化工学报 ›› 2021, Vol. 72 ›› Issue (8): 4166-4176.DOI: 10.11949/0438-1157.20210172
李燕1( ),蹇亮1,茅沁怡1,潘成思1,蒋平平1,朱永法2,董玉明1(
),蹇亮1,茅沁怡1,潘成思1,蒋平平1,朱永法2,董玉明1( )
)
收稿日期:2021-01-26
修回日期:2021-05-07
出版日期:2021-08-05
发布日期:2021-08-05
通讯作者:
董玉明
作者简介:李燕(1996—),女,硕士研究生,基金资助:
Yan LI1( ),Liang JIAN1,Qinyi MAO1,Chengsi PAN1,Pingping JIANG1,Yongfa ZHU2,Yuming DONG1(
),Liang JIAN1,Qinyi MAO1,Chengsi PAN1,Pingping JIANG1,Yongfa ZHU2,Yuming DONG1( )
)
Received:2021-01-26
Revised:2021-05-07
Online:2021-08-05
Published:2021-08-05
Contact:
Yuming DONG
摘要:
在保证选择性的前提下高效光催化氧化苯甲醇为苯甲醛仍然是当下面临的一个巨大挑战。g-C3N4的价带位置适中,具有温和的氧化能力,已被开发用来光催化氧化苯甲醇以保证反应的选择性,但由于其电子空穴复合率高导致反应的转化率难以提升。由于Bi2O2CO3的超薄片层结构不仅可以增加催化剂的比表面积形成更多的活性中心,同时可以形成局部电场,更有效地分离光生电子-空穴对,因此通过构建Bi2O2CO3/g-C3N4异质结来加快光生载流子分离进而提升反应速率。其中最优的催化剂可以在反应9 h后使苯甲醇完全氧化为苯甲醛,降低了分离成本。
中图分类号:
李燕, 蹇亮, 茅沁怡, 潘成思, 蒋平平, 朱永法, 董玉明. 构建Bi2O2CO3/g-C3N4异质结光催化完全氧化苯甲醇至苯甲醛[J]. 化工学报, 2021, 72(8): 4166-4176.
Yan LI, Liang JIAN, Qinyi MAO, Chengsi PAN, Pingping JIANG, Yongfa ZHU, Yuming DONG. Construction of Bi2O2CO3/g-C3N4 heterojunction photocatalytic complete oxidation of benzyl alcohol to benzaldehyde[J]. CIESC Journal, 2021, 72(8): 4166-4176.
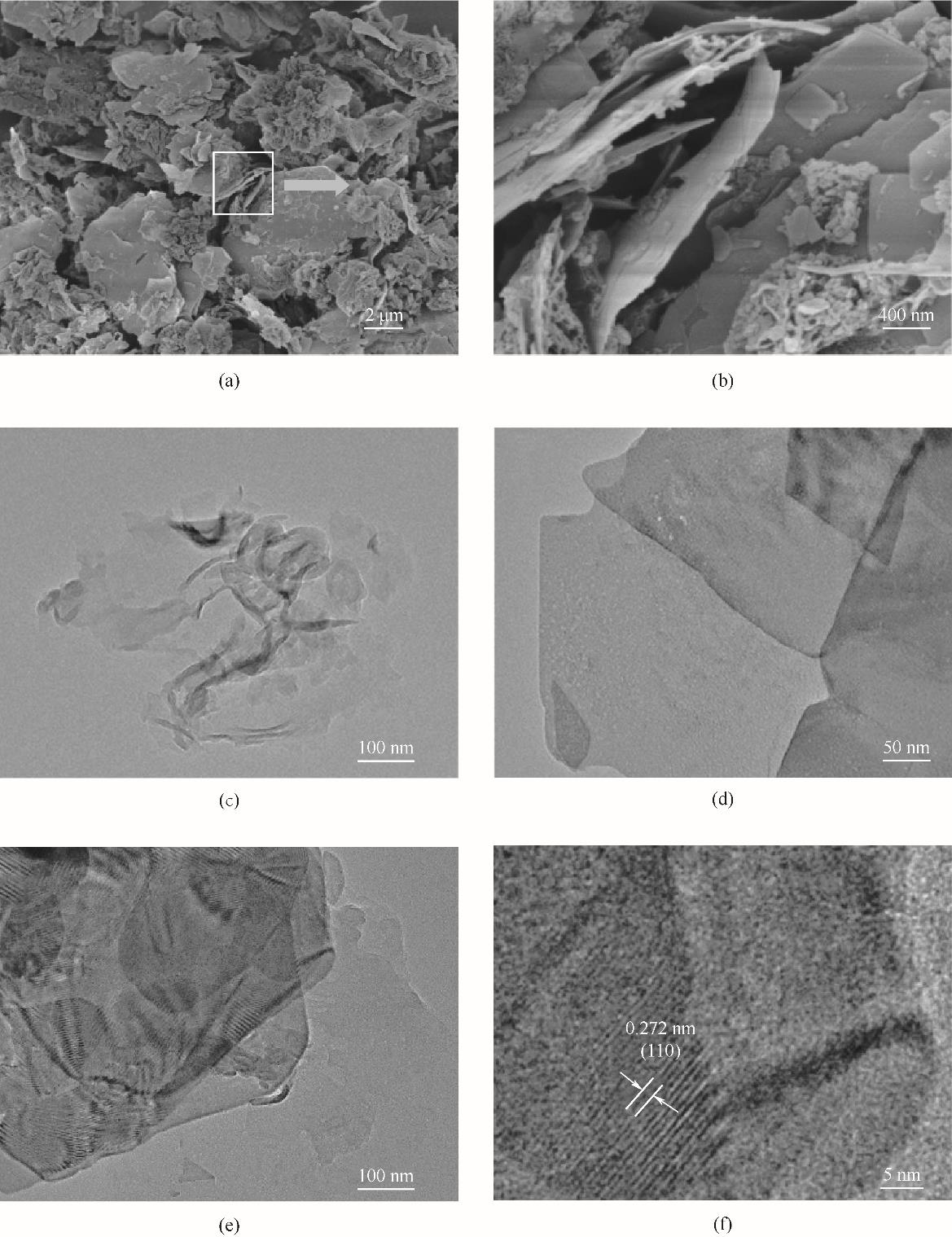
图1 催化剂形貌表征(a),(b) 1.5-Bi2O2CO3/g-C3N4 的SEM图;(c)~(e) g-C3N4、Bi2O2CO3、1.5-Bi2O2CO3/g-C3N4 的TEM图; (f) 1.5-Bi2O2CO3/g-C3N4 的高分辨透射电镜图
Fig.1 Morphology of catalysts
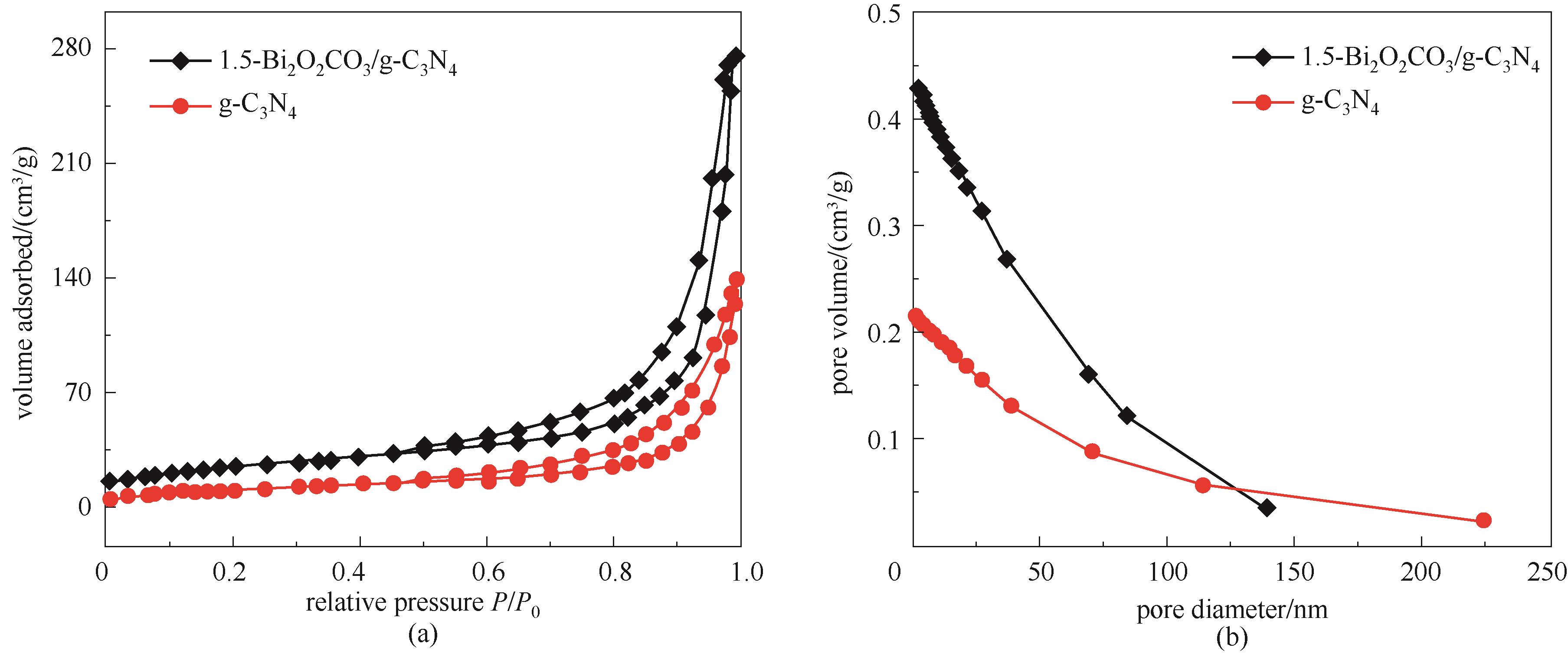
图2 g-C3N4与1.5-Bi2O2CO3/g-C3N4的N2吸附-解吸等温线(a)和相应的孔径分布曲线(b)
Fig.2 N2 adsorption-desorption isotherms (a) and BJH pore size distribution (b) of g-C3N4 and 1.5-Bi2O2CO3/g-C3N4
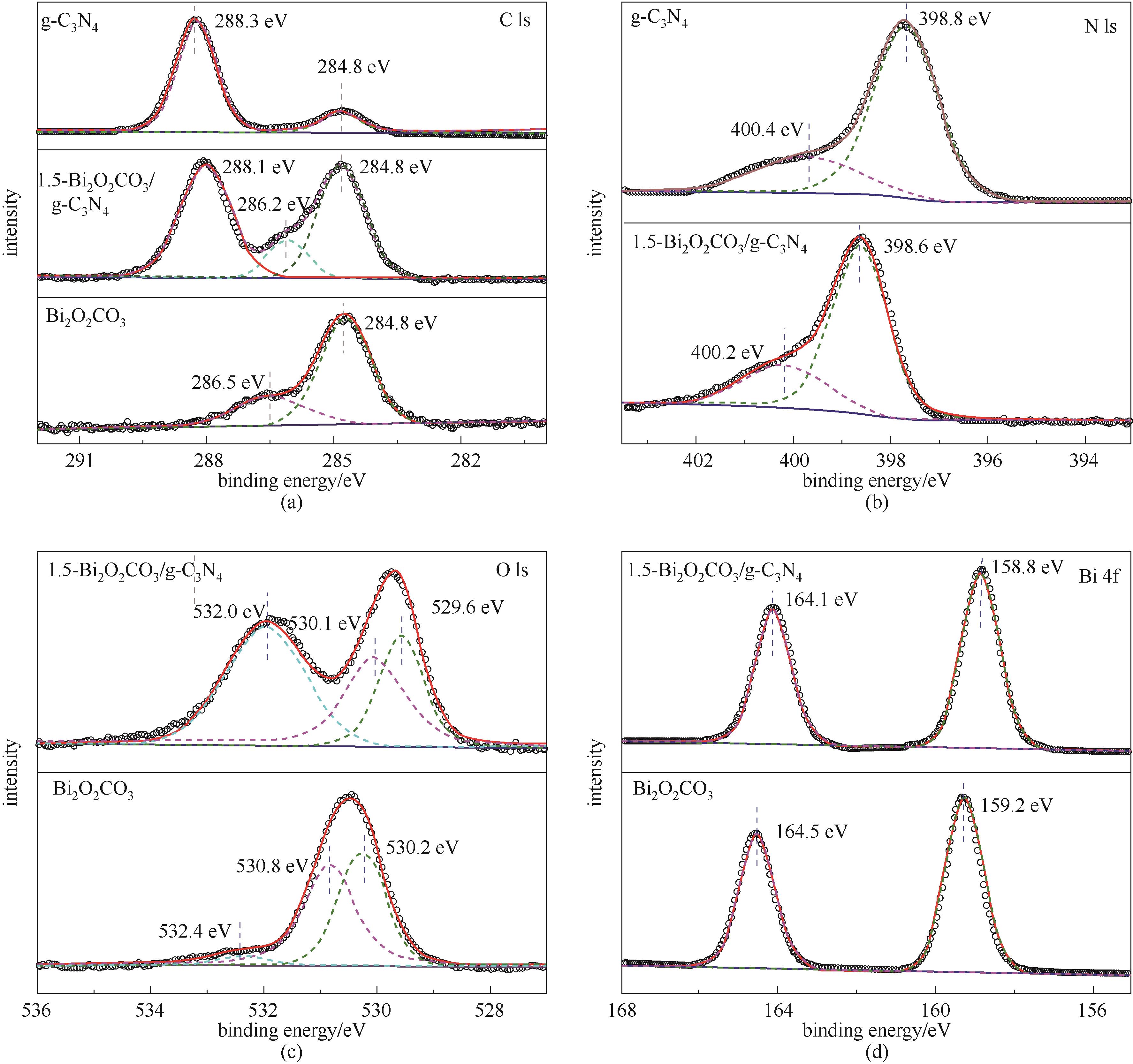
图4 1.5-Bi2O2CO3/g-C3N4 中 C 1s (a)、 N 1s (b)、 O 1s (c)、 Bi 4f (d)的高分辨率XPS光谱图
Fig.4 XPS spectra of C 1s (a), N 1s (b), O 1s (c), Bi 4f (d) in 1.5-Bi2O2CO3/g-C3N4
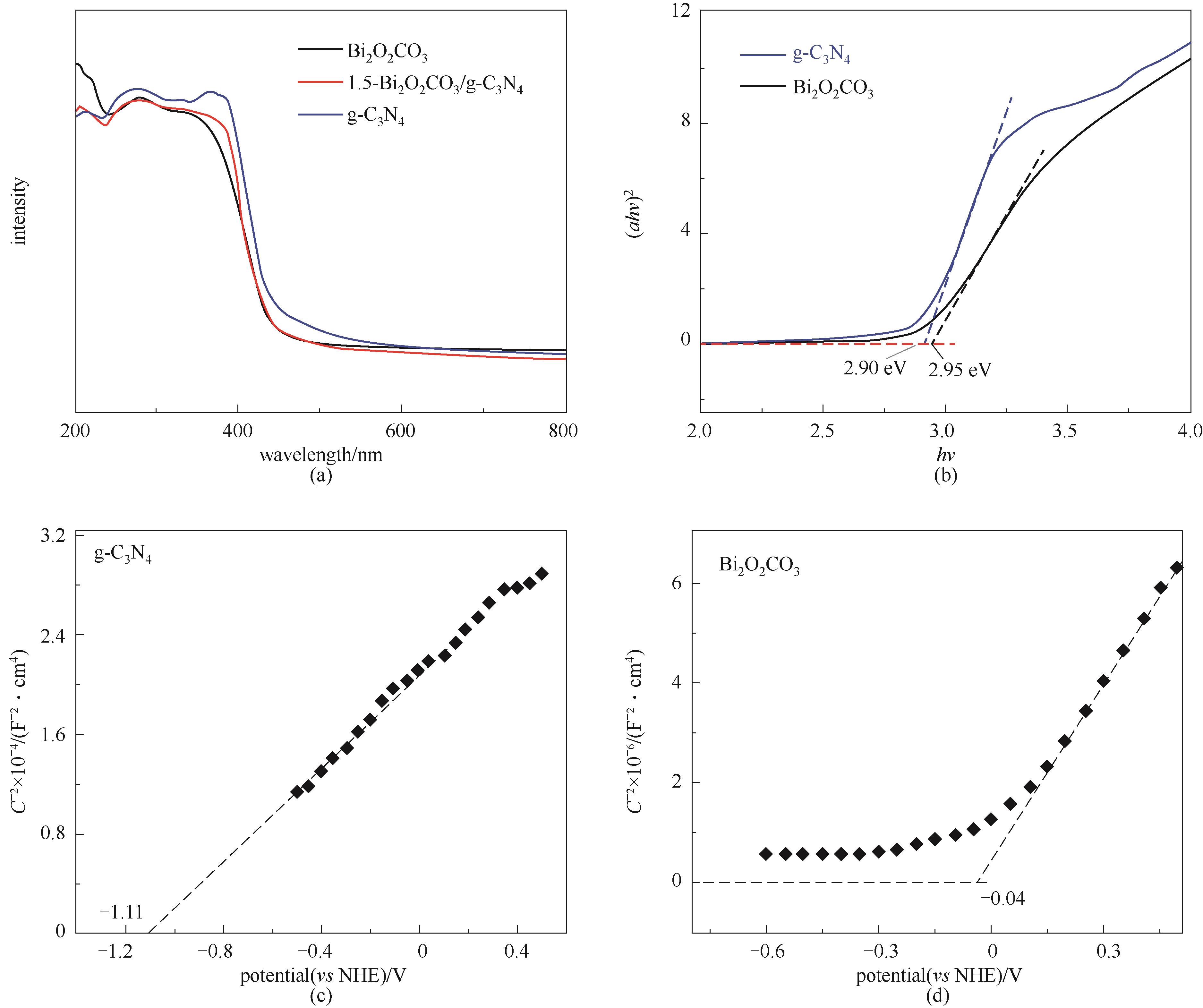
图5 Bi2O2CO3、g-C3N4及1.5-Bi2O2CO3/g-C3N4的紫外-可见漫反射吸收光谱图(a); Bi2O2CO3、g-C3N4的Tauc曲线 (b); g-C3N4 (c)和 Bi2O2CO3 (d) 的Mott-Schottky图
Fig.5 UV-Vis diffuse reflection spectra of Bi2O2CO3, g-C3N4 and 1.5-Bi2O2CO3/g-C3N4 (a); Tauc curves of Bi2O2CO3 and g-C3N4 (b); Mott-Schottky diagram of g-C3N4 (c) and Bi2O2CO3 (d)
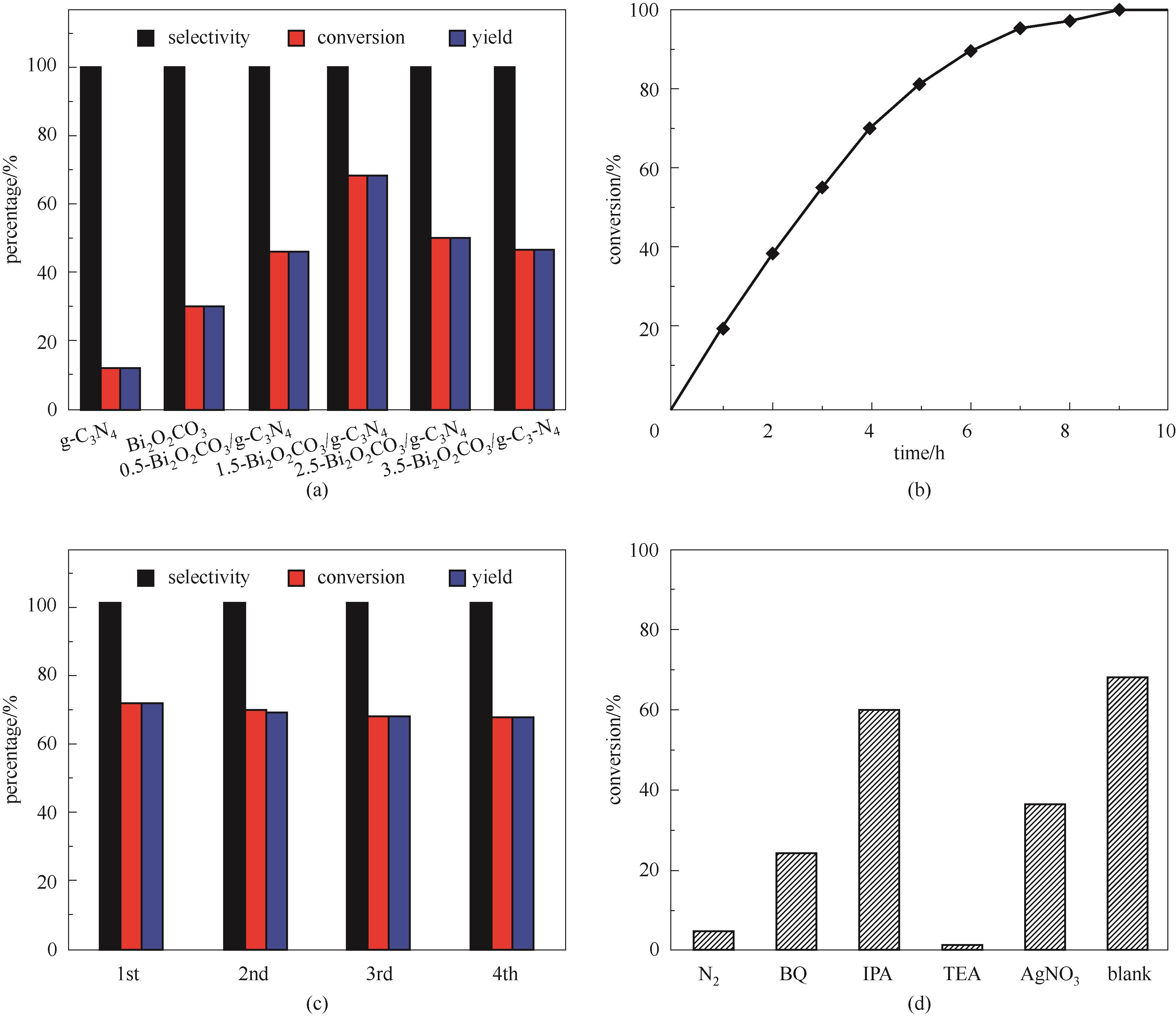
图6 g-C3N4、Bi2O2CO3、x-Bi2O2CO3/g-C3N4 (x=0.5、1.5、2.5、3.5)反应活性对比[测试条件:30 mg催化剂,300 W氙灯(AM 1.5G),光照时间4 h](a); 1.5-Bi2O2CO3/g-C3N4在相同条件下的延长反应时间转化率(b); 1.5-Bi2O2CO3/g-C3N4的稳定性测试[测试条件:30 mg催化剂,300 W氙灯(AM 1.5G),光照时间4 h](c); 1.5-Bi2O2CO3/g-C3N4在反应过程中的自由基捕获实验 (d)
Fig.6 Comparison of reaction activity of g-C3N4, Bi2O2CO3, x-Bi2O2CO3/g-C3N4 (x=0.5, 1.5, 2.5, 3.5) [test conditions: 30 mg catalysts, 300 W xenon lamp (AM 1.5G), illumination time 4 h](a); The conversion of benzyl alcohol on 1.5-Bi2O2CO3/g-C3N4 in 9 h under the same conditions (b); Stability test of 1.5-Bi2O2CO3/g-C3N4 30 mg catalyst [test conditions: 30 mg catalysts, 300 W xenon lamp (AM 1.5G), illumination time 4 h] (c); The conversion of benzyl alcohol after radical capture experiment on 1.5-Bi2O2CO3/g-C3N4 (d)
| 序号 | 催化剂 | 反应条件 | 选择性/% | 转化率/% | 文献 |
|---|---|---|---|---|---|
| 1 | TiO2@COF | white light LED, 30 h | 99.9 | 92.5 | [ |
| 2 | Au-Pd/ZnIn2S4 | λ> 420 nm, 10 h | >99 | 90.6 | [ |
| 3 | NH2-MIL-125(Ti) | white light LED, 40 h | >99 | 88 | [ |
| 4 | N-vacancy-g-C3N4 | AM 1.5, 9 h | >99 | 68.3 | [ |
| 5 | CdS@SnO2; | λ> 420 nm, 8 h | 98 | 78 | [ |
| 6 | Au-BiOCl-OV | λ> 420 nm, 8 h | >99 | 75.6 | [ |
| 7 | Bi4O5Br2 | blue LED, 24 h | >99 | 99.1 | [ |
| 8 | Bi2O2CO3/g-C3N4 | AM 1.5, 9 h | >99.9 | >99.9 | 本工作 |
表1 选择性光催化苯甲醇氧化的相关文献
Table 1 Overview of the literature on selective photocatalytic oxidation of benzyl alcohol
| 序号 | 催化剂 | 反应条件 | 选择性/% | 转化率/% | 文献 |
|---|---|---|---|---|---|
| 1 | TiO2@COF | white light LED, 30 h | 99.9 | 92.5 | [ |
| 2 | Au-Pd/ZnIn2S4 | λ> 420 nm, 10 h | >99 | 90.6 | [ |
| 3 | NH2-MIL-125(Ti) | white light LED, 40 h | >99 | 88 | [ |
| 4 | N-vacancy-g-C3N4 | AM 1.5, 9 h | >99 | 68.3 | [ |
| 5 | CdS@SnO2; | λ> 420 nm, 8 h | 98 | 78 | [ |
| 6 | Au-BiOCl-OV | λ> 420 nm, 8 h | >99 | 75.6 | [ |
| 7 | Bi4O5Br2 | blue LED, 24 h | >99 | 99.1 | [ |
| 8 | Bi2O2CO3/g-C3N4 | AM 1.5, 9 h | >99.9 | >99.9 | 本工作 |
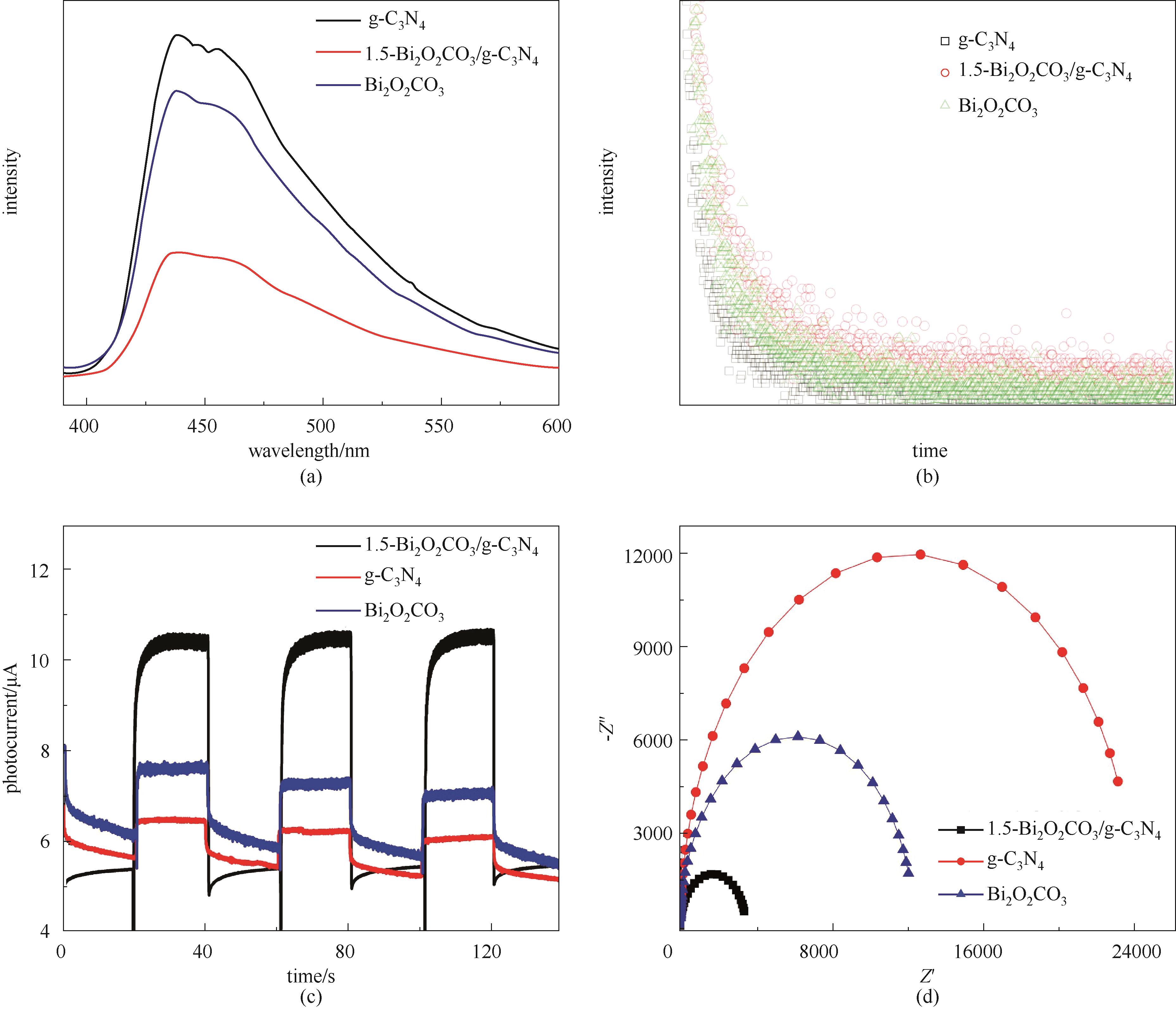
图7 g-C3N4、Bi2O2CO3及1.5-Bi2O2CO3/g-C3N4光催化剂的稳态荧光光谱(a)、时间分辨荧光光谱(b)、瞬态光电流谱图(c)和阻抗谱图(d)
Fig.7 Steady-state fluorescence spectra (a), time-resolved PL decay spectra (b), transient photocurrent spectra (c), the EIS Nyquist plots (d) of photocatalysts for g-C3N4, Bi2O2CO3 and 1.5-Bi2O2CO3/g-C3N4
| 1 | Yang Z W, Xu X Q, Liang X X, et al. MIL-53(Fe)-graphene nanocomposites: efficient visible-light photocatalysts for the selective oxidation of alcohols[J]. Applied Catalysis B: Environmental, 2016, 198: 112-123. |
| 2 | Chen X L, Zhong X, Yuan B W, et al. Defect engineering of nickel hydroxide nanosheets by Ostwald ripening for enhanced selective electrocatalytic alcohol oxidation[J]. Green Chemistry, 2019, 21(3): 578-588. |
| 3 | She H D, Zhou H, Li L S, et al. Nickel-doped excess oxygen defect titanium dioxide for efficient selective photocatalytic oxidation of benzyl alcohol[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(9): 11939-11948. |
| 4 | Sun L Q, Li B, Chu X Y, et al. Synthesis of Si-O-bridged g-C3N4/WO3 2D-heterojunctional nanocomposites as efficient photocatalysts for aerobic alcohol oxidation and mechanism insight[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(11): 9916-9927. |
| 5 | 马淳安, 廖艳梅, 朱英红, 等. Ni-Cu合金电极上苯甲醇的选择性电氧化[J]. 化工学报, 2011, 62(1): 142-146. |
| Ma C A, Liao Y M, Zhu Y H, et al. Selective electro-oxidation of benzyl alcohol on Ni-Cu alloy electrodes[J]. CIESC Journal, 2011, 62(1): 142-146. | |
| 6 | McClelland K P, Weiss E A. Selective photocatalytic oxidation of benzyl alcohol to benzaldehyde or C—C coupled products by visible-light-absorbing quantum dots[J]. ACS Applied Energy Materials, 2019, 2(1): 92-96. |
| 7 | Hao H C, Zhang L, Wang W Z, et al. Photocatalytic hydrogen evolution coupled with efficient selective benzaldehyde production from benzyl alcohol aqueous solution over ZnS-NixSy composites[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(12): 10501-10508. |
| 8 | Zou J H, Wang Z T, Guo W, et al. Photocatalytic selective oxidation of benzyl alcohol over ZnTi-LDH: the effect of surface OH groups[J]. Applied Catalysis B: Environmental, 2020, 260: 118185. |
| 9 | Jing K Q, Ma W, Ren Y H, et al. Hierarchical Bi2MoO6 spheres in situ assembled by monolayer nanosheets toward photocatalytic selective oxidation of benzyl alcohol[J]. Applied Catalysis B: Environmental, 2019, 243: 10-18. |
| 10 | Li X R, Wang J G, Men Y, et al. TiO2 mesocrystal with exposed (001) facets and CdS quantum dots as an active visible photocatalyst for selective oxidation reactions[J]. Applied Catalysis B: Environmental, 2016, 187: 115-121. |
| 11 | Xu C, Yang F, Deng B J, et al. Ti3C2/TiO2 nanowires with excellent photocatalytic performance for selective oxidation of aromatic alcohols to aldehydes[J]. Journal of Catalysis, 2020, 383: 1-12. |
| 12 | Lu G L, Huang X B, Wu Z Y, et al. Construction of covalently integrated core-shell TiO2 nanobelts@COF hybrids for highly selective oxidation of alcohols under visible light[J]. Applied Surface Science, 2019, 493: 551-560. |
| 13 | Wang Z, Feng J J, Li X L, et al. Au-Pd nanoparticles immobilized on TiO2 nanosheet as an active and durable catalyst for solvent-free selective oxidation of benzyl alcohol[J]. Journal of Colloid and Interface Science, 2021, 588: 787-794. |
| 14 | Lv Y, Xu Z L, Kobayashi H, et al. Novel Pd-loaded urchin-like (NH4)xWO3/WO3 as an efficient visible-light-driven photocatalyst for partial conversion of benzyl alcohol[J]. Journal of Alloys and Compounds, 2020, 845: 156225. |
| 15 | Ren Z Y, Zhang J Y, Xiao F X, et al. Revisiting the construction of graphene–CdS nanocomposites as efficient visible-light-driven photocatalysts for selective organic transformation[J]. J.Mater. Chem. A, 2014, 2(15): 5330-5339. |
| 16 | Samanta S, Khilari S, Pradhan D, et al. An efficient, visible light driven, selective oxidation of aromatic alcohols and amines with O2 using BiVO4/g-C3N4 nanocomposite: a systematic and comprehensive study toward the development of a photocatalytic process[J]. ACS Sustainable Chemistry & Engineering, 2017, 5(3): 2562-2577. |
| 17 | Dai Y T, Ren P J, Li Y R, et al. Solid base Bi24O31Br10(OH)δ with active lattice oxygen for the efficient photo-oxidation of primary alcohols to aldehydes[J]. Angewandte Chemie, 2019, 131(19): 6331-6336. |
| 18 | Zhang R Q, Liu Y Y, Wang Z Y, et al. Selective photocatalytic conversion of alcohol to aldehydes by singlet oxygen over Bi-based metal-organic frameworks under UV-Vis light irradiation[J]. Applied Catalysis B: Environmental, 2019, 254: 463-470. |
| 19 | Li H, Qin F, Yang Z, et al. New reaction pathway induced by plasmon for selective benzyl alcohol oxidation on BiOCl possessing oxygen vacancies[J]. Journal of the American Chemical Society, 2017, 139(9): 3513-3521. |
| 20 | Ding J, Xu W, Wan H, et al. Nitrogen vacancy engineered graphitic C3N4-based polymers for photocatalytic oxidation of aromatic alcohols to aldehydes[J]. Applied Catalysis B: Environmental, 2018, 221: 626-634. |
| 21 | 张开莲, 杨凯, 李笑笑, 等. 一步水热合成In2S3/CdIn2S4异质结微球及其光催化性能[J]. 化工学报, 2020, 71(8): 3602-3613. |
| Zhang K L, Yang K, Li X X, et al. One-step hydrothermal synthesis of In2S3/CdIn2S4 heterojunction microsphere and its photocatalytic performance[J]. CIESC Journal, 2020, 71(8): 3602-3613. | |
| 22 | 孙丹阳, 翟婷婷, 黎汉生, 等. g-C3N4的改性策略以及g-C3N4/Ti3C2异质结研究进展[J]. 化工学报, 2020, 71: 1-11. |
| Sun D Y, Zhai T T, Li H S, et al. Research progress on modification strategy of g-C3N4 and g-C3N4/Ti3C2 heterojunction[J]. CIESC Journal, 2020, 71: 1-11. | |
| 23 | Zhang K N, Zhang T N, Cheng G H, et al. Interlayer transition and infrared photodetection in atomically thin type-Ⅱ MoTe₂/MoS₂ van der Waals heterostructures[J]. ACS Nano, 2016, 10(3): 3852-3858. |
| 24 | Yang G, Chen D M, Ding H, et al. Well-designed 3D ZnIn2S4 nanosheets/TiO2 nanobelts as direct Z-scheme photocatalysts for CO2 photoreduction into renewable hydrocarbon fuel with high efficiency[J]. Applied Catalysis B: Environmental, 2017, 219: 611-618. |
| 25 | Liao G F, Gong Y, Zhang L, et al. Semiconductor polymeric graphitic carbon nitride photocatalysts: the “holy grail” for the photocatalytic hydrogen evolution reaction under visible light[J]. Energy & Environmental Science, 2019, 12(7): 2080-2147. |
| 26 | Chen D M, Wang K W, Xiang D G, et al. Significantly enhancement of photocatalytic performances via core-shell structure of ZnO@mpg-C3N4[J]. Applied Catalysis B: Environmental, 2014, 147: 554-561. |
| 27 | Chen D M, Wang K W, Hong W Z, et al. Visible light photoactivity enhancement via CuTCPP hybridized g-C3N4 nanocomposite[J]. Applied Catalysis B: Environmental, 2015, 166/167: 366-373. |
| 28 | Zhang W Y, Bariotaki A, Smonou I, et al. Visible-light-driven photooxidation of alcohols using surface-doped graphitic carbon nitride[J]. Green Chemistry, 2017, 19(9): 2096-2100. |
| 29 | Xing C S, Wu Z D, Jiang D L, et al. Hydrothermal synthesis of In2S3/g-C3N4 heterojunctions with enhanced photocatalytic activity[J]. Journal of Colloid and Interface Science, 2014, 433: 9-15. |
| 30 | 何志桥, 陈锦萍, 童丽丽, 等. BiOCl/g-C3N4异质结催化剂可见光催化还原CO2[J]. 化工学报, 2016, 67(11): 4634-4642. |
| He Z Q, Chen J P, Tong L L, et al. BiOCl/g-C3N4 heterojunction catalyst for efficient photocatalytic reduction of CO2 under visible light[J]. CIESC Journal, 2016, 67(11): 4634-4642. | |
| 31 | Chen L, Hua H, Yang Q, et al. Visible-light photocatalytic activity of Ag2O coated Bi2WO6 hierarchical microspheres assembled by nanosheets[J]. Applied Surface Science, 2015, 327: 62-67. |
| 32 | Kim K, Nam S K, Park J H, et al. Growth of BiVO4 nanoparticles on a WO3 porous scaffold: improved water-splitting by high band-edge light harvesting[J]. Journal of Materials Chemistry A, 2019, 7(9): 4480-4485. |
| 33 | Wang G Z, Luo X K, Huang Y H, et al. BiOX/BiOY (X, Y = F, Cl, Br, I) superlattices for visible light photocatalysis applications[J]. RSC Advances, 2016, 6(94): 91508-91516. |
| 34 | Zhang G Y, Wang J J, Shen X Q, et al. Br-doped Bi2O2CO3 nanosheets with improved electronic structure and accelerated charge migration for outstanding photocatalytic behavior[J]. Applied Surface Science, 2019, 470: 63-73. |
| 35 | Zhao H P, Li G F, Tian F, et al. g-C3N4 surface-decorated Bi2O2CO3 for improved photocatalytic performance: theoretical calculation and photodegradation of antibiotics in actual water matrix[J]. Chemical Engineering Journal, 2019, 366: 468-479. |
| 36 | Lan Y L, Li Z S, Xie W, et al. In situ fabrication of I-doped Bi2O2CO3/g-C3N4 heterojunctions for enhanced photodegradation activity under visible light[J]. Journal of Hazardous Materials, 2020, 385: 121622. |
| 37 | Ma Y J, Bian Y, Tan P F, et al. Simple and facile ultrasound-assisted fabrication of Bi2O2CO3/g-C3N4 composites with excellent photoactivity[J]. Journal of Colloid and Interface Science, 2017, 497: 144-154. |
| 38 | 陈克龙, 黄建花. g-C3N4-CdS-NiS2复合纳米管的制备及可见光催化分解水制氢[J]. 化工学报, 2020, 71(1): 397-408. |
| Chen K L, Huang J H. G-C3N4-CdS-NiS2 composite nanotube: synthesis and its photocatalytic activity for H2 generation under visible light[J]. CIESC Journal, 2020, 71(1): 397-408. | |
| 39 | Zhang R Y, Ran T, Cao Y H, et al. Oxygen activation of noble-metal-free g-C3N4/α-Ni(OH)2 to control the toxic byproduct of photocatalytic nitric oxide removal[J]. Chemical Engineering Journal, 2020, 382: 123029. |
| 40 | Yang B, Lv K, Li Q, et al. Photosensitization of Bi2O2CO3 nanoplates with amorphous Bi2S3 to improve the visible photoreactivity towards NO oxidation[J]. Applied Surface Science, 2019, 495: 143561. |
| 41 | Hao Q, Xie C A, Huang Y M, et al. Accelerated separation of photogenerated charge carriers and enhanced photocatalytic performance of g-C3N4 by Bi2S3 nanoparticles[J]. Chinese Journal of Catalysis, 2020, 41(2): 249-258. |
| 42 | Liu S, Zhao M Y, He Z T, et al. Preparation of a p-n heterojunction 2D BiOI nanosheet/1DBiPO4 nanorod composite electrode for enhanced visible light photoelectrocatalysis[J]. Chinese Journal of Catalysis, 2019, 40(3): 446-457. |
| 43 | Jin J, Yu J G, Guo D P, et al. A hierarchical Z-scheme CdS-WO3 photocatalyst with enhanced CO2 reduction activity[J]. Small, 2015, 11(39): 5262-5271. |
| 44 | Heidari S, Haghighi M, Shabani M. Sono-photodeposition of Ag over sono-fabricated mesoporous Bi2Sn2O7-two dimensional carbon nitride: type-Ⅱ plasmonic nano-heterojunction with simulated sunlight-driven elimination of drug[J]. Chemical Engineering Journal, 2020, 389: 123418. |
| 45 | Feng C J, Yang X L, Sun Z L, et al. Dual interfacial synergism in Au-Pd/ZnIn2S4 for promoting photocatalytic selective oxidation of aromatic alcohol[J]. Applied Surface Science, 2020, 501: 144018. |
| 46 | Wu Z Y, Huang X B, Zheng H Y, et al. Aromatic heterocycle-grafted NH2-MIL-125(Ti) via conjugated linker with enhanced photocatalytic activity for selective oxidation of alcohols under visible light[J]. Applied Catalysis B: Environmental, 2018, 224: 479-487. |
| 47 | Liu Y, Zhang P, Tian B Z, et al. Core-shell structural CdS@SnO2 nanorods with excellent visible-light photocatalytic activity for the selective oxidation of benzyl alcohol to benzaldehyde[J]. ACS Applied Materials & Interfaces, 2015, 7(25): 13849-13858. |
| 48 | Zheng C X, He G P, Xiao X, et al. Selective photocatalytic oxidation of benzyl alcohol into benzaldehyde with high selectivity and conversion ratio over Bi4O5Br2 nanoflakes under blue LED irradiation[J]. Applied Catalysis B: Environmental, 2017, 205: 201-210. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [3] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [4] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [5] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [6] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [7] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [8] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [9] | 杨百玉, 寇悦, 姜峻韬, 詹亚力, 王庆宏, 陈春茂. 炼化碱渣湿式氧化预处理过程DOM的化学转化特征[J]. 化工学报, 2023, 74(9): 3912-3920. |
| [10] | 陈哲文, 魏俊杰, 张玉明. 超临界水煤气化耦合SOFC发电系统集成及其能量转化机制[J]. 化工学报, 2023, 74(9): 3888-3902. |
| [11] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [12] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [13] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [14] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [15] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号