × |
| [N4444]BF4 | × | × | × | × |
| [N4444]Br | ? | ? | ? | ? |
| [N4444]Cl | ? | ? | ? | ? |
| [N4444]CF3COO | LCST | LCST | LCST | LCST |
表2 热可逆离子液体-低共熔溶剂-水体系的构建
Table 2 Construction of thermo reversible ILs-DESs-H2O system
| ILs | [ChCl][Rib] | [ChCl][Glu] | [ChCl][Fru] | [ChCl][Sur] |
|---|---|---|---|---|
| [C4mim]BF4 | ? | UCST | UCST | UCST |
| [C4mim]Br | ? | ? | ? | ? |
| [C4mim]Cl | ? | ? | ? | ? |
| [C4mim]CF3COO | ? | ? | ? | ? |
| [P4444]BF4 | × | × | × | × |
| [P4444]Br | LCST | LCST | LCST | LCST |
| [P4444]Cl | LCST | ? | LCST | LCST |
| [P4444]CF3COO | ? | ? | ? | ? |
| [P4448]BF4 | × | × | × | × |
| [P4448]Br | LCST | LCST | LCST | LCST |
| [P4448]Cl | LCST | LCST | LCST | LCST |
| [P4448]CF3COO | × | × | × | × |
| [N4444]BF4 | × | × | × | × |
| [N4444]Br | ? | ? | ? | ? |
| [N4444]Cl | ? | ? | ? | ? |
| [N4444]CF3COO | LCST | LCST | LCST | LCST |
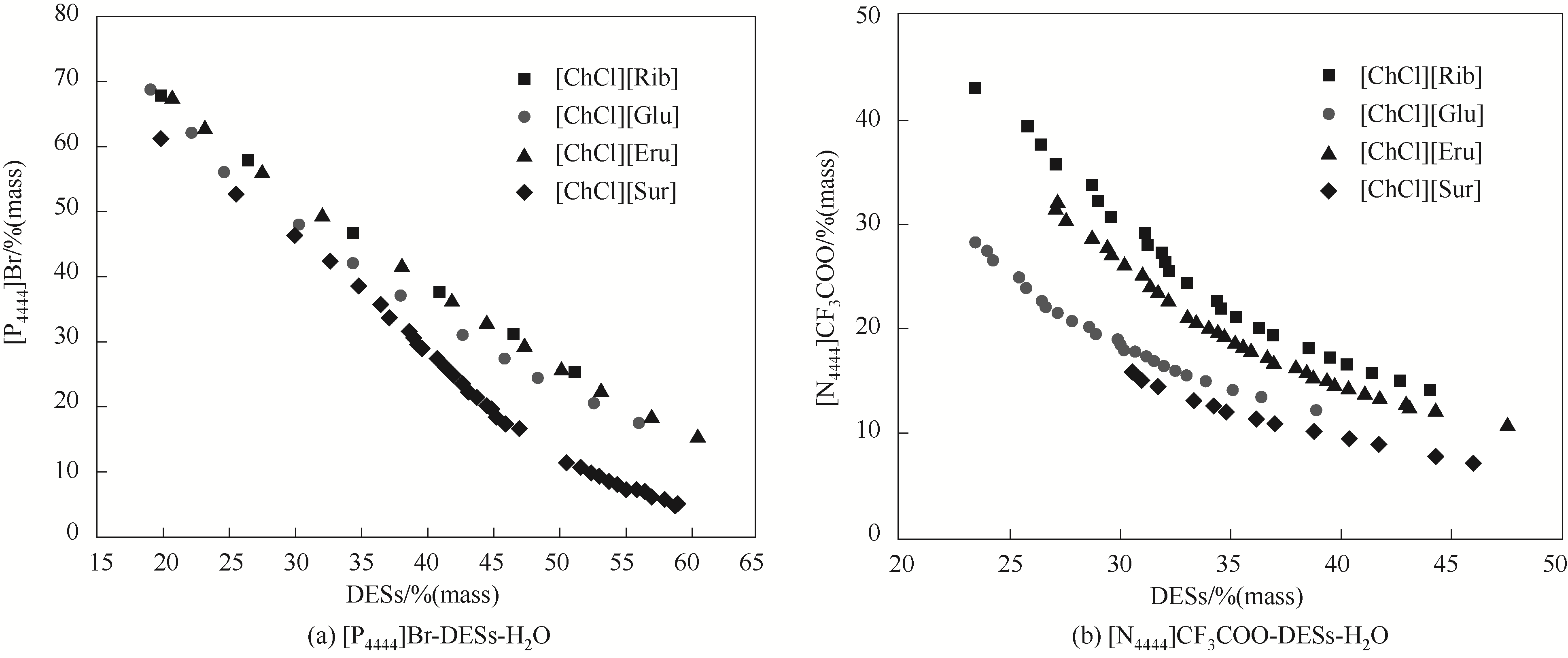
图4 [P4444]Br-低共熔溶剂(a)和[N4444]CF3COO-低共熔溶剂(b)在308.15 K下的双水相相图
Fig.4 Phase diagrams of DESs-[P4444]Br-H2O (a) and DESs-[N4444]CF3COO-H2O (b) at 308.15 K
| ILs | DESs | T/K | A | B | C | R2 |
|---|---|---|---|---|---|---|
| [C4mim]BF4 | [ChCl][Fru] | 298.15 | 1.00 | -1.02 | 9.75 | 0.9999 |
| [ChCl][Glu] | 298.15 | 1.19 | -2.05 | 8.86 | 0.9941 | |
| [ChCl][Glu] | 328.15 | 1.23 | -1.67 | 0.31 | 0.9982 | |
| [ChCl][Sur] | 308.15 | 1.21 | -1.99 | 1.08 | 0.9929 | |
| [P4444]Br | [ChCl][Rib] | 308.15 | 1.48 | -1.70 | 4.17 | 1.0000 |
| [ChCl][Glu] | 308.15 | 2.10 | -2.54 | 3.44 | 0.9994 | |
| [ChCl][Fru] | 308.15 | 1.58 | -1.81 | 4.30 | 0.9998 | |
| [ChCl][Sur] | 308.15 | 1.04 | -0.91 | 11.69 | 0.9983 | |
| [P4444]Cl | [ChCl][Fru] | 308.15 | 0.07 | 3.83 | 8.64 | 0.9996 |
| [ChCl][Sur] | 308.15 | 0.62×10-17 | 59.49 | 65.87 | 0.9928 | |
| [P4448]Br | [ChCl][Fru] | 308.15 | 0.58 | -4.97 | 24.29 | 0.9976 |
| [ChCl][Sur] | 308.15 | 0.10 | -2.04 | 133.00 | 0.9912 | |
| [P4448]Cl | [ChCl][Fru] | 308.15 | 0.72 | -0.61 | 63.26 | 0.9890 |
| [ChCl][Sur] | 308.15 | 1.47 | -2.31 | 51.95 | 0.9971 | |
| [N4444]CF3COO | [ChCl][Rib] | 308.15 | 8.05 | -5.90 | 2.34 | 0.9916 |
| [ChCl][Glu] | 308.15 | 2.66 | -4.36 | 9.64 | 0.9978 | |
| [ChCl][Fru] | 298.15 | 3.65 | -4.23 | 5.02 | 0.9961 | |
| [ChCl][Fru] | 308.15 | 28.17 | -8.74 | -3.85 | 0.9976 | |
| [ChCl][Fru] | 328.15 | 3.57 | -5.40 | -1.52 | 0.9976 | |
| [ChCl][Sur] | 308.15 | 3696.29 | -19.26 | -23.64 | 0.9782 |
表3 离子液体-低共熔溶剂体系双水相相图实验数据相关性拟合
Table 3 Phase diagram of ILs-DESs-H2O with the corresponding fitting of the experimental data
| ILs | DESs | T/K | A | B | C | R2 |
|---|---|---|---|---|---|---|
| [C4mim]BF4 | [ChCl][Fru] | 298.15 | 1.00 | -1.02 | 9.75 | 0.9999 |
| [ChCl][Glu] | 298.15 | 1.19 | -2.05 | 8.86 | 0.9941 | |
| [ChCl][Glu] | 328.15 | 1.23 | -1.67 | 0.31 | 0.9982 | |
| [ChCl][Sur] | 308.15 | 1.21 | -1.99 | 1.08 | 0.9929 | |
| [P4444]Br | [ChCl][Rib] | 308.15 | 1.48 | -1.70 | 4.17 | 1.0000 |
| [ChCl][Glu] | 308.15 | 2.10 | -2.54 | 3.44 | 0.9994 | |
| [ChCl][Fru] | 308.15 | 1.58 | -1.81 | 4.30 | 0.9998 | |
| [ChCl][Sur] | 308.15 | 1.04 | -0.91 | 11.69 | 0.9983 | |
| [P4444]Cl | [ChCl][Fru] | 308.15 | 0.07 | 3.83 | 8.64 | 0.9996 |
| [ChCl][Sur] | 308.15 | 0.62×10-17 | 59.49 | 65.87 | 0.9928 | |
| [P4448]Br | [ChCl][Fru] | 308.15 | 0.58 | -4.97 | 24.29 | 0.9976 |
| [ChCl][Sur] | 308.15 | 0.10 | -2.04 | 133.00 | 0.9912 | |
| [P4448]Cl | [ChCl][Fru] | 308.15 | 0.72 | -0.61 | 63.26 | 0.9890 |
| [ChCl][Sur] | 308.15 | 1.47 | -2.31 | 51.95 | 0.9971 | |
| [N4444]CF3COO | [ChCl][Rib] | 308.15 | 8.05 | -5.90 | 2.34 | 0.9916 |
| [ChCl][Glu] | 308.15 | 2.66 | -4.36 | 9.64 | 0.9978 | |
| [ChCl][Fru] | 298.15 | 3.65 | -4.23 | 5.02 | 0.9961 | |
| [ChCl][Fru] | 308.15 | 28.17 | -8.74 | -3.85 | 0.9976 | |
| [ChCl][Fru] | 328.15 | 3.57 | -5.40 | -1.52 | 0.9976 | |
| [ChCl][Sur] | 308.15 | 3696.29 | -19.26 | -23.64 | 0.9782 |
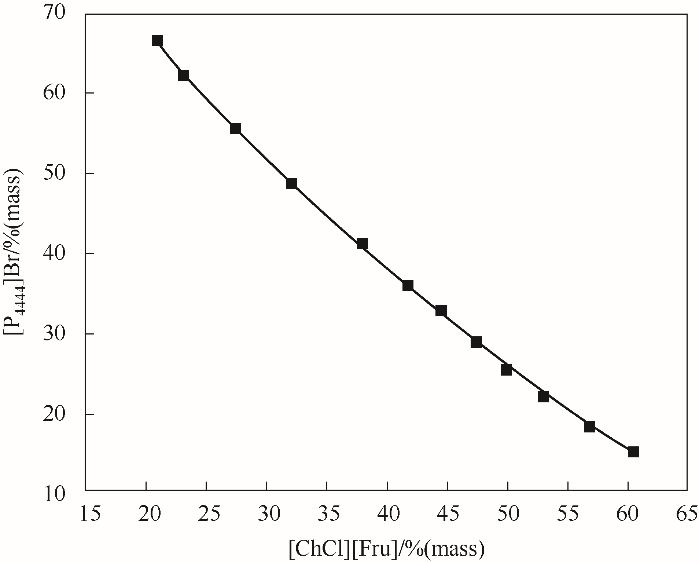
图5 308.15 K下[P4444]Br-[ChCl][Fru]双水相相图实验数据相关性拟合
Fig.5 Phase diagram of [P4444]Br-[ChCl][Fru]-H2O with the corresponding fitting of the experimental data at 308.15 K
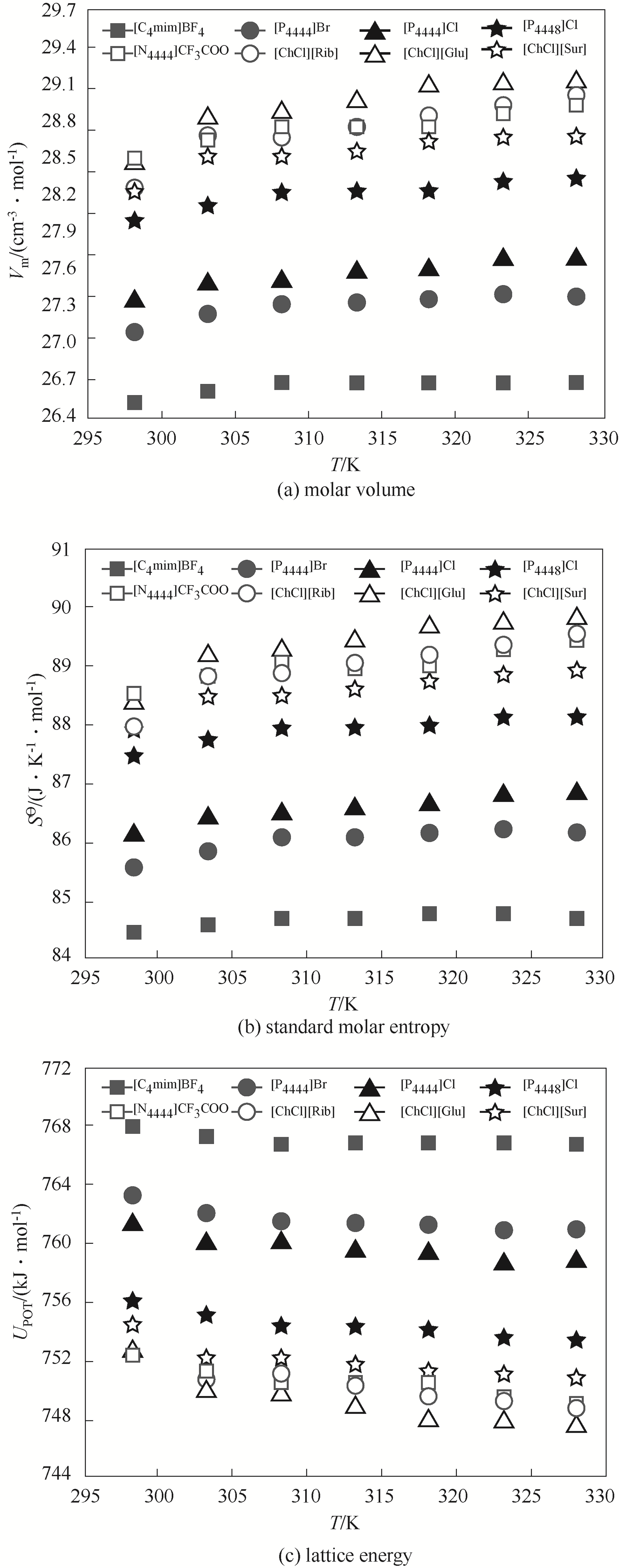
图7 不同温度下离子液体-低共熔溶剂-水三元体系摩尔体积(Vm)、标准摩尔熵(S?)和晶格能(UPOT)的变化趋势
Fig.7 Molar volume (Vm), standard molar entropy (S?) and lattice energy (UPOT) for ILs-DESs-H2O as a function of temperature
| System | Ecomplex/(kJ·mol-1) | E[ChCl][Fru]/(kJ·mol-1) | EBSSE/(kJ·mol-1) | ΔG/(kJ·mol-1) | ||
|---|---|---|---|---|---|---|
| [P4448]+-Cl- | -998.19×103 | -709.25×103 | -288.84×103 | — | 4.79 | -91.01 |
| [P4448]Cl-H2O | -1046.19×103 | -998.19×103 | -47.98×103 | — | 1.36 | -16.39 |
| [Ch]+-Cl- | -495.19×103 | -206.35×103 | -288.84×103 | — | 4.05 | -110.71 |
| [ChCl][Fru] | -926.74×103 | -495.29×103 | -431.41×103 | — | 2.10 | -43.25 |
| [ChCl][Fru]-H2O | -974.74×103 | -926.73×103 | -47.98×103 | — | 1.68 | -20.95 |
| [P4448]Cl-[ChCl][Fru]-H2O | -1972.96×103 | -926.73×103 | -998.18×103 | -47.98×103 | 3.70 | -60.43 |
表4 离子液体-低共熔溶剂-水三元体系的相互作用能
Table 4 Interaction energy of ILs-DESs-H2O ternary system
| System | Ecomplex/(kJ·mol-1) | E[ChCl][Fru]/(kJ·mol-1) | EBSSE/(kJ·mol-1) | ΔG/(kJ·mol-1) | ||
|---|---|---|---|---|---|---|
| [P4448]+-Cl- | -998.19×103 | -709.25×103 | -288.84×103 | — | 4.79 | -91.01 |
| [P4448]Cl-H2O | -1046.19×103 | -998.19×103 | -47.98×103 | — | 1.36 | -16.39 |
| [Ch]+-Cl- | -495.19×103 | -206.35×103 | -288.84×103 | — | 4.05 | -110.71 |
| [ChCl][Fru] | -926.74×103 | -495.29×103 | -431.41×103 | — | 2.10 | -43.25 |
| [ChCl][Fru]-H2O | -974.74×103 | -926.73×103 | -47.98×103 | — | 1.68 | -20.95 |
| [P4448]Cl-[ChCl][Fru]-H2O | -1972.96×103 | -926.73×103 | -998.18×103 | -47.98×103 | 3.70 | -60.43 |
| 1 | Wang B, Qin L, Mu T, et al. Are ionic liquids chemically stable?[J]. Chemical Reviews, 2017, 117(10): 7113-7131. |
| 2 | Xue Z M, Qin L, Jiang J Y, et al. Thermal, electrochemical and radiolytic stabilities of ionic liquids[J]. Physical Chemistry Chemical Physics, 2018, 20(13): 8382-8402. |
| 3 | Gutowski K E, Broker G A, Willauer H D, et al. Controlling the aqueous miscibility of ionic liquids: aqueous biphasic systems of water-miscible ionic liquids and water-structuring salts for recycle, metathesis, and separations[J]. Journal of the American Chemical Society, 2003, 125(22): 6632-6633. |
| 4 | Santana-Mayor Á, Socas-Rodríguez B, Rodríguez-Ramos R, et al. A green and simple procedure based on deep eutectic solvents for the extraction of phthalates from beverages[J]. Food Chemistry, 2020, 312: 125798. |
| 5 | Yu D K, Mou H Y, Fu H, et al. “Inverted” deep eutectic solvents based on host-guest interactions[J]. Chemistry — An Asian Journal, 2019, 14(23): 4183-4188. |
| 6 | Yu D K, Mu T C. Strategy to form eutectic molecular liquids based on noncovalent interactions[J]. The Journal of Physical Chemistry B, 2019, 123(23): 4958-4966. |
| 7 | Yu D K, Mou H Y, Zhao X H, et al. Eutectic molecular liquids based on hydrogen bonding and π-π interaction for exfoliating two-dimensional materials and recycling polymers[J]. Chemistry — An Asian Journal, 2019, 14(19): 3350-3356. |
| 8 | Zeng Q, Wang Y, Huang Y, et al. Deep eutectic solvents as novel extraction media for protein partitioning[J]. The Analyst, 2014, 139(10): 2565-2573. |
| 9 | Xu K J, Wang Y Z, Huang Y H, et al. A green deep eutectic solvent-based aqueous two-phase system for protein extracting[J]. Analytica Chimica Acta, 2015, 864: 9-20. |
| 10 | Xu P L, Wang Y Z, Chen J, et al. Development of deep eutectic solvent-based aqueous biphasic system for the extraction of lysozyme[J]. Talanta, 2019, 202: 1-10. |
| 11 | Zhang H M, Wang Y Z, Zhou Y G, et al. Aqueous biphasic systems containing PEG-based deep eutectic solvents for high-performance partitioning of RNA[J]. Talanta, 2017, 170: 266-274. |
| 12 | Li N, Wang Y Z, Xu K J, et al. High-performance of deep eutectic solvent based aqueous bi-phasic systems for the extraction of DNA[J]. RSC Advances, 2016, 6(87): 84406-84414. |
| 13 | Reichardt C. Solvatochromic dyes as solvent polarity indicators[J]. Chemical Reviews, 1994, 94(8): 2319-2358. |
| 14 | Li Y, Lu X, He W, et al. Influence of the salting-out ability and temperature on the liquid-liquid equilibria of aqueous two-phase systems based on ionic liquid-organic salts-water[J]. Journal of Chemical and Engineering Data, 2016, 61: 475–486. |
| 15 | Freire M G, Cláudio A F, Araújo J M, et al. Aqueous biphasic systems: a boost brought about by using ionic liquids[J]. Chemical Society Reviews, 2012, 41(14): 4966-4995. |
| 16 | Dilip M, Bridges N J, Rodríguez H, et al. Effect of temperature on salt-salt aqueous biphasic systems: manifestations of upper critical solution temperature[J]. Journal of Solution Chemistry, 2015, 44(3/4): 454-468. |
| 17 | Zafarani-Moattar M T, Hamzehzadeh S. Phase diagrams for the aqueous two-phase ternary system containing the ionic liquid 1-butyl-3-methylimidazolium bromide and tri-potassium citrate at T= (278.15, 298.15, and 318.15) K[J]. Journal of Chemical & Engineering Data, 2009, 54(3): 833-841. |
| 18 | Pang J Y, Han C R, Chao Y H, et al. Partitioning behavior of tetracycline in hydrophilic ionic liquids two-phase systems[J]. Separation Science and Technology, 2015, 50(13): 1993-1998. |
| 19 | Freire M G, Teles A R R, Canongia Lopes J N, et al. Partition coefficients of alkaloids in biphasic ionic-liquid-aqueous systems and their dependence on the hofmeister series[J]. Separation Science and Technology, 2012, 47(2): 284-291. |
| 20 | Sadeghi R, Golabiazar R, Shekaari H. The salting-out effect and phase separation in aqueous solutions of tri-sodium citrate and 1-butyl-3-methylimidazolium bromide[J]. The Journal of Chemical Thermodynamics, 2010, 42(4): 441-453. |
| 21 | Gao J, Chen L, Yan Z C. Extraction of dimethyl sulfoxide using ionic-liquid-based aqueous biphasic systems[J]. Separation and Purification Technology, 2014, 124: 107-116. |
| 22 | Taha M, Quental M V, Correia I, et al. Extraction and stability of bovine serum albumin (BSA) using cholinium-based Good's buffers ionic liquids[J]. Process Biochemistry, 2015, 50(7): 1158-1166. |
| 23 | Pereira M M, Pedro S N, Quental M V, et al. Enhanced extraction of bovine serum albumin with aqueous biphasic systems of phosphonium- and ammonium-based ionic liquids[J]. Journal of Biotechnology, 2015, 206: 17-25. |
| 24 | Deive F J, Rodríguez A, Pereiro A B, et al. Ionic liquid-based aqueous biphasic system for lipase extraction[J]. Green Chemistry, 2011, 13(2): 390-396. |
| 25 | Gao J, Guo J Y, Nie F H, et al. LCST-type phase behavior of aqueous biphasic systems composed of phosphonium-based ionic liquids and potassium phosphate[J]. Journal of Chemical & Engineering Data, 2017, 62(4): 1335-1340. |
| 26 | Pandey A, Pandey S. Solvatochromic probe behavior within choline chloride-based deep eutectic solvents: effect of temperature and water[J]. The Journal of Physical Chemistry B, 2014, 118(50): 14652-14661. |
| 27 | Guan W, Chang N, Yang L L, et al. Determination and prediction for the polarity of ionic liquids[J]. Journal of Chemical & Engineering Data, 2017, 62(9): 2610-2616. |
| 28 | Gao J, Chen L, Xin Y, et al. Ionic liquid-based aqueous biphasic systems with controlled hydrophobicity: the polar solvent effect[J]. Journal of Chemical & Engineering Data, 2014, 59(7): 2150-2158. |
| 29 | Sánchez P B, González B, Salgado J, et al. Physical properties of seven deep eutectic solvents based on l-proline or betaine[J]. The Journal of Chemical Thermodynamics, 2019, 131: 517-523. |
| 30 | Shafie M H, Yusof R, Gan C Y. Synthesis of citric acid monohydrate-choline chloride based deep eutectic solvents (DES) and characterization of their physicochemical properties[J]. Journal of Molecular Liquids, 2019, 288: 111081. |
| 31 | Zhao Y, Truhlar D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals[J]. Theoretical Chemistry Accounts, 2008, 120(1/2/3): 215-241. |
| 32 | Stewart J J P. MOPAC: a semiempirical molecular orbital program[J]. Journal of Computer-Aided Molecular Design, 1990, 4(1): 1-103. |
| 33 | Grimme S, Antony J, Ehrlich S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. |
| 34 | Rappoport D, Furche F. Property-optimized Gaussian basis sets for molecular response calculations[J]. The Journal of Chemical Physics, 2010, 133(13): 134105. |
| 35 | Boys S F, Bernardi F. The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors[J]. Molecular Physics, 1970, 19(4): 553-566. |
| 36 | Simon S, Duran M, Dannenberg J J. How does basis set superposition error change the potential surfaces for hydrogen-bonded dimers?[J]. The Journal of Chemical Physics, 1996, 105(24): 11024-11031. |
| 37 | Zafarani-Moattar M T, Shekaari H, Ghaffari F. Evaluation of solute-solvent interaction and phase separation for aqueous polymers solutions containing choline chloride/D-sucrose natural deep eutectic solvent through vapor-liquid equilibria, volumetric and acoustic studies[J]. The Journal of Chemical Thermodynamics, 2020, 142: 105963. |
| 38 | 金文彬, 李雪楠, 张依, 等. 离子液体在结构相似物分离中的进展[J]. 中国科学: 化学, 2016, 46(12): 1251-1263. |
| Jin W B, Li X N, Zhang Y, et al. Separation of structurally-related compounds with ionic liquids[J]. Scientia Sinica (Chimica), 2016, 46(12): 1251-1263. | |
| 39 | 胡丽华, 陈砺, 方泳华, 等. 低共熔溶剂的分子结构及物性估算的研究进展[J]. 化学试剂, 2017, 39(9): 937-941. |
| Hu L H, Chen L, Fang Y H, et al. Molecular structure and physical property estimation of deep eutectic solvents(DESs)[J]. Chemical Reagents, 2017, 39(9): 937-941. | |
| 40 | Passos H, Luís A, Coutinho J A P, et al. Thermoreversible (ionic-liquid-based) aqueous biphasic systems[J]. Scientific Reports, 2016, 6: 20276. |
| 41 | Silva F A E, Pereira J F B, Kurnia K A, et al. Temperature dependency of aqueous biphasic systems: an alternative approach for exploring the differences between coulombic-dominated salts and ionic liquids[J]. Chemical Communications, 2017, 53(53): 7298-7301. |
| 42 | Griffin S T, Dilip M, Spear S K, et al. The opposite effect of temperature on polyethylene glycol-based aqueous biphasic systems versus aqueous biphasic extraction chromatographic resins[J]. Journal of Chromatography B, 2006, 844(1): 23-31. |
| 43 | Schaeffer N, Pérez-Sánchez G, Passos H, et al. Mechanisms of phase separation in temperature-responsive acidic aqueous biphasic systems[J]. Physical Chemistry Chemical Physics, 2019, 21(14): 7462-7473. |
| 44 | Gao J, Chen L, Yan Z C. Phase behavior of aqueous biphasic systems composed of ionic liquids and organic salts[J]. Journal of Chemical & Engineering Data, 2015, 60(3): 464-470. |
| 45 | Qin M Y, Zhong F, Sun Y, et al. Experimental and DFT studies on surface properties of sulfonate-based surface active ionic liquids[J]. Journal of Molecular Structure, 2020, 1215: 128258. |
| 46 | Gao J, Fang C L, Lin Y Z, et al. Enhanced extraction of astaxanthin using aqueous biphasic systems composed of ionic liquids and potassium phosphate[J]. Food Chemistry, 2020, 309: 125672. |
| 47 | 张莉莉, 高静, 魏媛仪, 等. LCST型离子液体-盐双水相体系提取虾青素[J]. 中国食品学报, 2020, 20(4): 170-178. |
| Zhang L L, Gao J, Wei Y Y, et al. Extraction of astaxanthin by LCST-type ionic liquid-salt aqueous biphasic systems[J]. Journal of Chinese Institute of Food Science and Technology, 2020, 20(4): 170-178. | |
| 48 | Cai G M, Yang S Q, Wang X X, et al. Densities and viscosities of binary mixtures containing the polyhydric protic ionic liquid(2-hydroxy-N-(2-hydroxyethyl)-N-methylethanaminium methanesulfonate) and water or alcohols[J]. Journal of Solution Chemistry, 2020, 49(4): 423-457. |
| 49 | Boli E, Katsavrias T, Voutsas E. Viscosities of pure protic ionic liquids and their binary and ternary mixtures with water and ethanol[J]. Fluid Phase Equilibria, 2020, 520: 112663. |
| 50 | Guo S, Chen F, Liu L, et al. Effects of the water content on the transport properties of ionic liquids[J]. Industrial & Engineering Chemistry Research, 2019, 58(42): 19661-19669. |
| 51 | Bounsiar R, Gascón I, Amireche F, et al. Volumetric properties of three pyridinium-based ionic liquids with a common cation or anion[J]. Fluid Phase Equilibria, 2020, 521: 112732. |
| 52 | Sardar S, Wilfred C D, Mumtaz A, et al. Physicochemical properties, Brönsted acidity and ecotoxicity of imidazolium-based organic salts: non-toxic variants of protic ionic liquids[J]. Journal of Molecular Liquids, 2018, 269: 178-186. |
| 53 | Bessa A M M, Venerando M S C, Feitosa F X, et al. Low viscosity lactam-based ionic liquids with carboxylate anions: synthesis, characterization, thermophysical properties and mutual miscibility of ionic liquid with alcohol, water, and hydrocarbons[J]. Journal of Molecular Liquids, 2020, 313: 113586. |
| 54 | Xue Z M, Yan C Y, Zhao X H, et al. How Hofmeister ions change the local environment around thermoresponsive polymers in aqueous solutions: an NMR study[J]. Acta Physico-Chimica Sinica, 2019, 35(1): 49-57. |
| 55 | Yan C, Xue Z M, Zhao W C, et al. Surprising Hofmeister effects on the bending vibration of water[J]. ChemPhysChem, 2016, 17(20): 3309-3314. |
| 56 | 郭晶涛. 离子液体结构性质与相互作用的计算[D]. 兰州: 西北师范大学, 2009. |
| Guo J T. The calculations of structural properties and the interaction between the anions and cations in ionic liquids[D]. Lanzhou: Northwest Normal University, 2009. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [4] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [5] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [6] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [7] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [8] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [9] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [10] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [11] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [12] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [13] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [14] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [15] | 杨灿, 孙雪琦, 尚明华, 张建, 张香平, 曾少娟. 相变离子液体体系吸收分离CO2的研究现状及展望[J]. 化工学报, 2023, 74(4): 1419-1432. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号