化工学报 ›› 2021, Vol. 72 ›› Issue (9): 4708-4717.DOI: 10.11949/0438-1157.20210239
李海涛( ),孟平凡,张因,武瑞芳,黄鑫,班丽君,韩旭东,席琳,王兴皓,田博辉,赵永祥(
),孟平凡,张因,武瑞芳,黄鑫,班丽君,韩旭东,席琳,王兴皓,田博辉,赵永祥( )
)
收稿日期:2021-02-07
修回日期:2021-06-29
出版日期:2021-09-05
发布日期:2021-09-05
通讯作者:
赵永祥
作者简介:李海涛(1982—),男,博士,副教授,基金资助:
Haitao LI( ),Pingfan MENG,Yin ZHANG,Ruifang WU,Xin HUANG,Lijun BAN,Xudong HAN,Lin XI,Xinghao WANG,Bohui TIAN,Yongxiang ZHAO(
),Pingfan MENG,Yin ZHANG,Ruifang WU,Xin HUANG,Lijun BAN,Xudong HAN,Lin XI,Xinghao WANG,Bohui TIAN,Yongxiang ZHAO( )
)
Received:2021-02-07
Revised:2021-06-29
Online:2021-09-05
Published:2021-09-05
Contact:
Yongxiang ZHAO
摘要:
甲醛与乙炔缩合制取1,4-丁炔二醇是乙炔化工的重要方向。探讨铜基催化剂在甲醛乙炔化反应中的演变及催化作用机制,并开发更高效的甲醛乙炔化催化剂是一个值得科学与产业界关注的课题。本工作在前期页硅酸铜催化剂制备及甲醛乙炔化性能研究基础上,进一步通过热处理温度的调整,在焙烧温度为650℃时,构筑了限域于SiO2网络结构中的CuO纳米晶催化剂。CuO纳米晶适宜的化学环境,使其在甲醛乙炔化反应初始阶段快速形成活性炔化亚铜,获得了1,4-丁炔二醇收率80%左右的结果,克服了页硅酸铜物种转化为炔化亚铜速率慢、诱导期长的弊端。SiO2网络结构的限域作用也进一步抑制了活性组分的流失,在6次套用实验中1,4-丁炔二醇收率几乎不变,呈现出良好的使用稳定性。
中图分类号:
李海涛, 孟平凡, 张因, 武瑞芳, 黄鑫, 班丽君, 韩旭东, 席琳, 王兴皓, 田博辉, 赵永祥. SiO2网络限域CuO纳米晶的甲醛乙炔化性能研究[J]. 化工学报, 2021, 72(9): 4708-4717.
Haitao LI, Pingfan MENG, Yin ZHANG, Ruifang WU, Xin HUANG, Lijun BAN, Xudong HAN, Lin XI, Xinghao WANG, Bohui TIAN, Yongxiang ZHAO. Study on formaldehyde ethynylation performance of CuO nanocrystalline confined in SiO2 networks[J]. CIESC Journal, 2021, 72(9): 4708-4717.
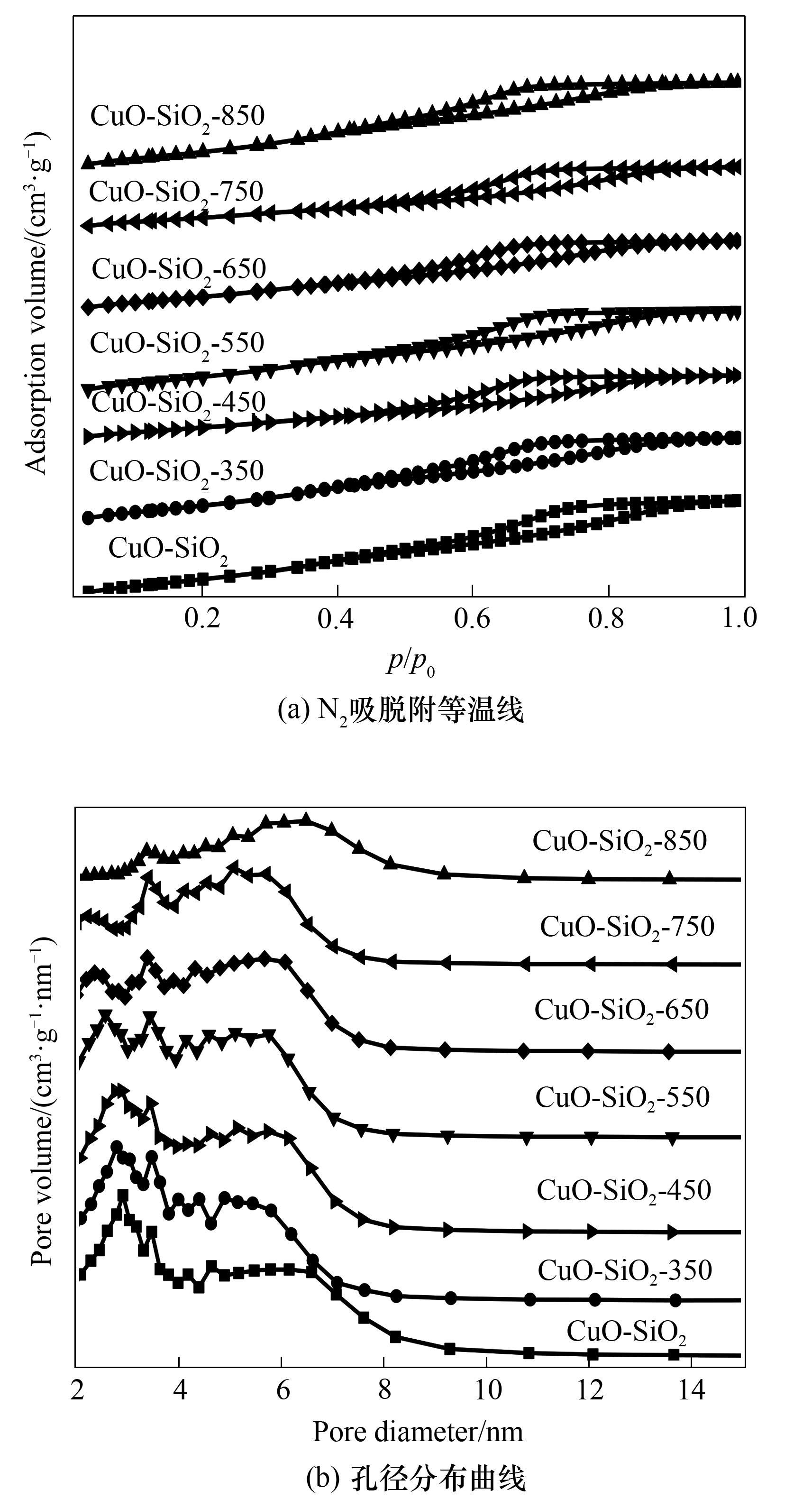
图2 不同温度处理的CuO-SiO2催化剂的N2吸、脱附等温线与孔径分布曲线
Fig.2 N2 adsorption/desorption isotherm and pore size distribution curves of CuO-SiO2 catalyst treated with different temperatures
| Catalyst | 比表面积ABET /(m2 ·g-1)① | 孔径Dpore/nm① | 孔体积VTotal/(cm3·g-1)① | Cuo晶粒尺寸DCuO/nm② |
|---|---|---|---|---|
| CuO-SiO2 | 549 | 5.05 | 0.69 | — |
| CuO-SiO2-350 | 532 | 4.75 | 0.63 | — |
| CuO-SiO2-450 | 502 | 4.94 | 0.62 | — |
| CuO-SiO2-550 | 499 | 4.89 | 0.61 | — |
| CuO-SiO2-650 | 392 | 5.43 | 0.51 | 5.6 |
| CuO-SiO2-750 | 312 | 5.63 | 0.45 | 8.6 |
| CuO-SiO2-850 | 150 | 7.36 | 0.28 | 11.4 |
表1 催化剂的织构性能和CuO晶粒尺寸
Table 1 Textural properties and CuO crystalline size of catalysts
| Catalyst | 比表面积ABET /(m2 ·g-1)① | 孔径Dpore/nm① | 孔体积VTotal/(cm3·g-1)① | Cuo晶粒尺寸DCuO/nm② |
|---|---|---|---|---|
| CuO-SiO2 | 549 | 5.05 | 0.69 | — |
| CuO-SiO2-350 | 532 | 4.75 | 0.63 | — |
| CuO-SiO2-450 | 502 | 4.94 | 0.62 | — |
| CuO-SiO2-550 | 499 | 4.89 | 0.61 | — |
| CuO-SiO2-650 | 392 | 5.43 | 0.51 | 5.6 |
| CuO-SiO2-750 | 312 | 5.63 | 0.45 | 8.6 |
| CuO-SiO2-850 | 150 | 7.36 | 0.28 | 11.4 |
| Catalyst | Before reaction | After reaction | |||||
|---|---|---|---|---|---|---|---|
| Peak binding energy/eV | Cu2+(Ⅰ)/Cu2+(Ⅱ) | Cu/Si | Peak kinetic energy/eV | Cu+/Cu2+ | |||
| Cu2+(Ⅰ) | Cu2+(Ⅱ) | Cu+ | Cu2+ | ||||
| CuO-SiO2-450 | 934.1 | 936.3 | 0.05 | 0.54 | 915.0 | 917.3 | 1.71 |
| CuO-SiO2-650 | 934.4 | 936.6 | 0.17 | 0.36 | 915.1 | 917.7 | 4.82 |
| CuO-SiO2-850 | 934.7 | 936.8 | 2.08 | 0.13 | 915.0 | 917.5 | 3.48 |
表2 CuO-SiO2中Cu形态的化学环境
Table 2 Chemical environment of Cu species in CuO-SiO2
| Catalyst | Before reaction | After reaction | |||||
|---|---|---|---|---|---|---|---|
| Peak binding energy/eV | Cu2+(Ⅰ)/Cu2+(Ⅱ) | Cu/Si | Peak kinetic energy/eV | Cu+/Cu2+ | |||
| Cu2+(Ⅰ) | Cu2+(Ⅱ) | Cu+ | Cu2+ | ||||
| CuO-SiO2-450 | 934.1 | 936.3 | 0.05 | 0.54 | 915.0 | 917.3 | 1.71 |
| CuO-SiO2-650 | 934.4 | 936.6 | 0.17 | 0.36 | 915.1 | 917.7 | 4.82 |
| CuO-SiO2-850 | 934.7 | 936.8 | 2.08 | 0.13 | 915.0 | 917.5 | 3.48 |
| Catalyst | Cu in mother liquid/(mg/L) | ||
|---|---|---|---|
| 1 Cycle | 3 Cycles | 6 Cycles | |
| CuO-SiO2-450 | 33.8 | 35.2 | 36.5 |
| CuO-SiO2-650 | 35.7 | 37.3 | 36.2 |
| CuO-SiO2-850 | 37.9 | 39.5 | 38.4 |
表3 铜在不同催化剂中的浸出量
Table 3 Leaching content of Cu in different catalysts
| Catalyst | Cu in mother liquid/(mg/L) | ||
|---|---|---|---|
| 1 Cycle | 3 Cycles | 6 Cycles | |
| CuO-SiO2-450 | 33.8 | 35.2 | 36.5 |
| CuO-SiO2-650 | 35.7 | 37.3 | 36.2 |
| CuO-SiO2-850 | 37.9 | 39.5 | 38.4 |
| 1 | Dudzińska A. Analysis of sorption and desorption of unsaturated hydrocarbons: ethylene, propylene and acetylene on hard coals[J]. Fuel, 2019, 246: 232-243. |
| 2 | Cai Y C, Liu X C. Mechanical properties test of pavement base or subbase made of solid waste stabilized by acetylene sludge and fly ash[J]. AIP Advances, 2020, 10(6): 065022. |
| 3 | 杨冲, 林旭枫, 张金锋, 等. 正己烷–异丙醇共沸体系液液相平衡数据测定及关联[J]. 化工学报, 2020, 71(7): 3009-3017. |
| Yang C, Lin X F, Zhang J F, et al. Measurement and correlation of liquid-liquid equilibrium data for n-hexane-isopropanol azeotropic system[J]. CIESC Journal, 2020, 71(7): 3009-3017. | |
| 4 | Heisig C, Diedenhoven J, Jensen C, et al. Selective hydrogenation of biomass-derived succinic acid: reaction network and kinetics[J]. Chemical Engineering & Technology, 2020, 43(3): 484-492. |
| 5 | 蒋瑞, 胡冬冬, 刘涛, 等. 热塑性聚醚酯弹性体硬段含量对其超临界CO2发泡行为的影响[J]. 化工学报, 2020, 71(2): 871-878. |
| Jiang R, Hu D D, Liu T, et al. Effect of hard segment content on microcellular foaming process of thermoplastic polyether ester elastomer using supercritical CO2 as blowing agent[J]. CIESC Journal, 2020, 71(2): 871-878. | |
| 6 | Le S D, Nishimura S. Highly selective synthesis of 1, 4-butanediol via hydrogenation of succinic acid with supported Cu-Pd alloy nanoparticles[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(22): 18483-18492. |
| 7 | Raju M A, Gidyonu P, Nagaiah P, et al. Mesoporous silica-supported copper catalysts for dehydrogenation of biomass-derived 1, 4-butanediol to gamma butyrolactone in a continuous process at atmospheric pressure[J]. Biomass Conversion and Biorefinery, 2019, 9(4): 719-726. |
| 8 | Chaudhari R V, Rode C V, Jaganathan R, et al. Process for the conversion of 1, 4 butynediol to 1, 4butanediol, or a mixture of 1, 4 butenediol and 1, 4 butanediol: US6469221[P]. 2002-10-22. |
| 9 | Lu C Y, Wang Y, Zhang R G, et al. Preparation of an unsupported copper-based catalyst for selective hydrogenation of acetylene from Cu2O nanocubes[J]. ACS Applied Materials & Interfaces, 2020, 12(41): 46027-46036. |
| 10 | Zak D. Butynediol production: US4085151[P]. 1978-04-18.. |
| 11 | Fremont J. Malachite preparation: US4107082 A[P]. 1978-08-15. |
| 12 | 郑艳, 孙自瑾, 王永钊, 等. CuO-Bi2O3/SiO2-MgO催化剂的制备及炔化性能[J]. 分子催化, 2012, 26(3): 233-238. |
| Zheng Y, Sun Z J, Wang Y Z, et al. Preparation of CuO-Bi2O3/SiO2-MgO catalyst and its ethynylation performance[J]. Journal of Molecular Catalysis, 2012, 26(3): 233-238. | |
| 13 | 王俊俊, 李海涛, 马志强, 等. 磁性CuO-Bi2O3/Fe3O4-SiO2-MgO催化剂的制备及甲醛乙炔化性能[J]. 化工学报, 2015, 66(6): 2098-2104. |
| Wang J J, Li H T, Ma Z Q, et al. Preparation of magnetic CuO-Bi2O3/Fe3O4-SiO2-MgO catalyst and its catalytic performance for formaldehyde ethynylation[J]. CIESC Journal, 2015, 66(6): 2098-2104. | |
| 14 | 马志强, 张洪喜, 李海涛, 等. 核壳结构CuO-Bi2O3@meso-SiO2催化剂的制备及甲醛乙炔化性能[J]. 工业催化, 2015, 23(5): 344-348. |
| Ma Z Q, Zhang H X, Li H T, et al. Preparation of core-shell CuO-Bi2O3@meso-SiO2 catalyst and its catalytic performance for formaldehyde ethynylation[J]. Industrial Catalysis, 2015, 23(5): 344-348. | |
| 15 | 杨国峰, 李海涛, 张鸿喜, 等. NaOH浓度对Cu2O结构及甲醛乙炔化性能的影响[J]. 分子催化, 2016, 30(6): 540-546. |
| Yang G F, Li H T, Zhang H X, et al. Effect of Na OH concentration on structure and catalytic performance of Cu2O for formaldehyde ethynylation[J]. Journal of Molecular Catalysis (China), 2016, 30(6): 540-546. | |
| 16 | 李海涛, 牛珠珠, 杨国峰, 等. Cu2O/TiO2催化甲醛乙炔化反应的载体效应[J]. 化工学报, 2018, 69(6): 2512-2518. |
| Li H T, Niu Z Z, Yang G F, et al. Effect of Cu2O/TiO2 catalyst support in formaldehyde ethynylation[J]. CIESC Journal, 2018, 69(6): 2512-2518. | |
| 17 | 李海涛, 郝全爱, 王志鹏, 等. 不同沉淀剂制备CuO-ZnO催化剂甲醛乙炔化反应性能[J]. 分子催化, 2019, 33(2): 124-131. |
| LI H T, HAO Q A, WANG Z P, et al. Study on catalytic performance of CuO-ZnO catalyst prepared by different precipitants[J]. Journal of Molecular Catalysis (China), 2019, 33(2): 124-131. | |
| 18 | Wang Z P, Ban L J, Meng P F, et al. Ethynylation of formaldehyde over binary Cu-based catalysts: study on synergistic effect between Cu+ species and acid/base sites[J]. Nanomaterials, 2019, 9(7): 1038. |
| 19 | Wang Z P, Ban L J, Meng P F, et al. Ethynylation of formaldehyde over CuO/SiO2 catalysts modified by Mg species: effects of the existential states of Mg species[J]. Nanomaterials, 2019, 9(8): 1137. |
| 20 | Li H T, Ban L J, Niu Z Z, et al. Application of CuxO-FeyOz nanocatalysts in ethynylation of formaldehyde[J]. Nanomaterials, 2019, 9(9): 1301. |
| 21 | Guerreiro E D, Gorriz O F, Larsen G, et al. Cu/SiO2 catalysts for methanol to methyl formate dehydrogenation: a comparative study using different preparation techniques[J]. Applied Catalysis A: General, 2000, 204(1): 33-48. |
| 22 | Brands D S, Poels E K, Bliek A. Ester hydrogenolysis over promoted Cu/SiO2 catalysts[J]. Applied Catalysis A: General, 1999, 184(2): 279-289. |
| 23 | Chen L, Guo P, Qiao M, et al. Cu/SiO2 catalysts prepared by the ammonia-evaporation method: Texture, structure, and catalytic performance in hydrogenation of dimethyl oxalate to ethylene glycol[J]. Journal of Catalysis, 2008, 257(1): 172-180. |
| 24 | Wang Z Q, Xu Z N, Peng S Y, et al. High-performance and long-lived Cu/SiO2 nanocatalyst for CO2 hydrogenation[J]. ACS Catalysis, 2015, 5(7): 4255-4259. |
| 25 | Ding T M, Tian H S, Liu J C, et al. Highly active Cu/SiO2 catalysts for hydrogenation of diethyl malonate to 1, 3-propanediol[J]. Chinese Journal of Catalysis, 2016, 37(4): 484-493. |
| 26 | 杨亚玲, 张博, 李伟, 等. 焙烧温度对草酸二甲酯加氢制乙二醇催化剂Cu/SiO2的影响[J]. 工业催化, 2010, 18(6): 28-31. |
| Yang Y L, Zhang B, Li W, et al. Effects of calcinations temperature on the properties of Cu/SiO2 catalyst for hydrogenation of dimethyl oxalate to ethylene glycol[J]. Industrial Catalysis, 2010, 18(6): 28-31. | |
| 27 | Li H T, Ban L J, Wang Z P, et al. Regulation of Cu species in CuO/SiO2 and its structural evolution in ethynylation reaction[J]. Nanomaterials, 2019, 9(6): 842. |
| 28 | Wang Z P, Niu Z Z, Hao Q, et al. Enhancing the ethynylation performance of CuO-Bi2O3 nanocatalysts by tuning Cu-Bi interactions and phase structures[J]. Catalysts, 2019, 9(1): 35. |
| 29 | 王志鹏, 牛珠珠, 班丽君, 等. 不同晶相TiO2负载Cu2O催化甲醛乙炔化反应[J]. 高等学校化学学报, 2019, 40(2): 334-341. |
| Wang Z P, Niu Z Z, Ban L J, et al. Formaldehyde ethynylation reaction over Cu2O supported on TiO2 with different phases[J]. Chemical Journal of Chinese Universities, 2019, 40(2): 334-341. | |
| 30 | 李海涛, 班丽君, 牛珠珠, 等. 制备条件对Cu2O结构及甲醛乙炔化性能的影响[J]. 分子催化, 2019, 33(3): 237-244. |
| Li H T, Ban L J, Niu Z Z, et al. Effect of preparation condition on structure and catalytic performance of Cu2O for formaldehyde ethynylation[J]. Journal of Molecular Catalysis (China), 2019, 33(3): 237-244. | |
| 31 | Dong F, Ding G Q, Zheng H Y, et al. Highly dispersed Cu nanoparticles as an efficient catalyst for the synthesis of the biofuel 2-methylfuran[J]. Catalysis Science & Technology, 2016, 6(3): 767-779. |
| 32 | Gong J L, Yue H R, Zhao Y J, et al. Synthesis of ethanol via syngas on Cu/SiO2 catalysts with balanced Cu0-Cu+ sites[J]. Journal of the American Chemical Society, 2012, 134(34): 13922-13925. |
| 33 | Zou G, Li H, Zhang D, et al. Well-aligned arrays of CuO nanoplatelets[J]. The Journal of Physical Chemistry B, 2006, 110(4): 1632-1637. |
| 34 | Wang Z, Liu Q S, Yu J F, et al. Surface structure and catalytic behavior of silica-supported copper catalysts prepared by impregnation and Sol-gel methods[J]. Applied Catalysis A: General, 2003, 239(1/2): 87-94. |
| 35 | Cordoba G, Arroyo R, Fierro J L G, et al. Study of xerogel-glass transition of CuO/SiO2[J]. Journal of Solid State Chemistry, 1996, 123(1): 93-99. |
| 36 | Díaz G, Pérez-Hernández R, Gómez-Cortés A, et al. CuO-SiO2 sol-gel catalysts: characterization and catalytic properties for NO reduction[J]. Journal of Catalysis, 1999, 187(1): 1-14. |
| 37 | Toupance T, Kermarec M, Lambert J F, et al. Conditions of formation of copper phyllosilicates in silica-supported copper catalysts prepared by selective adsorption[J]. The Journal of Physical Chemistry B, 2002, 106(9): 2277-2286. |
| 38 | Kliche G, Popovic Z V. Far-infrared spectroscopic investigations on CuO[J]. Physical Review B, Condensed Matter, 1990, 42(16): 10060-10066. |
| 39 | Dunning T H, McKoy V. Nonempirical calculations on excited states: the ethylene molecule[J]. The Journal of Chemical Physics, 1967, 47(5): 1735-1747. |
| 40 | Degen I A, Newman G A. Raman spectra of inorganic ions[J]. Spectrochimica Acta Part A: Molecular Spectroscopy, 1993, 49(5/6): 859-887. |
| 41 | Goldstein H F, Kim D S, Yu P Y, et al. Raman study of CuO single crystals[J]. Physical Review B, 1990, 41(10): 7192-7194. |
| 42 | Irwin J C, Chrzanowski J, Wei T, et al. Raman scattering from single crystals of cupric oxide[J]. Physica C: Superconductivity, 1990, 166(5/6): 456-464. |
| 43 | Huang Z W, Liu H L, Cui F, et al. Effects of the precipitation agents and rare earth additives on the structure and catalytic performance in glycerol hydrogenolysis of Cu/SiO2 catalysts prepared by precipitation-gel method[J]. Catalysis Today, 2014, 234: 223-232. |
| 44 | Huang Z W, Cui F, Xue J J, et al. Cu/SiO2 catalysts prepared by hom- and heterogeneous deposition-precipitation methods: texture, structure, and catalytic performance in the hydrogenolysis of glycerol to 1, 2-propanediol[J]. Catalysis Today, 2012, 183(1): 42-51. |
| 45 | Huang Z W, Cui F, Xue J J, et al. Synthesis and structural characterization of silica dispersed copper nanomaterials with unusual thermal stability prepared by precipitation-gel method[J]. The Journal of Physical Chemistry C, 2010, 114(39): 16104-16113. |
| 46 | Oosterwyck-Gastuche M C V. La structure de la chrysocolle[EB/OL]. [2021-01-05]. . |
| 47 | Wang C, Cheng Q P, Wang X L, et al. Enhanced catalytic performance for CO preferential oxidation over CuO catalysts supported on highly defective CeO2 nanocrystals[J]. Applied Surface Science, 2017, 422: 932-943. |
| 48 | Cocco F, Elsener B, Fantauzzi M, et al. Nanosized surface films on brass alloys by XPS and XAES[J]. RSC Advances, 2016, 6(37): 31277-31289. |
| [1] | 江河, 袁俊飞, 王林, 邢谷雨. 均流腔结构对微细通道内相变流动特性影响的实验研究[J]. 化工学报, 2023, 74(S1): 235-244. |
| [2] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [5] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [6] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| [7] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [8] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [9] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [10] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [11] | 孟令玎, 崇汝青, 孙菲雪, 孟子晖, 刘文芳. 改性聚乙烯膜和氧化硅固定化碳酸酐酶[J]. 化工学报, 2023, 74(8): 3472-3484. |
| [12] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [13] | 陈雅鑫, 袁航, 刘冠章, 毛磊, 杨纯, 张瑞芳, 张光亚. 蛋白质纳米笼介导的酶自固定化研究进展[J]. 化工学报, 2023, 74(7): 2773-2782. |
| [14] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [15] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号