化工学报 ›› 2021, Vol. 72 ›› Issue (4): 1874-1884.DOI: 10.11949/0438-1157.20201138
曹燕1( ),丁延1,郭义仓1,汪城2,刘英杰1,陶磊2,李进龙1(
),丁延1,郭义仓1,汪城2,刘英杰1,陶磊2,李进龙1( )
)
收稿日期:2020-08-10
修回日期:2020-09-21
出版日期:2021-04-05
发布日期:2021-04-05
通讯作者:
李进龙
作者简介:曹燕 (1993—),女,硕士研究生,基金资助:
CAO Yan1( ),DING Yan1,GUO Yicang1,WANG Cheng2,LIU Yingjie1,TAO Lei2,LI Jinlong1(
),DING Yan1,GUO Yicang1,WANG Cheng2,LIU Yingjie1,TAO Lei2,LI Jinlong1( )
)
Received:2020-08-10
Revised:2020-09-21
Online:2021-04-05
Published:2021-04-05
Contact:
LI Jinlong
摘要:
常压条件下,实验测定了不同温度(283.15~343.15 K)下吸收制冷/热泵工质对——溴化锂(LiBr)、1-乙基-3-甲基咪唑醋酸盐([EMIM][OAC])和1-丁基-3-甲基咪唑硫氰酸盐([BMIM][SCN])水溶液的密度、黏度和表面张力,借助线性方程和Vogel-Tammann-Fulcher(VTF)模型,分别成功关联了密度和黏度实验值。研究结果表明:在相同条件下,溴化锂水溶液的密度大于离子液体水溶液的密度,而前者的黏度小于后者;对表面张力,随着溴化锂含量增加,其水溶液的表面张力值增加,而少量离子液体可使水的表面张力快速下降。根据实验黏度和表面张力分别获得了能量势垒和表面熵/焓,表明各水溶液中分子或离子迁移难易程度遵循[EMIM][OAC] > [BMIM][SCN] > LiBr,表面有序性遵循[BMIM][SCN] > [EMIM][OAC] > LiBr。研究结果可为吸收制冷/热泵工质对及低温余热回收系统的设计和计算提供可靠的数据支撑。
中图分类号:
曹燕,丁延,郭义仓,汪城,刘英杰,陶磊,李进龙. 溴化锂及离子液体水溶液密度、黏度和表面张力测定与计算[J]. 化工学报, 2021, 72(4): 1874-1884.
CAO Yan,DING Yan,GUO Yicang,WANG Cheng,LIU Yingjie,TAO Lei,LI Jinlong. Measurement and calculations of density, viscosity and surface tension for lithium bromide and ionic liquid aqueous solutions[J]. CIESC Journal, 2021, 72(4): 1874-1884.
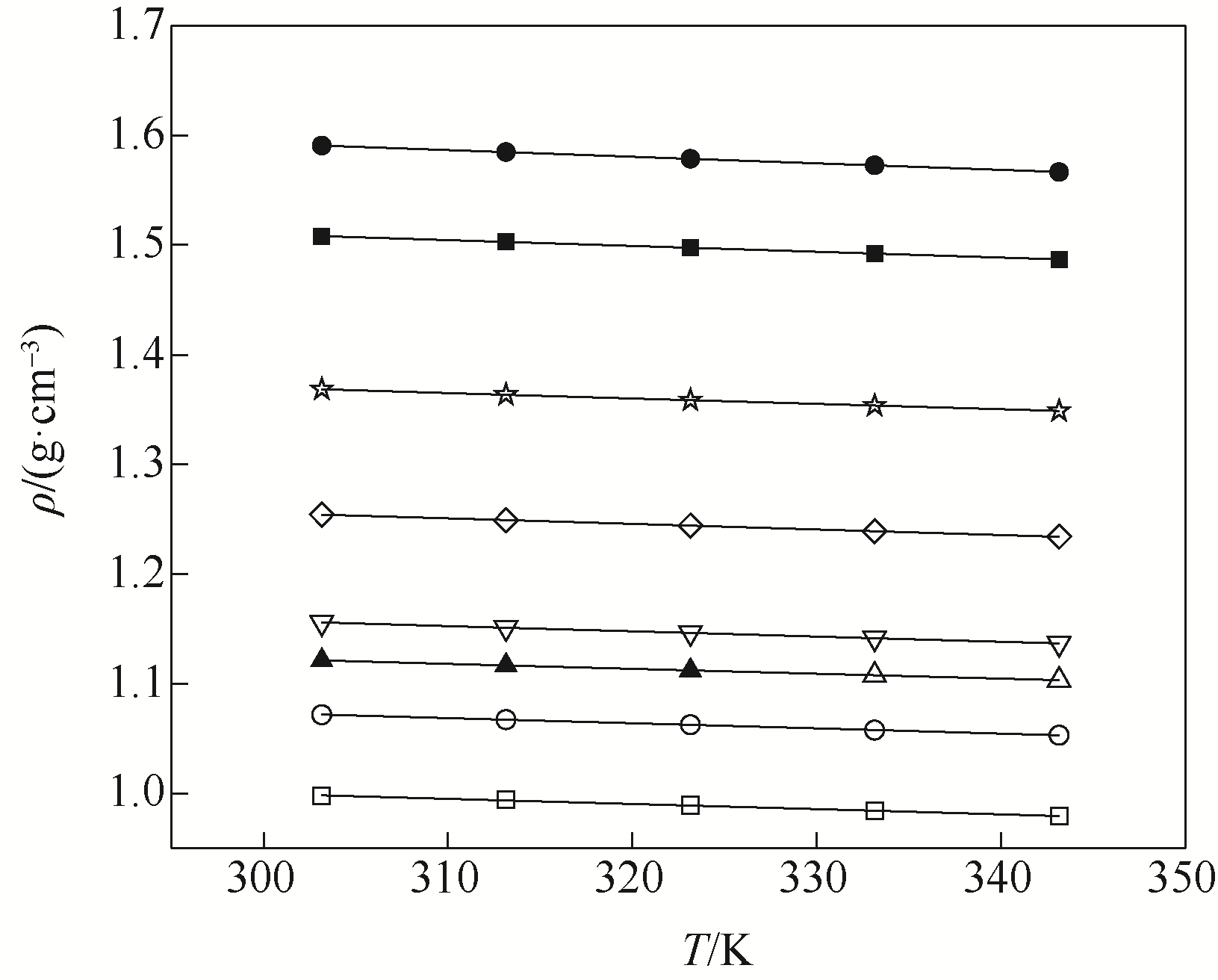
图1 不同温度下LiBr ( xA)+H2O (1-xA)密度的实验值(点)和计算值(线)□ 0; ○ 0.0225; △ 0.0366; ▽ 0.0492; ◇ 0.0815; ☆ 0.1216; ■ 0.1719; ● 0.2022; ▲ 0.0362(literature data)
Fig.1 Experimental (symbols) and calculated (lines) density of LiBr ( xA)+H2O (1-xA)
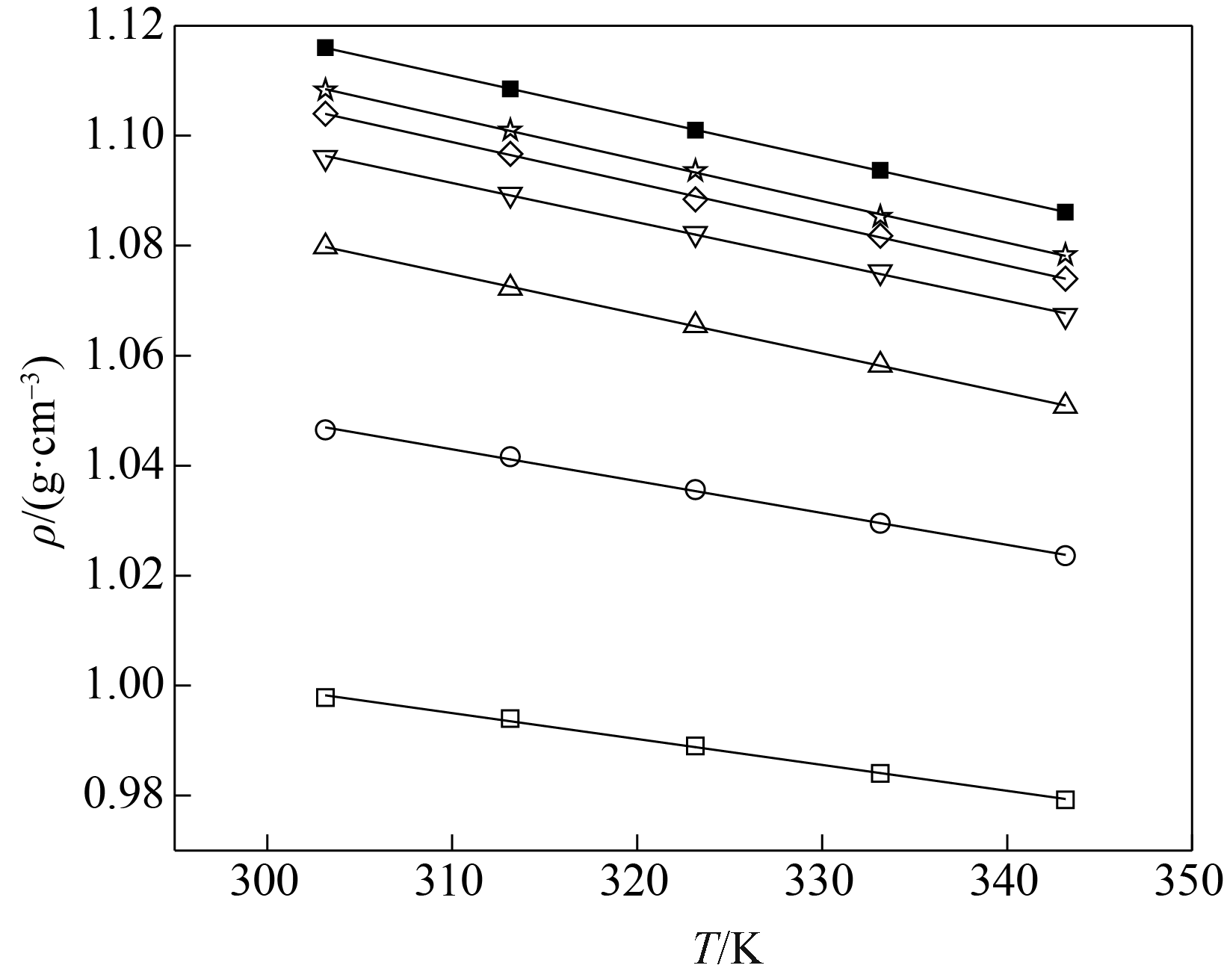
图2 不同温度下[EMIM][OAC]( xA)+H2O (1-xA)密度的实验值(点)和计算值(线)□ 0; ○ 0.0500; △ 0.1001; ▽ 0.1499; ◇ 0.2002; ☆ 0.2502; ■ 0.4000
Fig.2 Experimental (symbols) and calculated (lines) density of [EMIM][OAC]( xA)+H2O (1-xA)
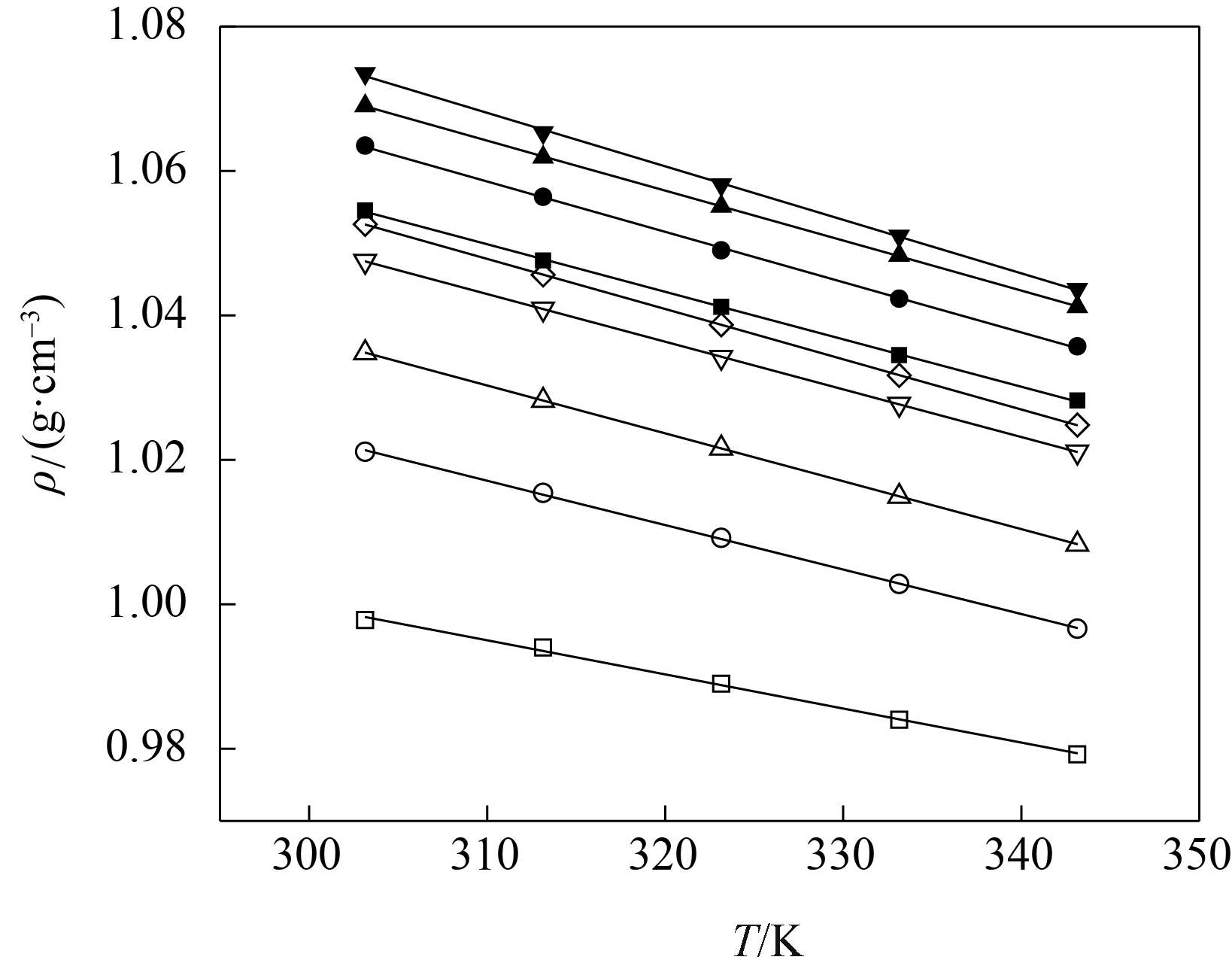
图3 不同温度下[BMIM][SCN]( xA)+H2O (1-xA)密度的实验值(点)和计算值(线)□ 0; ○ 0.0501; △ 0.0998; ▽ 0.1500; ◇ 0.2002; ■ 0.2501; ● 0.4000; ▲ 0.5989; ▼ 0.8002
Fig.3 Experimental (symbols) and calculated (lines) density of [BMIM][SCN]( xA)+H2O (1-xA)
| Solution | Aρa | Aρb | Aρc | Bρa | Bρb | Bρc | AAD |
|---|---|---|---|---|---|---|---|
| LiBr+H2O | -0.0039 | 2.2353×10-4 | -4.7187×10-4 | -0.1529 | 3.1040 | 1.1467 | 0.78% |
| [EMIM][OAC]+H2O | 0.0030 | -0.0017 | -5.2964×10-4 | -1.7728 | 1.0911 | 1.1862 | 0.02% |
| [BMIM][SCN]+H2O | 6.5232×10-4 | -1.7491×10-4 | -6.3246×10-4 | -0.1505 | 0.2213 | 1.2113 | 0.12% |
表1 水溶液密度模型化参数
Table 1 Correlated model parameters of densities for aqueous solution
| Solution | Aρa | Aρb | Aρc | Bρa | Bρb | Bρc | AAD |
|---|---|---|---|---|---|---|---|
| LiBr+H2O | -0.0039 | 2.2353×10-4 | -4.7187×10-4 | -0.1529 | 3.1040 | 1.1467 | 0.78% |
| [EMIM][OAC]+H2O | 0.0030 | -0.0017 | -5.2964×10-4 | -1.7728 | 1.0911 | 1.1862 | 0.02% |
| [BMIM][SCN]+H2O | 6.5232×10-4 | -1.7491×10-4 | -6.3246×10-4 | -0.1505 | 0.2213 | 1.2113 | 0.12% |
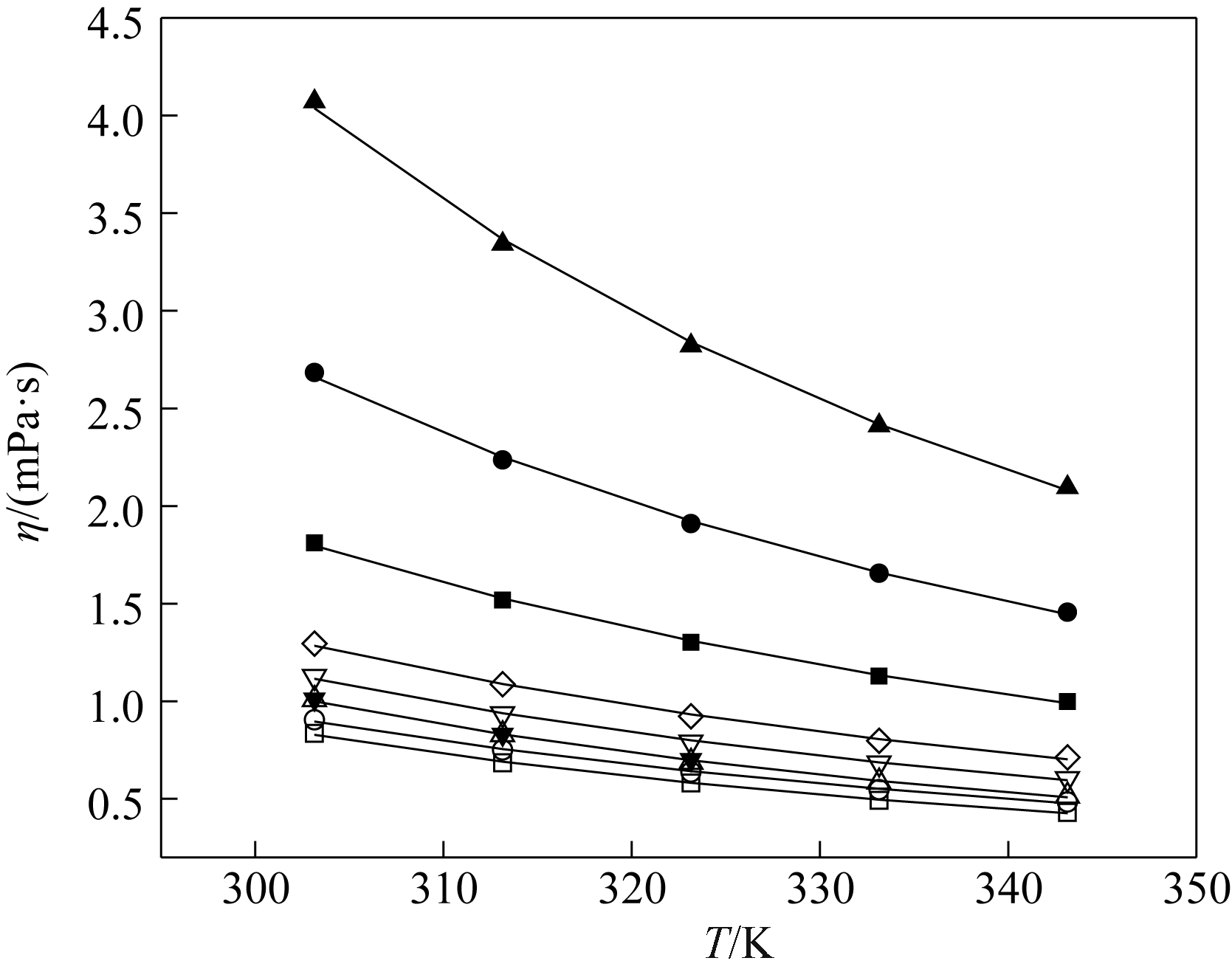
图4 不同温度下LiBr ( xA)+H2O (1-xA)黏度的实验值(点)和计算值(线)□ 0; ○ 0.0225; △ 0.0366; ▽ 0.0492; ◇ 0.0815; ■ 0.1216; ● 0.1719; ▲ 0.2022; ▼ 0.0362[39]
Fig.4 Experimental (symbols) and calculated (lines) viscosity of LiBr ( xA)+H2O (1-xA)
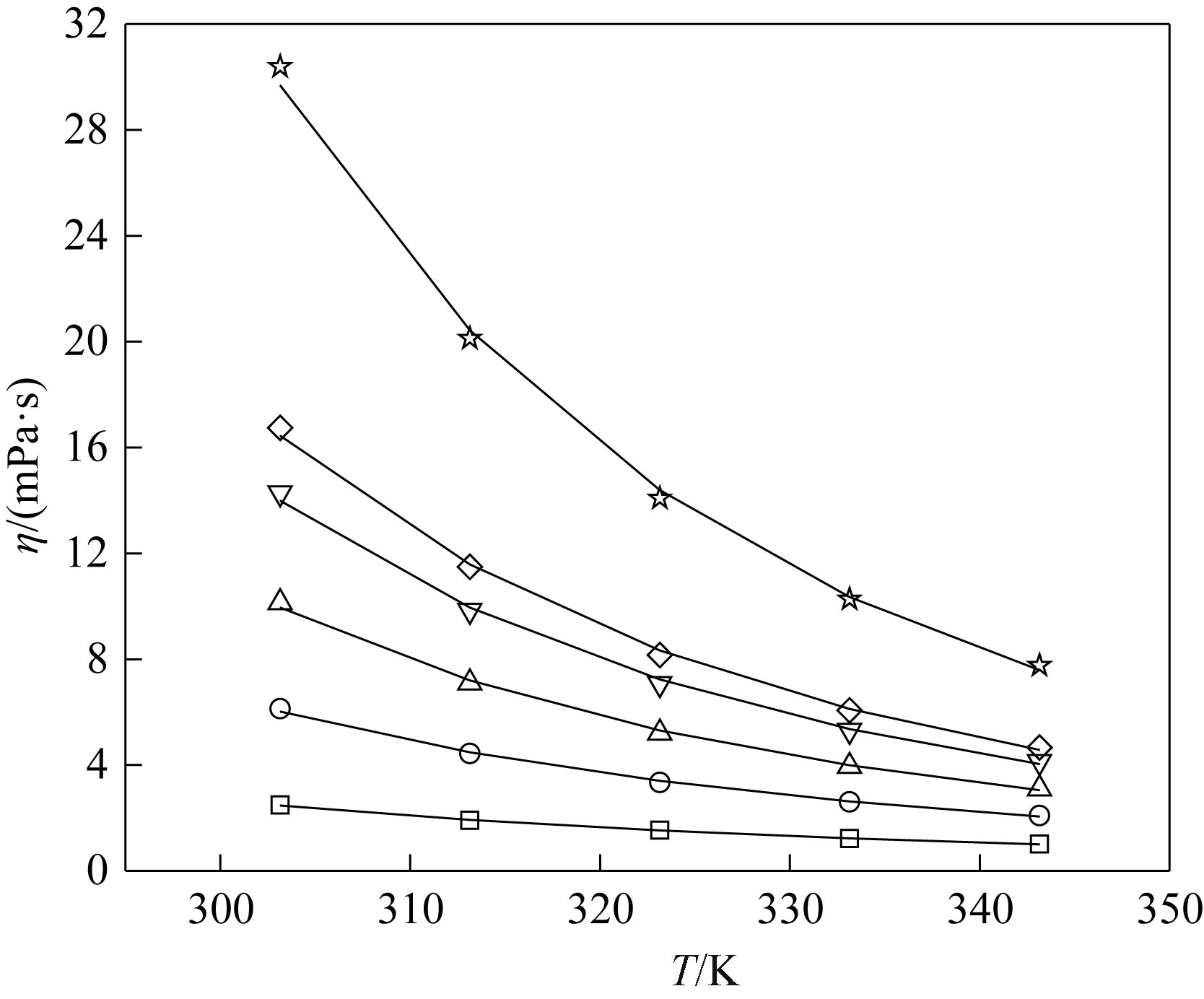
图5 不同温度下[EMIM][OAC]( xA)+H2O (1-xA)黏度的实验值(点)和计算值(线)□ 0.0500;○ 0.1001;△ 0.1499;▽ 0.2002;◇ 0.2502;☆ 0.4000
Fig.5 Experimental (symbols) and calculated (lines) viscosity of [EMIM][OAC]( xA)+H2O (1-xA)
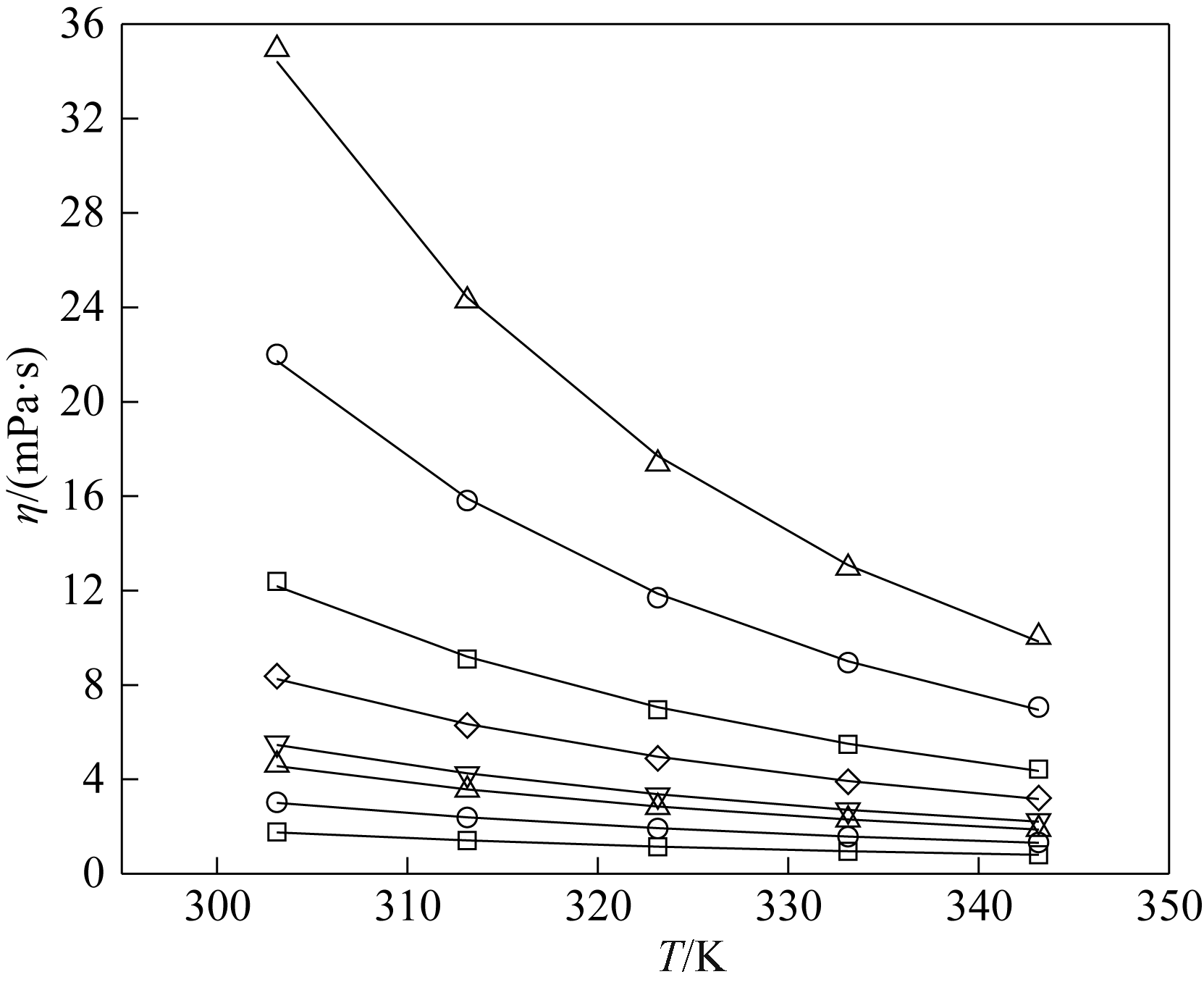
图6 不同温度下[BMIM][SCN]( xA)+H2O (1-xA)黏度的实验值(点)和计算值(线)□ 0.0501; ○ 0.0998; △ 0.1500; ▽ 0.2002; ◇ 0.2501; ■ 0.4000; ● 0.5989; ▲ 0.8002
Fig.6 Experimental (symbols) and calculated (lines) viscosity of [BMIM][SCN]( xA)+H2O (1-xA)
| Solution | Aηa | Aηb | Aηc | Bηa | Bηb | Bηc | AAD |
|---|---|---|---|---|---|---|---|
| LiBr+H2O | -43.3531 | 17.9018 | -6.0990 | 18818.5269 | -4278.8401 | 1797.4374 | 0.77% |
| [EMIM][OAC]+H2O | 19.2837 | -12.7411 | -6.2758 | -11958.8667 | 8625.6431 | 1991.0684 | 1.48% |
| [BMIM][SCN]+H2O | -2.3485 | 0.4841 | -6.1061 | -589.9570 | 2057.7217 | 1969.7655 | 1.07% |
表2 水溶液黏度模型化参数
Table 2 Correlated model parameters of viscosities for aqueous solution
| Solution | Aηa | Aηb | Aηc | Bηa | Bηb | Bηc | AAD |
|---|---|---|---|---|---|---|---|
| LiBr+H2O | -43.3531 | 17.9018 | -6.0990 | 18818.5269 | -4278.8401 | 1797.4374 | 0.77% |
| [EMIM][OAC]+H2O | 19.2837 | -12.7411 | -6.2758 | -11958.8667 | 8625.6431 | 1991.0684 | 1.48% |
| [BMIM][SCN]+H2O | -2.3485 | 0.4841 | -6.1061 | -589.9570 | 2057.7217 | 1969.7655 | 1.07% |
| System | xA | Sσ×10-5/ (J·m-2·K-1) | Hσ×10-2/ (J·m-2) |
|---|---|---|---|
| LiBr | 0.0225 | 15.29 | 16.02 |
| 0.0492 | 14.81 | 15.56 | |
| 0.0815 | 14.25 | 15.03 | |
| 0.1216 | 10.82 | 11.63 | |
| 0.1719 | 15.27 | 16.14 | |
| [EMIM][OAC] | 0.0010 | 15.58 | 16.29 |
| 0.0051 | 9.68 | 10.23 | |
| 0.0100 | 9.27 | 9.74 | |
| 0.0199 | 5.92 | 6.37 | |
| 0.0298 | 6.22 | 6.66 | |
| 0.0396 | 6.11 | 6.54 | |
| 0.0502 | 5.91 | 6.34 | |
| [BMIM][SCN] | 0.0009 | 6.81 | 7.41 |
| 0.0048 | 5.14 | 5.67 | |
| 0.0101 | 5.05 | 5.54 | |
| 0.0198 | 4.60 | 5.09 | |
| 0.0300 | 5.17 | 5.66 | |
| 0.0400 | 5.18 | 5.67 | |
| 0.0501 | 5.26 | 5.75 |
表3 表面热力学性质
Table 3 Surface thermodynamic properties
| System | xA | Sσ×10-5/ (J·m-2·K-1) | Hσ×10-2/ (J·m-2) |
|---|---|---|---|
| LiBr | 0.0225 | 15.29 | 16.02 |
| 0.0492 | 14.81 | 15.56 | |
| 0.0815 | 14.25 | 15.03 | |
| 0.1216 | 10.82 | 11.63 | |
| 0.1719 | 15.27 | 16.14 | |
| [EMIM][OAC] | 0.0010 | 15.58 | 16.29 |
| 0.0051 | 9.68 | 10.23 | |
| 0.0100 | 9.27 | 9.74 | |
| 0.0199 | 5.92 | 6.37 | |
| 0.0298 | 6.22 | 6.66 | |
| 0.0396 | 6.11 | 6.54 | |
| 0.0502 | 5.91 | 6.34 | |
| [BMIM][SCN] | 0.0009 | 6.81 | 7.41 |
| 0.0048 | 5.14 | 5.67 | |
| 0.0101 | 5.05 | 5.54 | |
| 0.0198 | 4.60 | 5.09 | |
| 0.0300 | 5.17 | 5.66 | |
| 0.0400 | 5.18 | 5.67 | |
| 0.0501 | 5.26 | 5.75 |
| No. | xA | ρ/(g·cm-3) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.9972 | 0.9940 | 0.9897 | 0.9840 | 0.9792 |
| 2 | 0.0225 | 1.0717 | 1.0672 | 1.0624 | 1.0577 | 1.0530 |
| 3 | 0.0366 | 1.1212 | 1.1168 | 1.1121 | 1.1076 | 1.1032 |
| 3 | 0.0492 | 1.1557 | 1.1510 | 1.1465 | 1.1416 | 1.1365 |
| 4 | 0.0815 | 1.2543 | 1.2491 | 1.2441 | 1.2390 | 1.2341 |
| 5 | 0.1216 | 1.3684 | 1.3635 | 1.3586 | 1.3536 | 1.3487 |
| 6 | 0.1719 | 1.5080 | 1.5028 | 1.4976 | 1.4922 | 1.4868 |
| 7 | 0.2022 | 1.5907 | 1.5847 | 1.5787 | 1.5727 | 1.5666 |
表A1 不同温度下LiBr(xA)+H2O (1-xA)密度的实验值
Table A1 Experimental density of LiBr(xA)+H2O (1-xA) at different temperatures
| No. | xA | ρ/(g·cm-3) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.9972 | 0.9940 | 0.9897 | 0.9840 | 0.9792 |
| 2 | 0.0225 | 1.0717 | 1.0672 | 1.0624 | 1.0577 | 1.0530 |
| 3 | 0.0366 | 1.1212 | 1.1168 | 1.1121 | 1.1076 | 1.1032 |
| 3 | 0.0492 | 1.1557 | 1.1510 | 1.1465 | 1.1416 | 1.1365 |
| 4 | 0.0815 | 1.2543 | 1.2491 | 1.2441 | 1.2390 | 1.2341 |
| 5 | 0.1216 | 1.3684 | 1.3635 | 1.3586 | 1.3536 | 1.3487 |
| 6 | 0.1719 | 1.5080 | 1.5028 | 1.4976 | 1.4922 | 1.4868 |
| 7 | 0.2022 | 1.5907 | 1.5847 | 1.5787 | 1.5727 | 1.5666 |
| No. | xA | ρ/(g·cm-3) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.9972 | 0.9940 | 0.9897 | 0.9840 | 0.9792 |
| 2 | 0.0500 | 1.0465 | 1.0416 | 1.0356 | 1.0295 | 1.0236 |
| 3 | 0.1001 | 1.0798 | 1.0723 | 1.0655 | 1.0583 | 1.0508 |
| 4 | 0.1499 | 1.0960 | 1.0893 | 1.0822 | 1.0752 | 1.0673 |
| 5 | 0.2002 | 1.1040 | 1.0967 | 1.0884 | 1.0818 | 1.0740 |
| 6 | 0.2502 | 1.1083 | 1.1010 | 1.0936 | 1.0853 | 1.0783 |
| 7 | 0.4000 | 1.1160 | 1.1085 | 1.1010 | 1.0937 | 1.0861 |
表A2 不同温度下[EMIM][OAC]( xA)+H2O (1-xA)密度的实验值
Table A2 Experimental density of [EMIM][OAC]( xA)+H2O (1-xA) at different temperatures
| No. | xA | ρ/(g·cm-3) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.9972 | 0.9940 | 0.9897 | 0.9840 | 0.9792 |
| 2 | 0.0500 | 1.0465 | 1.0416 | 1.0356 | 1.0295 | 1.0236 |
| 3 | 0.1001 | 1.0798 | 1.0723 | 1.0655 | 1.0583 | 1.0508 |
| 4 | 0.1499 | 1.0960 | 1.0893 | 1.0822 | 1.0752 | 1.0673 |
| 5 | 0.2002 | 1.1040 | 1.0967 | 1.0884 | 1.0818 | 1.0740 |
| 6 | 0.2502 | 1.1083 | 1.1010 | 1.0936 | 1.0853 | 1.0783 |
| 7 | 0.4000 | 1.1160 | 1.1085 | 1.1010 | 1.0937 | 1.0861 |
| No. | xA | ρ/(g·cm-3) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.9972 | 0.9940 | 0.9897 | 0.9840 | 0.9792 |
| 2 | 0.0501 | 1.0211 | 1.0154 | 1.0092 | 1.0028 | 0.9966 |
| 3 | 0.0998 | 1.0348 | 1.0282 | 1.0216 | 1.0149 | 1.0083 |
| 4 | 0.1500 | 1.0475 | 1.0409 | 1.0342 | 1.0277 | 1.0211 |
| 5 | 0.2002 | 1.0526 | 1.0456 | 1.0387 | 1.0317 | 1.0248 |
| 6 | 0.2501 | 1.0545 | 1.0476 | 1.0412 | 1.0345 | 1.0282 |
| 7 | 0.4000 | 1.0635 | 1.0564 | 1.0490 | 1.0423 | 1.0357 |
| 8 | 0.5989 | 1.0690 | 1.0619 | 1.0551 | 1.0483 | 1.0412 |
| 9 | 0.8002 | 1.0735 | 1.0653 | 1.0581 | 1.0510 | 1.0436 |
表A3 不同温度下[BMIM][SCN]( xA)+H2O (1-xA)密度的实验值
Table A3 Experimental density of [BMIM][SCN(xA)+H2O (1-xA) at different temperatures
| No. | xA | ρ/(g·cm-3) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.9972 | 0.9940 | 0.9897 | 0.9840 | 0.9792 |
| 2 | 0.0501 | 1.0211 | 1.0154 | 1.0092 | 1.0028 | 0.9966 |
| 3 | 0.0998 | 1.0348 | 1.0282 | 1.0216 | 1.0149 | 1.0083 |
| 4 | 0.1500 | 1.0475 | 1.0409 | 1.0342 | 1.0277 | 1.0211 |
| 5 | 0.2002 | 1.0526 | 1.0456 | 1.0387 | 1.0317 | 1.0248 |
| 6 | 0.2501 | 1.0545 | 1.0476 | 1.0412 | 1.0345 | 1.0282 |
| 7 | 0.4000 | 1.0635 | 1.0564 | 1.0490 | 1.0423 | 1.0357 |
| 8 | 0.5989 | 1.0690 | 1.0619 | 1.0551 | 1.0483 | 1.0412 |
| 9 | 0.8002 | 1.0735 | 1.0653 | 1.0581 | 1.0510 | 1.0436 |
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0225 | 0.9045 | 0.7498 | 0.6373 | 0.5470 | 0.4829 |
| 3 | 0.0366 | 1.0089 | 0.8286 | 0.6878 | 0.5873 | 0.5153 |
| 4 | 0.0492 | 1.1263 | 0.9346 | 0.7901 | 0.6834 | 0.6028 |
| 5 | 0.0815 | 1.2947 | 1.0872 | 0.9226 | 0.7969 | 0.7121 |
| 6 | 0.1216 | 1.8105 | 1.5173 | 1.3008 | 1.1288 | 0.9980 |
| 7 | 0.1719 | 2.6830 | 2.2359 | 1.9092 | 1.6548 | 1.4556 |
| 8 | 0.2022 | 4.0714 | 3.3417 | 2.8213 | 2.4116 | 2.0955 |
表A4 不同温度下LiBr(xA)+H2O (1-xA)黏度的实验值
Table A4 Experimental viscosity of LiBr(xA)+H2O (1-xA) at different temperatures
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0225 | 0.9045 | 0.7498 | 0.6373 | 0.5470 | 0.4829 |
| 3 | 0.0366 | 1.0089 | 0.8286 | 0.6878 | 0.5873 | 0.5153 |
| 4 | 0.0492 | 1.1263 | 0.9346 | 0.7901 | 0.6834 | 0.6028 |
| 5 | 0.0815 | 1.2947 | 1.0872 | 0.9226 | 0.7969 | 0.7121 |
| 6 | 0.1216 | 1.8105 | 1.5173 | 1.3008 | 1.1288 | 0.9980 |
| 7 | 0.1719 | 2.6830 | 2.2359 | 1.9092 | 1.6548 | 1.4556 |
| 8 | 0.2022 | 4.0714 | 3.3417 | 2.8213 | 2.4116 | 2.0955 |
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0225 | 0.9045 | 0.7498 | 0.6373 | 0.5470 | 0.4829 |
| 3 | 0.0366 | 1.0089 | 0.8286 | 0.6878 | 0.5873 | 0.5153 |
| 4 | 0.0492 | 1.1263 | 0.9346 | 0.7901 | 0.6834 | 0.6028 |
| 5 | 0.0815 | 1.2947 | 1.0872 | 0.9226 | 0.7969 | 0.7121 |
| 6 | 0.1216 | 1.8105 | 1.5173 | 1.3008 | 1.1288 | 0.9980 |
| 7 | 0.1719 | 2.6830 | 2.2359 | 1.9092 | 1.6548 | 1.4556 |
| 8 | 0.2022 | 4.0714 | 3.3417 | 2.8213 | 2.4116 | 2.0955 |
表A4 不同温度下LiBr(xA)+H2O (1-xA)黏度的实验值
Table A4 Experimental viscosity of LiBr(xA)+H2O (1-xA) at different temperatures
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0225 | 0.9045 | 0.7498 | 0.6373 | 0.5470 | 0.4829 |
| 3 | 0.0366 | 1.0089 | 0.8286 | 0.6878 | 0.5873 | 0.5153 |
| 4 | 0.0492 | 1.1263 | 0.9346 | 0.7901 | 0.6834 | 0.6028 |
| 5 | 0.0815 | 1.2947 | 1.0872 | 0.9226 | 0.7969 | 0.7121 |
| 6 | 0.1216 | 1.8105 | 1.5173 | 1.3008 | 1.1288 | 0.9980 |
| 7 | 0.1719 | 2.6830 | 2.2359 | 1.9092 | 1.6548 | 1.4556 |
| 8 | 0.2022 | 4.0714 | 3.3417 | 2.8213 | 2.4116 | 2.0955 |
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0500 | 2.4907 | 1.9084 | 1.5423 | 1.2218 | 1.0152 |
| 3 | 0.1001 | 6.1310 | 4.4364 | 3.3443 | 2.6091 | 2.0926 |
| 4 | 0.1499 | 10.1404 | 7.1074 | 5.2156 | 3.9575 | 3.1090 |
| 5 | 0.2002 | 14.2839 | 9.8433 | 7.0754 | 5.3088 | 4.1285 |
| 6 | 0.2502 | 16.7417 | 11.4878 | 8.1586 | 6.0638 | 4.6674 |
| 7 | 0.4000 | 30.3731 | 20.1181 | 14.0798 | 10.2631 | 7.7607 |
表A5 不同温度下[EMIM][OAC]( xA)+H2O (1-xA)黏度的实验值
Table A5 Experimental viscosity of [EMIM][OAC]( xA)+H2O (1-xA) at different temperatures
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0500 | 2.4907 | 1.9084 | 1.5423 | 1.2218 | 1.0152 |
| 3 | 0.1001 | 6.1310 | 4.4364 | 3.3443 | 2.6091 | 2.0926 |
| 4 | 0.1499 | 10.1404 | 7.1074 | 5.2156 | 3.9575 | 3.1090 |
| 5 | 0.2002 | 14.2839 | 9.8433 | 7.0754 | 5.3088 | 4.1285 |
| 6 | 0.2502 | 16.7417 | 11.4878 | 8.1586 | 6.0638 | 4.6674 |
| 7 | 0.4000 | 30.3731 | 20.1181 | 14.0798 | 10.2631 | 7.7607 |
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0500 | 2.4907 | 1.9084 | 1.5423 | 1.2218 | 1.0152 |
| 3 | 0.1001 | 6.1310 | 4.4364 | 3.3443 | 2.6091 | 2.0926 |
| 4 | 0.1499 | 10.1404 | 7.1074 | 5.2156 | 3.9575 | 3.1090 |
| 5 | 0.2002 | 14.2839 | 9.8433 | 7.0754 | 5.3088 | 4.1285 |
| 6 | 0.2502 | 16.7417 | 11.4878 | 8.1586 | 6.0638 | 4.6674 |
| 7 | 0.4000 | 30.3731 | 20.1181 | 14.0798 | 10.2631 | 7.7607 |
表A5 不同温度下[EMIM][OAC]( xA)+H2O (1-xA)黏度的实验值
Table A5 Experimental viscosity of [EMIM][OAC]( xA)+H2O (1-xA) at different temperatures
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0500 | 2.4907 | 1.9084 | 1.5423 | 1.2218 | 1.0152 |
| 3 | 0.1001 | 6.1310 | 4.4364 | 3.3443 | 2.6091 | 2.0926 |
| 4 | 0.1499 | 10.1404 | 7.1074 | 5.2156 | 3.9575 | 3.1090 |
| 5 | 0.2002 | 14.2839 | 9.8433 | 7.0754 | 5.3088 | 4.1285 |
| 6 | 0.2502 | 16.7417 | 11.4878 | 8.1586 | 6.0638 | 4.6674 |
| 7 | 0.4000 | 30.3731 | 20.1181 | 14.0798 | 10.2631 | 7.7607 |
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0501 | 1.7689 | 1.4078 | 1.1433 | 0.9510 | 0.8091 |
| 3 | 0.0998 | 3.0283 | 2.3871 | 1.9139 | 1.5694 | 1.3266 |
| 4 | 0.1500 | 4.6148 | 3.5508 | 2.8167 | 2.2870 | 1.8994 |
| 5 | 0.2002 | 5.5270 | 4.2265 | 3.3339 | 2.6966 | 2.2395 |
| 6 | 0.2501 | 8.3727 | 6.2903 | 4.8910 | 3.9063 | 3.2129 |
| 7 | 0.4000 | 12.3906 | 9.1011 | 6.9448 | 5.4842 | 4.4314 |
| 8 | 0.5989 | 22.0023 | 15.8051 | 11.6956 | 8.9455 | 7.0548 |
| 9 | 0.8002 | 34.9379 | 24.2818 | 17.3683 | 12.9612 | 10.0297 |
表A6 不同温度下[BMIM][SCN]( xA)+H2O (1-xA)黏度的实验值
Table A6 Experimental viscosity of [BMIM][SCN](xA)+H2O (1-xA) at different temperatures
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0501 | 1.7689 | 1.4078 | 1.1433 | 0.9510 | 0.8091 |
| 3 | 0.0998 | 3.0283 | 2.3871 | 1.9139 | 1.5694 | 1.3266 |
| 4 | 0.1500 | 4.6148 | 3.5508 | 2.8167 | 2.2870 | 1.8994 |
| 5 | 0.2002 | 5.5270 | 4.2265 | 3.3339 | 2.6966 | 2.2395 |
| 6 | 0.2501 | 8.3727 | 6.2903 | 4.8910 | 3.9063 | 3.2129 |
| 7 | 0.4000 | 12.3906 | 9.1011 | 6.9448 | 5.4842 | 4.4314 |
| 8 | 0.5989 | 22.0023 | 15.8051 | 11.6956 | 8.9455 | 7.0548 |
| 9 | 0.8002 | 34.9379 | 24.2818 | 17.3683 | 12.9612 | 10.0297 |
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0501 | 1.7689 | 1.4078 | 1.1433 | 0.9510 | 0.8091 |
| 3 | 0.0998 | 3.0283 | 2.3871 | 1.9139 | 1.5694 | 1.3266 |
| 4 | 0.1500 | 4.6148 | 3.5508 | 2.8167 | 2.2870 | 1.8994 |
| 5 | 0.2002 | 5.5270 | 4.2265 | 3.3339 | 2.6966 | 2.2395 |
| 6 | 0.2501 | 8.3727 | 6.2903 | 4.8910 | 3.9063 | 3.2129 |
| 7 | 0.4000 | 12.3906 | 9.1011 | 6.9448 | 5.4842 | 4.4314 |
| 8 | 0.5989 | 22.0023 | 15.8051 | 11.6956 | 8.9455 | 7.0548 |
| 9 | 0.8002 | 34.9379 | 24.2818 | 17.3683 | 12.9612 | 10.0297 |
表A6 不同温度下[BMIM][SCN]( xA)+H2O (1-xA)黏度的实验值
Table A6 Experimental viscosity of [BMIM][SCN](xA)+H2O (1-xA) at different temperatures
| No. | xA | η/(mPa·s) | ||||
|---|---|---|---|---|---|---|
| 303.15 K | 313.15 K | 323.15 K | 333.15 K | 343.15 K | ||
| 1 | 0.0000 | 0.8351 | 0.6847 | 0.5813 | 0.4923 | 0.4295 |
| 2 | 0.0501 | 1.7689 | 1.4078 | 1.1433 | 0.9510 | 0.8091 |
| 3 | 0.0998 | 3.0283 | 2.3871 | 1.9139 | 1.5694 | 1.3266 |
| 4 | 0.1500 | 4.6148 | 3.5508 | 2.8167 | 2.2870 | 1.8994 |
| 5 | 0.2002 | 5.5270 | 4.2265 | 3.3339 | 2.6966 | 2.2395 |
| 6 | 0.2501 | 8.3727 | 6.2903 | 4.8910 | 3.9063 | 3.2129 |
| 7 | 0.4000 | 12.3906 | 9.1011 | 6.9448 | 5.4842 | 4.4314 |
| 8 | 0.5989 | 22.0023 | 15.8051 | 11.6956 | 8.9455 | 7.0548 |
| 9 | 0.8002 | 34.9379 | 24.2818 | 17.3683 | 12.9612 | 10.0297 |
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0225 | 76.44±0.12 | 74.31 ±0.10 | 72.86±0.16 | 71.48 ±0.09 | 70.21±0.11 |
| 3 | 0.0492 | 78.57±0.10 | 76.97±0.16 | 75.48±0.09 | 74.02 ±0.12 | 72.64±0.15 |
| 4 | 0.0815 | 81.13±0.04 | 79.93±0.06 | 78.79 ±0.16 | 77.10 ±0.18 | 75.42±0.19 |
| 5 | 0.1216 | 84.09±0.15 | 82.93 ±0.11 | 82.01±0.09 | 80.83 ±0.14 | 79.73±0.20 |
| 6 | 0.1719 | 91.08±0.17 | 88.05±0.06 | 86.47 ±0.09 | 85.74 ±0.15 | 84.60±0.16 |
表A7 不同温度下LiBr(xA)+H2O (1-xA)的表面张力
Table A7 Surface tension of LiBr(xA)+H2O (1-xA) at different temperatures
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0225 | 76.44±0.12 | 74.31 ±0.10 | 72.86±0.16 | 71.48 ±0.09 | 70.21±0.11 |
| 3 | 0.0492 | 78.57±0.10 | 76.97±0.16 | 75.48±0.09 | 74.02 ±0.12 | 72.64±0.15 |
| 4 | 0.0815 | 81.13±0.04 | 79.93±0.06 | 78.79 ±0.16 | 77.10 ±0.18 | 75.42±0.19 |
| 5 | 0.1216 | 84.09±0.15 | 82.93 ±0.11 | 82.01±0.09 | 80.83 ±0.14 | 79.73±0.20 |
| 6 | 0.1719 | 91.08±0.17 | 88.05±0.06 | 86.47 ±0.09 | 85.74 ±0.15 | 84.60±0.16 |
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0225 | 76.44±0.12 | 74.31 ±0.10 | 72.86±0.16 | 71.48 ±0.09 | 70.21±0.11 |
| 3 | 0.0492 | 78.57±0.10 | 76.97±0.16 | 75.48±0.09 | 74.02 ±0.12 | 72.64±0.15 |
| 4 | 0.0815 | 81.13±0.04 | 79.93±0.06 | 78.79 ±0.16 | 77.10 ±0.18 | 75.42±0.19 |
| 5 | 0.1216 | 84.09±0.15 | 82.93 ±0.11 | 82.01±0.09 | 80.83 ±0.14 | 79.73±0.20 |
| 6 | 0.1719 | 91.08±0.17 | 88.05±0.06 | 86.47 ±0.09 | 85.74 ±0.15 | 84.60±0.16 |
表A7 不同温度下LiBr(xA)+H2O (1-xA)的表面张力
Table A7 Surface tension of LiBr(xA)+H2O (1-xA) at different temperatures
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0225 | 76.44±0.12 | 74.31 ±0.10 | 72.86±0.16 | 71.48 ±0.09 | 70.21±0.11 |
| 3 | 0.0492 | 78.57±0.10 | 76.97±0.16 | 75.48±0.09 | 74.02 ±0.12 | 72.64±0.15 |
| 4 | 0.0815 | 81.13±0.04 | 79.93±0.06 | 78.79 ±0.16 | 77.10 ±0.18 | 75.42±0.19 |
| 5 | 0.1216 | 84.09±0.15 | 82.93 ±0.11 | 82.01±0.09 | 80.83 ±0.14 | 79.73±0.20 |
| 6 | 0.1719 | 91.08±0.17 | 88.05±0.06 | 86.47 ±0.09 | 85.74 ±0.15 | 84.60±0.16 |
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0010 | 73.58±0.16 | 72.43±0.12 | 70.85±0.08 | 69.17±0.10 | 67.42±0.12 |
| 3 | 0.0051 | 56.59±0.14 | 55.73±0.15 | 54.37±0.06 | 53.59±0.09 | 52.82±0.06 |
| 4 | 0.0100 | 48.37±0.14 | 47.54±0.16 | 46.36±0.11 | 45.45±0.15 | 44.78±0.09 |
| 5 | 0.0199 | 46.76±0.16 | 46.02±0.20 | 45.52±0.16 | 44.92±0.18 | 44.35±0.15 |
| 6 | 0.0298 | 45.37±0.09 | 44.45±0.14 | 43.97±0.13 | 43.25±0.18 | 42.86±0.12 |
| 7 | 0.0396 | 44.74±0.12 | 44.20±0.09 | 43.37±0.20 | 42.95±0.16 | 42.31±0.06 |
| 8 | 0.0502 | 44.58±0.09 | 43.94±0.07 | 43.21±0.10 | 42.85±0.11 | 42.17±0.15 |
表A8 不同温度下[EMIM][OAC]( xA)+H2O (1-xA)的表面张力
Table A8 Surface tension of [EMIM][OAC]( xA)+H2O (1-xA) at different temperatures
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0010 | 73.58±0.16 | 72.43±0.12 | 70.85±0.08 | 69.17±0.10 | 67.42±0.12 |
| 3 | 0.0051 | 56.59±0.14 | 55.73±0.15 | 54.37±0.06 | 53.59±0.09 | 52.82±0.06 |
| 4 | 0.0100 | 48.37±0.14 | 47.54±0.16 | 46.36±0.11 | 45.45±0.15 | 44.78±0.09 |
| 5 | 0.0199 | 46.76±0.16 | 46.02±0.20 | 45.52±0.16 | 44.92±0.18 | 44.35±0.15 |
| 6 | 0.0298 | 45.37±0.09 | 44.45±0.14 | 43.97±0.13 | 43.25±0.18 | 42.86±0.12 |
| 7 | 0.0396 | 44.74±0.12 | 44.20±0.09 | 43.37±0.20 | 42.95±0.16 | 42.31±0.06 |
| 8 | 0.0502 | 44.58±0.09 | 43.94±0.07 | 43.21±0.10 | 42.85±0.11 | 42.17±0.15 |
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0010 | 73.58±0.16 | 72.43±0.12 | 70.85±0.08 | 69.17±0.10 | 67.42±0.12 |
| 3 | 0.0051 | 56.59±0.14 | 55.73±0.15 | 54.37±0.06 | 53.59±0.09 | 52.82±0.06 |
| 4 | 0.0100 | 48.37±0.14 | 47.54±0.16 | 46.36±0.11 | 45.45±0.15 | 44.78±0.09 |
| 5 | 0.0199 | 46.76±0.16 | 46.02±0.20 | 45.52±0.16 | 44.92±0.18 | 44.35±0.15 |
| 6 | 0.0298 | 45.37±0.09 | 44.45±0.14 | 43.97±0.13 | 43.25±0.18 | 42.86±0.12 |
| 7 | 0.0396 | 44.74±0.12 | 44.20±0.09 | 43.37±0.20 | 42.95±0.16 | 42.31±0.06 |
| 8 | 0.0502 | 44.58±0.09 | 43.94±0.07 | 43.21±0.10 | 42.85±0.11 | 42.17±0.15 |
表A8 不同温度下[EMIM][OAC]( xA)+H2O (1-xA)的表面张力
Table A8 Surface tension of [EMIM][OAC]( xA)+H2O (1-xA) at different temperatures
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0010 | 73.58±0.16 | 72.43±0.12 | 70.85±0.08 | 69.17±0.10 | 67.42±0.12 |
| 3 | 0.0051 | 56.59±0.14 | 55.73±0.15 | 54.37±0.06 | 53.59±0.09 | 52.82±0.06 |
| 4 | 0.0100 | 48.37±0.14 | 47.54±0.16 | 46.36±0.11 | 45.45±0.15 | 44.78±0.09 |
| 5 | 0.0199 | 46.76±0.16 | 46.02±0.20 | 45.52±0.16 | 44.92±0.18 | 44.35±0.15 |
| 6 | 0.0298 | 45.37±0.09 | 44.45±0.14 | 43.97±0.13 | 43.25±0.18 | 42.86±0.12 |
| 7 | 0.0396 | 44.74±0.12 | 44.20±0.09 | 43.37±0.20 | 42.95±0.16 | 42.31±0.06 |
| 8 | 0.0502 | 44.58±0.09 | 43.94±0.07 | 43.21±0.10 | 42.85±0.11 | 42.17±0.15 |
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0009 | 61.23±0.06 | 60.78±0.06 | 60.21±0.06 | 59.45±0.14 | 58.49±0.14 |
| 3 | 0.0048 | 54.32±0.14 | 53.87±0.17 | 53.06±0.13 | 52.75±0.12 | 52.31±0.12 |
| 4 | 0.0101 | 50.12±0.12 | 49.76±0.13 | 49.17±0.16 | 48.61±0.21 | 48.17±0.09 |
| 5 | 0.0198 | 49.76±0.09 | 49.24±0.23 | 48.81±0.21 | 48.34±0.16 | 47.91±0.13 |
| 6 | 0.0300 | 49.69±0.11 | 49.14±0.15 | 48.75±0.19 | 48.21±0.17 | 47.57±0.11 |
| 7 | 0.0400 | 49.61±0.15 | 49.07±0.19 | 48.64±0.15 | 48.13±0.15 | 47.49±0.06 |
| 8 | 0.0501 | 49.55±0.11 | 49.01±0.09 | 48.57±0.08 | 48.01±0.12 | 47.42±0.14 |
表A9 不同温度下[BMIM][SCN]( xA)+H2O (1-xA)的表面张力
Table A9 Surface tension of [BMIM][SCN]( xA)+H2O (1-xA) at different temperatures
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0009 | 61.23±0.06 | 60.78±0.06 | 60.21±0.06 | 59.45±0.14 | 58.49±0.14 |
| 3 | 0.0048 | 54.32±0.14 | 53.87±0.17 | 53.06±0.13 | 52.75±0.12 | 52.31±0.12 |
| 4 | 0.0101 | 50.12±0.12 | 49.76±0.13 | 49.17±0.16 | 48.61±0.21 | 48.17±0.09 |
| 5 | 0.0198 | 49.76±0.09 | 49.24±0.23 | 48.81±0.21 | 48.34±0.16 | 47.91±0.13 |
| 6 | 0.0300 | 49.69±0.11 | 49.14±0.15 | 48.75±0.19 | 48.21±0.17 | 47.57±0.11 |
| 7 | 0.0400 | 49.61±0.15 | 49.07±0.19 | 48.64±0.15 | 48.13±0.15 | 47.49±0.06 |
| 8 | 0.0501 | 49.55±0.11 | 49.01±0.09 | 48.57±0.08 | 48.01±0.12 | 47.42±0.14 |
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0009 | 61.23±0.06 | 60.78±0.06 | 60.21±0.06 | 59.45±0.14 | 58.49±0.14 |
| 3 | 0.0048 | 54.32±0.14 | 53.87±0.17 | 53.06±0.13 | 52.75±0.12 | 52.31±0.12 |
| 4 | 0.0101 | 50.12±0.12 | 49.76±0.13 | 49.17±0.16 | 48.61±0.21 | 48.17±0.09 |
| 5 | 0.0198 | 49.76±0.09 | 49.24±0.23 | 48.81±0.21 | 48.34±0.16 | 47.91±0.13 |
| 6 | 0.0300 | 49.69±0.11 | 49.14±0.15 | 48.75±0.19 | 48.21±0.17 | 47.57±0.11 |
| 7 | 0.0400 | 49.61±0.15 | 49.07±0.19 | 48.64±0.15 | 48.13±0.15 | 47.49±0.06 |
| 8 | 0.0501 | 49.55±0.11 | 49.01±0.09 | 48.57±0.08 | 48.01±0.12 | 47.42±0.14 |
表A9 不同温度下[BMIM][SCN]( xA)+H2O (1-xA)的表面张力
Table A9 Surface tension of [BMIM][SCN]( xA)+H2O (1-xA) at different temperatures
| No. | xA | σ/(mN·m-1) | ||||
|---|---|---|---|---|---|---|
| 283.15 K | 293.15 K | 303.15 K | 313.15 K | 323.15 K | ||
| 1 | 0.0000 | 74.18±0.06 | 72.70±0.04 | 71.10±0.01 | 69.48±0.02 | 67.83±0.03 |
| 2 | 0.0009 | 61.23±0.06 | 60.78±0.06 | 60.21±0.06 | 59.45±0.14 | 58.49±0.14 |
| 3 | 0.0048 | 54.32±0.14 | 53.87±0.17 | 53.06±0.13 | 52.75±0.12 | 52.31±0.12 |
| 4 | 0.0101 | 50.12±0.12 | 49.76±0.13 | 49.17±0.16 | 48.61±0.21 | 48.17±0.09 |
| 5 | 0.0198 | 49.76±0.09 | 49.24±0.23 | 48.81±0.21 | 48.34±0.16 | 47.91±0.13 |
| 6 | 0.0300 | 49.69±0.11 | 49.14±0.15 | 48.75±0.19 | 48.21±0.17 | 47.57±0.11 |
| 7 | 0.0400 | 49.61±0.15 | 49.07±0.19 | 48.64±0.15 | 48.13±0.15 | 47.49±0.06 |
| 8 | 0.0501 | 49.55±0.11 | 49.01±0.09 | 48.57±0.08 | 48.01±0.12 | 47.42±0.14 |
| 1 | 卞宜峰, 何国庚, 蔡德华, 等. 吸收式制冷工质对的研究进展[J]. 制冷学报, 2015, 36(6): 17-26. |
| Bian Y F, He G G, Cai D H, et al. Research progress of absorption refrigeration working pairs[J]. Journal of Refrigeration, 2015, 36(6): 17-26. | |
| 2 | Kim Y J, Kim S, Joshi Y K, et al. Thermodynamic analysis of an absorption refrigeration system with ionic-liquid/refrigerant mixture as a working fluid[J]. Energy, 2012, 44(1): 1005-1016. |
| 3 | Zheng D X, Dong L, Huang W J, et al. A review of imidazolium ionic liquids research and development towards working pair of absorption cycle[J]. Renewable and Sustainable Energy Reviews, 2014, 37: 47-68. |
| 4 | 李春喜. 离子液体的溶液热力学模型研究进展[J]. 化工学报, 2020, 71(1): 81-91. |
| Li C X. Recent advances in thermodynamic modelling of ionic liquid solutions[J]. CIESC Journal, 2020, 71(1): 81-91. | |
| 5 | Táboas F, Vallès M, Bourouis M, et al. Flow boiling heat transfer of ammonia/water mixture in a plate heat exchanger[J]. International Journal of Refrigeration, 2010, 33(4): 695-705. |
| 6 | Inoue T, Monde M, Teruya Y. Pool boiling heat transfer in binary mixtures of ammonia/water[J]. International Journal of Heat and Mass Transfer, 2002, 45(22): 4409-4415. |
| 7 | Gao N, Chen G M, Jiang Y Y, et al. Heat capacities of four promising alternatives to lithium bromide aqueous solution in absorption refrigerators[J]. Journal of Chemical & Engineering Data, 2013, 58(11): 3155-3159. |
| 8 | Nasirpour N, Mohammadpourfard M, Zeinali Heris S. Ionic liquids: promising compounds for sustainable chemical processes and applications[J]. Chemical Engineering Research and Design, 2020, 160: 264-300. |
| 9 | Ilconich J, Myers C, Pennline H, et al. Experimental investigation of the permeability and selectivity of supported ionic liquid membranes for CO2/He separation at temperatures up to 125℃[J]. Journal of Membrane Science, 2007, 298(1/2): 41-47. |
| 10 | Singh S K, Savoy A W. Ionic liquids synthesis and applications: an overview[J]. Journal of Molecular Liquids, 2020, 297: 112038. |
| 11 | 姚加, 王冠淇, 陈航, 等. 螯合型离子液体: 合成、性质以及应用[J]. 化工学报, 2018, 69(1): 203-217. |
| Yao J, Wang G Q, Chen H, et al. Chelate ionic liquids: synthesis, properties and applications[J]. CIESC Journal, 2018, 69(1): 203-217. | |
| 12 | Zeng S J, Cao Y K, Li P F, et al. Ionic liquid-based green processes for ammonia separation and recovery[J]. Current Opinion in Green and Sustainable Chemistry, 2020, 25: 100354. |
| 13 | Qu H N, Zhou Y M, Ma Y M, et al. A green catalyst for hydrolysis of cellulose: amino acid protic ionic liquid[J]. Journal of the Taiwan Institute of Chemical Engineers, 2018, 93: 667-673. |
| 14 | Yu L, Liu J Y, Yin W Y, et al. Ionic liquid combined with NiCo2O4/rGO enhances electrochemical oxygen sensing[J]. Talanta, 2020, 209: 120515. |
| 15 | Wang J Y, Jiang H C, Liu Y M, et al. Density and surface tension of pure 1-ethyl-3-methylimidazolium l-lactate ionic liquid and its binary mixtures with water[J]. The Journal of Chemical Thermodynamics, 2011, 43(5): 800-804. |
| 16 | Ghatee M H, Bahrami M, Khanjari N. Measurement and study of density, surface tension, and viscosity of quaternary ammonium-based ionic liquids ([N222(n)]Tf2N)[J]. The Journal of Chemical Thermodynamics, 2013, 65: 42-52. |
| 17 | Wu W, Zhang H Y, You T, et al. Thermodynamic investigation and comparison of absorption cycles using hydrofluoroolefins and ionic liquid[J]. Industrial & Engineering Chemistry Research, 2017, 56(35): 9906-9916. |
| 18 | Freire M G, Carvalho P J, Gardas R L, et al. Solubility of water in tetradecyltrihexylphosphonium-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2008, 53(10): 2378-2382. |
| 19 | 杨许召, 王军, 方云. 含非对称Gemini离子液体二元混合体系的体积和黏度性质[J]. 化工学报, 2019, 70(11): 4131-4142. |
| Yang X Z, Wang J, Fang Y. Volumetric and viscosity properties of binary mixtures containing asymmetrical Gemini ionic liquid[J]. CIESC Journal, 2019, 70(11): 4131-4142. | |
| 20 | de Lucas A, Donate M, Rodríguez J F. Absorption of water vapor into new working fluids for absorption refrigeration systems[J]. Industrial & Engineering Chemistry Research, 2007, 46(1): 345-350. |
| 21 | Kim S, Kohl P A. Theoretical and experimental investigation of an absorption refrigeration system using R134/[bmim][PF6] working fluid[J]. Industrial & Engineering Chemistry Research, 2013, 52(37): 13459-13465. |
| 22 | Castro M C, Arce A, Soto A, et al. Thermophysical characterization of the mixtures of the ionic liquid 1-ethyl-3-methylimidazolium acetate with 1-propanol or 2-propanol[J]. Journal of Chemical & Engineering Data, 2016, 61(7): 2299-2310. |
| 23 | Domańska U, Zawadzki M, Tshibangu M M, et al. Phase equilibria study of {n-hexylisoquinolinium bis{(trifluoromethyl)sulfonyl}imide + aromatic hydrocarbons or an alcohol} binary systems[J]. The Journal of Physical Chemistry B, 2011, 115(14): 4003-4010. |
| 24 | Kilaru P, Baker G A, Scovazzo P. Density and surface tension measurements of imidazolium-, quaternary phosphonium-, and ammonium-based room-temperature ionic liquids: data and correlations[J]. Journal of Chemical & Engineering Data, 2007, 52(6): 2306-2314. |
| 25 | Sánchez L G, Espel J R, Onink F, et al. Density, viscosity, and surface tension of synthesis grade imidazolium, pyridinium, and pyrrolidinium based room temperature ionic liquids[J]. Journal of Chemical & Engineering Data, 2009, 54(10): 2803-2812. |
| 26 | Ayou D S, Currás M R, Salavera D, et al. Performance analysis of absorption heat transformer cycles using ionic liquids based on imidazolium cation as absorbents with 2, 2, 2-trifluoroethanol as refrigerant[J]. Energy Conversion and Management, 2014, 84: 512-523. |
| 27 | Yang X G, Fu Y, Zhang X Q, et al. Liquid-liquid equilibria of benzene + cyclohexane + N, N-dimethyl acetamide + ammonium thiocyanate at 298.15 K and atmospheric pressure[J]. Journal of Chemical & Engineering Data, 2015, 60(4): 971-975. |
| 28 | Li J L, Zhu H, Peng C J, et al. Densities and viscosities for ionic liquids [BMIM][BF4] and [BMIM][Cl] and their binary mixtures at various temperatures and atmospheric pressure[J]. Chinese Journal of Chemical Engineering, 2019, 27(12): 2994-2999. |
| 29 | Ghalami-Choobar B, Fallahkar T N. Thermophysical properties of 1-ethyl-3-methylimidazolium bromide ionic liquid in water + ethylene carbonate mixtures at T = (298.2, 308.2 and 318.2) K[J]. Fluid Phase Equilibria, 2019, 496: 42-60. |
| 30 | Oster K, Goodrich P, Jacquemin J, et al. A new insight into pure and water-saturated quaternary phosphonium-based carboxylate ionic liquids: density, heat capacity, ionic conductivity, thermogravimetric analysis, thermal conductivity and viscosity[J]. The Journal of Chemical Thermodynamics, 2018, 121: 97-111. |
| 31 | Rilo E, Pico J, García-Garabal S, et al. Density and surface tension in binary mixtures of CnMIM-BF4 ionic liquids with water and ethanol[J]. Fluid Phase Equilibria, 2009, 285(1/2): 83-89. |
| 32 | Almeida H F D, Canongia Lopes J N, Rebelo L P N, et al. Densities and viscosities of mixtures of two ionic liquids containing a common cation[J]. Journal of Chemical & Engineering Data, 2016, 61(8): 2828-2843. |
| 33 | Freire M G, Carvalho P J, Fernandes A M, et al. Surface tensions of imidazolium based ionic liquids: anion, cation, temperature and water effect[J]. Journal of Colloid and Interface Science, 2007, 314(2): 621-630. |
| 34 | Martins M A R, Neves C M S S, Kurnia K A, et al. Densities, viscosities and derived thermophysical properties of water-saturated imidazolium-based ionic liquids[J]. Fluid Phase Equilibria, 2016, 407: 188-196. |
| 35 | Liu W W, Cheng L Y, Zhang Y M, et al. The physical properties of aqueous solution of room-temperature ionic liquids based on imidazolium: database and evaluation[J]. Journal of Molecular Liquids, 2008, 140(1/2/3): 68-72. |
| 36 | Xu H T, Zhao D C, Xu P, et al. Conductivity and viscosity of 1-allyl-3-methyl-imidazolium chloride + water and + ethanol from 293.15 K to 333.15 K[J]. Journal of Chemical & Engineering Data, 2005, 50(1): 133-135. |
| 37 | 粟航, 郭开华, 皇甫立霞, 等. 强吸水性离子液体-水工质对吸收式制冷循环性能分析[J]. 制冷学报, 2013, 34(3): 24-30. |
| Su H, Guo K H, Huangpu L X, et al. Study on absorption refrigeration cycle with a new working pair of ionic liquid and water[J]. Journal of Refrigeration, 2013, 34(3): 24-30. | |
| 38 | Zhang X D, Hu D P. Performance simulation of the absorption chiller using water and ionic liquid 1-ethyl-3-methylimidazolium dimethylphosphate as the working pair[J]. Applied Thermal Engineering, 2011, 31(16): 3316-3321. |
| 39 | Wimby J M, Berntsson T S. Viscosity and density of aqueous solutions of lithium bromide, lithium chloride, zinc bromide, calcium chloride and lithium nitrate (1): Single salt solutions[J]. Journal of Chemical & Engineering Data, 1994, 39(1): 68-72. |
| 40 | Okoturo O O, van der Noot T J. Temperature dependence of viscosity for room temperature ionic liquids[J]. Journal of Electroanalytical Chemistry, 2004, 568: 167-181. |
| 41 | Miran M S, Kinoshita H, Yasuda T, et al. Physicochemical properties determined by ΔpKa for protic ionic liquids based on an organic super-strong base with various Brønsted acids[J]. Physical Chemistry Chemical Physics: PCCP, 2012, 14(15): 5178-5186. |
| 42 | Rocha M A A, Neves C M S S, Freire M G, et al. Alkylimidazolium based ionic liquids: impact of cation symmetry on their nanoscale structural organization[J]. The Journal of Physical Chemistry B, 2013, 117(37): 10889-10897. |
| 43 | Yao W, Bjurstroem H, Setterwall F. Surface tension of lithium bromide solutions with heat-transfer additives[J]. Journal of Chemical & Engineering Data, 1991, 36(1): 96-98. |
| 44 | Cumicheo M C, Nobre L C S, Santos A F, et al. Thermophysical properties of 1-butyl-1-methyl-pyrrolidinium dicyanamide + H2O mixtures[J]. Journal of Chemical & Engineering Data, 2015, 60(12): 3766-3775. |
| 45 | Carvalho P J, Neves C M S S, Coutinho J A P. Surface tensions of bis(trifluoromethylsulfonyl)imide anion-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3807-3812. |
| 46 | Martino W, de la Mora J F, Yoshida Y, et al. Surface tension measurements of highly conducting ionic liquids[J]. Green Chemistry, 2006, 8(4): 390-397. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 张龙, 宋孟杰, 邵苛苛, 张旋, 沈俊, 高润淼, 甄泽康, 江正勇. 管翅式换热器迎风侧翅片末端霜层生长模拟研究[J]. 化工学报, 2023, 74(S1): 179-182. |
| [3] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [4] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [5] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [8] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [9] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [10] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [11] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [12] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [13] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [14] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [15] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号