化工学报 ›› 2020, Vol. 71 ›› Issue (1): 177-191.DOI: 10.11949/0438-1157.20191361
王薪薪1( ),周清1,2,张晓春1,张志博1,吕兴梅1,2,张锁江1,2(
),周清1,2,张晓春1,张志博1,吕兴梅1,2,张锁江1,2( )
)
收稿日期:2019-11-11
修回日期:2019-11-21
出版日期:2020-01-05
发布日期:2020-01-05
通讯作者:
张锁江
作者简介:王薪薪(1987—),女,硕士,基金资助:
Xinxin WANG1( ),Qing ZHOU1,2,Xiaochun ZHANG1,Zhibo ZHANG1,Xingmei LYU1,2,Suojiang ZHANG1,2(
),Qing ZHOU1,2,Xiaochun ZHANG1,Zhibo ZHANG1,Xingmei LYU1,2,Suojiang ZHANG1,2( )
)
Received:2019-11-11
Revised:2019-11-21
Online:2020-01-05
Published:2020-01-05
Contact:
Suojiang ZHANG
摘要:
离子液体因其特殊的内在结构及性能,使得其在纤维素预处理应用过程中展现出良好的效果,其相关体系的物性研究是推进工业化进程的基础必要数据。因此选用甲基咪唑和磷酸三甲酯合成了离子液体1,3-二甲基咪唑磷酸二甲酯([Mmim][DMP]),并测定了[Mmim][DMP] (1) + DMSO (2) 和 [Mmim][DMP] (1) + 乙腈 (2) 两个二元体系,在293.15~323.15 K时,全组成范围内的密度和黏度。计算了体积性质和超额性质数据。通过比较分析二元体系的超额摩尔体积(V E)、表观摩尔体积(
中图分类号:
王薪薪, 周清, 张晓春, 张志博, 吕兴梅, 张锁江. 离子液体[Mmim][DMP]与DMSO/乙腈二元体系的密度和黏度[J]. 化工学报, 2020, 71(1): 177-191.
Xinxin WANG, Qing ZHOU, Xiaochun ZHANG, Zhibo ZHANG, Xingmei LYU, Suojiang ZHANG. Densities and viscosities of binary system containing 1,3-dimethylimidazolium dimethylphosphate and dimethyl sulfoxide or acetonitrile[J]. CIESC Journal, 2020, 71(1): 177-191.
| Compound | T/K | ρ/(g·cm-3) | η/(mPa·s) | ||
|---|---|---|---|---|---|
| Exp. | Lit. | Exp. | Lit. | ||
| [Mmim][DMP] | 298.15 | 1.261314 | 1.2587[ 1.2612[ | 290.7599 | 271.86[ |
| 303.15 | 1.257795 | 1.2559[ 1.2441[ | 210.0863 | 188.30[ | |
| 308.15 | 1.254402 | 1.2530[ | 155.5793 | 139.49[ | |
| 313.15 | 1.250993 | 1.2491[ 1.2505[ 1.2368[ | 119.1838 | 110.25[ | |
| 318.15 | 1.247590 | 1.2452[ | 92.7692 | 84.44[ | |
| 323.15 | 1.244193 | 1.2415[ 1.2274[ | 74.2418 | 69.99[ | |
| DMSO | 293.15 | 1.100318 | 1.10076[ | 1.9937 | 2.210[ |
| 298.15 | 1.095326 | 1.0958[ | 1.8027 | 1.964[ | |
| 1.09574[ | |||||
| 303.15 | 1.090312 | 1.09073[ | 1.6362 | 1.787[ | |
| 1.0900[ | |||||
| 308.15 | 1.085265 | 1.0839[ | 1.4961 | 1.566[ | |
| 313.15 | 1.080264 | 1.08069[ | 1.3746 | 1.490[ | |
| 1.0783[ | |||||
| 318.15 | 1.075234 | 1.0731[ | 1.2682 | 1.3173[ | |
| 323.15 | 1.070217 | 1.07066[ | 1.1756 | 1.285[ | |
| 1.0693[ | |||||
| 乙腈 | 293.15 | 0.782374 | 0.7820[ | 0.3548 | 0.3645[ |
| 298.15 | 0.776984 | 0.7760[ | 0.3440 | 0.344[ | |
| 303.15 | 0.771554 | 0.7712[ | 0.3323 | 0.3307[ | |
| 308.15 | 0.766094 | 0.7663[ | 0.3220 | 0.313[ | |
| 313.15 | 0.760602 | 0.7603[ | 0.3124 | 0.3005[ | |
| 318.15 | 0.755076 | 0.7550[ | 0.3037 | 0.289[ | |
| 323.15 | 0.749510 | 0.7492[ | 0.2956 | 0.2746[ | |
表1 纯物质的密度、黏度实验值与文献值比较
Table 1 Comparison of measured with literature values of densities and viscosities of pure compounds
| Compound | T/K | ρ/(g·cm-3) | η/(mPa·s) | ||
|---|---|---|---|---|---|
| Exp. | Lit. | Exp. | Lit. | ||
| [Mmim][DMP] | 298.15 | 1.261314 | 1.2587[ 1.2612[ | 290.7599 | 271.86[ |
| 303.15 | 1.257795 | 1.2559[ 1.2441[ | 210.0863 | 188.30[ | |
| 308.15 | 1.254402 | 1.2530[ | 155.5793 | 139.49[ | |
| 313.15 | 1.250993 | 1.2491[ 1.2505[ 1.2368[ | 119.1838 | 110.25[ | |
| 318.15 | 1.247590 | 1.2452[ | 92.7692 | 84.44[ | |
| 323.15 | 1.244193 | 1.2415[ 1.2274[ | 74.2418 | 69.99[ | |
| DMSO | 293.15 | 1.100318 | 1.10076[ | 1.9937 | 2.210[ |
| 298.15 | 1.095326 | 1.0958[ | 1.8027 | 1.964[ | |
| 1.09574[ | |||||
| 303.15 | 1.090312 | 1.09073[ | 1.6362 | 1.787[ | |
| 1.0900[ | |||||
| 308.15 | 1.085265 | 1.0839[ | 1.4961 | 1.566[ | |
| 313.15 | 1.080264 | 1.08069[ | 1.3746 | 1.490[ | |
| 1.0783[ | |||||
| 318.15 | 1.075234 | 1.0731[ | 1.2682 | 1.3173[ | |
| 323.15 | 1.070217 | 1.07066[ | 1.1756 | 1.285[ | |
| 1.0693[ | |||||
| 乙腈 | 293.15 | 0.782374 | 0.7820[ | 0.3548 | 0.3645[ |
| 298.15 | 0.776984 | 0.7760[ | 0.3440 | 0.344[ | |
| 303.15 | 0.771554 | 0.7712[ | 0.3323 | 0.3307[ | |
| 308.15 | 0.766094 | 0.7663[ | 0.3220 | 0.313[ | |
| 313.15 | 0.760602 | 0.7603[ | 0.3124 | 0.3005[ | |
| 318.15 | 0.755076 | 0.7550[ | 0.3037 | 0.289[ | |
| 323.15 | 0.749510 | 0.7492[ | 0.2956 | 0.2746[ | |
| x 1 | A/(g·cm-3) | B×10-4/(g·cm-3·K-1) | R 2 |
|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | |||
| 0 | 1.394 | -10.000 | 1 |
| 0.1002 | 1.412 | -9.248 | 1 |
| 0.1997 | 1.426 | -8.732 | 1 |
| 0.2998 | 1.436 | -8.333 | 0.99999 |
| 0.4000 | 1.447 | -8.124 | 0.99988 |
| 0.5008 | 1.450 | -7.746 | 0.99999 |
| 0.6023 | 1.456 | -7.553 | 0.99995 |
| 0.7014 | 1.458 | -7.324 | 0.99999 |
| 0.7996 | 1.460 | -7.127 | 1 |
| 0.8964 | 1.463 | -6.984 | 0.99997 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |
| [Mmim][DMP] (1) + 乙腈 (2) | |||
| 0 | 1.103 | -11.000 | 0.99997 |
| 0.0901 | 1.203 | -9.771 | 0.99998 |
| 0.2010 | 1.285 | -8.998 | 0.99976 |
| 0.3001 | 1.329 | -8.316 | 1 |
| 0.4013 | 1.365 | -7.940 | 0.99998 |
| 0.5005 | 1.396 | -7.792 | 0.99994 |
| 0.5984 | 1.414 | -7.487 | 0.99999 |
| 0.6978 | 1.430 | -7.270 | 1 |
| 0.8007 | 1.444 | -7.097 | 0.99999 |
| 0.8995 | 1.462 | -7.184 | 0.99993 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |
表2 方程(1)拟合参数A和B的值
Table 2 Fitted values of empirical parameters A and B for densities of binary mixtures based on Eq. (1)
| x 1 | A/(g·cm-3) | B×10-4/(g·cm-3·K-1) | R 2 |
|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | |||
| 0 | 1.394 | -10.000 | 1 |
| 0.1002 | 1.412 | -9.248 | 1 |
| 0.1997 | 1.426 | -8.732 | 1 |
| 0.2998 | 1.436 | -8.333 | 0.99999 |
| 0.4000 | 1.447 | -8.124 | 0.99988 |
| 0.5008 | 1.450 | -7.746 | 0.99999 |
| 0.6023 | 1.456 | -7.553 | 0.99995 |
| 0.7014 | 1.458 | -7.324 | 0.99999 |
| 0.7996 | 1.460 | -7.127 | 1 |
| 0.8964 | 1.463 | -6.984 | 0.99997 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |
| [Mmim][DMP] (1) + 乙腈 (2) | |||
| 0 | 1.103 | -11.000 | 0.99997 |
| 0.0901 | 1.203 | -9.771 | 0.99998 |
| 0.2010 | 1.285 | -8.998 | 0.99976 |
| 0.3001 | 1.329 | -8.316 | 1 |
| 0.4013 | 1.365 | -7.940 | 0.99998 |
| 0.5005 | 1.396 | -7.792 | 0.99994 |
| 0.5984 | 1.414 | -7.487 | 0.99999 |
| 0.6978 | 1.430 | -7.270 | 1 |
| 0.8007 | 1.444 | -7.097 | 0.99999 |
| 0.8995 | 1.462 | -7.184 | 0.99993 |
| 1.0000 | 1.466 | -6.879 | 0.9999 |

图2 [Mmim][DMP]二元体系的密度随温度的变化
Fig.2 Densities for [Mmim][DMP] binary systems as a function of temperature at different mole fractions of IL ■ x 1 = 0; □ x 1 = 0.1002; ▲ x 1 = 0.1997; △ x 1 = 0.2998; ▼ x 1 = 0.4000; ▽ x 1 = 0.5008; ◆ x 1 = 0.6023; ◇ x 1 = 0.7014; ● x 1 = 0.7996; ○ x 1 = 0.8964; × x 1 = 1.0000

图3 [Mmim][DMP]二元体系的密度随组成的变化
Fig.3 Densities for [Mmim][DMP] binary system as a function of mole fractions of IL at different temperatures ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K
| T/K | | | | |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 293.15 | 170.85 | 39.10 | -76.28 | 0.11 |
| 298.15 | 171.22 | 40.07 | -78.19 | 0.12 |
| 303.15 | 171.59 | 41.16 | -80.62 | 0.12 |
| 308.15 | 171.93 | 42.51 | -83.45 | 0.13 |
| 313.15 | 172.28 | 43.62 | -85.68 | 0.13 |
| 318.15 | 172.62 | 44.97 | -88.46 | 0.14 |
| 323.15 | 172.95 | 46.30 | -91.13 | 0.14 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 293.15 | 162.96 | 85.85 | -122.79 | 0.54 |
| 298.15 | 162.83 | 89.72 | -128.38 | 0.55 |
| 303.15 | 162.70 | 93.54 | -133.90 | 0.57 |
| 308.15 | 162.54 | 97.57 | -139.64 | 0.58 |
| 313.15 | 163.21 | 75.33 | -107.73 | 0.70 |
| 318.15 | 163.11 | 78.95 | -112.86 | 0.71 |
| 323.15 | 163.05 | 82.34 | -117.73 | 0.73 |
表3 293.15~323.15 K下[Mmim][DMP]的无限稀释表观摩尔体积( V ? ∞ )
Table 3 Apparent molar volume at infinite dilution ( V ? ∞ ) of [Mmim][DMP] at temperatures from 293.15 to 323.15 K
| T/K | | | | |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 293.15 | 170.85 | 39.10 | -76.28 | 0.11 |
| 298.15 | 171.22 | 40.07 | -78.19 | 0.12 |
| 303.15 | 171.59 | 41.16 | -80.62 | 0.12 |
| 308.15 | 171.93 | 42.51 | -83.45 | 0.13 |
| 313.15 | 172.28 | 43.62 | -85.68 | 0.13 |
| 318.15 | 172.62 | 44.97 | -88.46 | 0.14 |
| 323.15 | 172.95 | 46.30 | -91.13 | 0.14 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 293.15 | 162.96 | 85.85 | -122.79 | 0.54 |
| 298.15 | 162.83 | 89.72 | -128.38 | 0.55 |
| 303.15 | 162.70 | 93.54 | -133.90 | 0.57 |
| 308.15 | 162.54 | 97.57 | -139.64 | 0.58 |
| 313.15 | 163.21 | 75.33 | -107.73 | 0.70 |
| 318.15 | 163.11 | 78.95 | -112.86 | 0.71 |
| 323.15 | 163.05 | 82.34 | -117.73 | 0.73 |
| x 1 | | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | |
| [Mmim][DMP] (1) + DMSO (2) | ||||||||||||||
| 0 | 201.93 | 202.85 | 203.78 | 204.73 | 205.68 | 206.64 | 207.61 | 70.63 | 70.94 | 71.26 | 71.58 | 71.90 | 72.23 | 72.55 |
| 0.1002 | 195.17 | 195.49 | 196.29 | 197.09 | 197.90 | 198.72 | 199.55 | 68.31 | 68.58 | 68.86 | 69.14 | 69.42 | 69.71 | 69.99 |
| 0.1997 | 190.64 | 190.62 | 191.34 | 192.07 | 192.79 | 193.52 | 194.26 | 66.72 | 66.97 | 67.23 | 67.48 | 67.74 | 67.99 | 68.25 |
| 0.2998 | 187.30 | 187.08 | 187.74 | 188.40 | 189.07 | 189.74 | 190.41 | 65.58 | 65.81 | 66.07 | 66.30 | 66.54 | 66.78 | 67.02 |
| 0.4000 | 184.77 | 184.42 | 185.05 | 185.70 | 186.32 | 186.95 | 187.58 | 64.66 | 64.88 | 65.08 | 65.30 | 65.52 | 65.74 | 65.96 |
| 0.5008 | 182.71 | 182.26 | 182.85 | 183.42 | 184.01 | 184.60 | 185.20 | 63.97 | 64.18 | 64.39 | 64.60 | 64.81 | 65.02 | 65.23 |
| 0.6023 | 181.05 | 180.55 | 181.12 | 181.67 | 182.23 | 182.79 | 183.36 | 63.41 | 63.59 | 63.79 | 63.99 | 64.19 | 64.39 | 64.59 |
| 0.7014 | 179.69 | 179.19 | 179.72 | 180.24 | 180.78 | 181.32 | 181.86 | 62.96 | 63.14 | 63.32 | 63.51 | 63.71 | 63.90 | 64.09 |
| 0.7996 | 178.55 | 178.07 | 178.57 | 179.07 | 179.59 | 180.11 | 180.63 | 62.58 | 62.77 | 62.95 | 63.13 | 63.31 | 63.50 | 63.68 |
| 0.8964 | 177.57 | 177.12 | 177.62 | 178.10 | 178.60 | 179.10 | 179.60 | 62.17 | 62.36 | 62.55 | 62.72 | 62.90 | 63.08 | 63.26 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 61.77 | 61.94 | 62.12 | 62.28 | 62.45 | 62.62 | 62.80 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||||||||||||
| 0 | 283.98 | 285.95 | 287.96 | 290.02 | 292.11 | 294.25 | 296.43 | 51.50 | 51.81 | 52.14 | 52.48 | 52.82 | 53.16 | 53.51 |
| 0.0901 | 243.49 | 243.74 | 245.05 | 246.39 | 247.74 | 249.10 | 250.48 | 44.40 | 44.62 | 44.85 | 45.07 | 45.30 | 45.56 | 45.81 |
| 0.2010 | 219.19 | 218.63 | 219.59 | 220.55 | 221.53 | 222.55 | 223.61 | 40.07 | 40.24 | 40.41 | 40.59 | 40.76 | 40.93 | 41.10 |
| 0.3001 | 206.58 | 205.63 | 206.43 | 207.23 | 208.04 | 208.85 | 209.67 | 37.88 | 38.02 | 38.18 | 38.33 | 38.48 | 38.64 | 38.79 |
| 0.4013 | 198.07 | 196.96 | 197.66 | 198.36 | 199.07 | 199.78 | 200.50 | 36.38 | 36.52 | 36.65 | 36.79 | 36.94 | 37.10 | 37.25 |
| 0.5005 | 192.11 | 190.97 | 191.60 | 192.23 | 192.88 | 193.55 | 194.22 | 35.35 | 35.48 | 35.61 | 35.74 | 35.86 | 35.98 | 36.11 |
| 0.5984 | 187.65 | 186.56 | 187.16 | 187.74 | 188.34 | 188.93 | 189.54 | 34.65 | 34.76 | 34.88 | 35.00 | 35.12 | 35.24 | 35.36 |
| 0.6978 | 184.11 | 183.17 | 183.72 | 184.26 | 184.82 | 185.38 | 185.95 | 34.06 | 34.17 | 34.28 | 34.39 | 34.50 | 34.62 | 34.74 |
| 0.8007 | 181.12 | 180.37 | 180.88 | 181.40 | 181.92 | 182.45 | 182.99 | 33.48 | 33.63 | 33.74 | 33.86 | 33.98 | 34.11 | 34.24 |
| 0.8995 | 178.72 | 178.11 | 178.64 | 179.14 | 179.65 | 180.17 | 180.70 | 33.05 | 33.14 | 33.25 | 33.34 | 33.44 | 33.53 | 33.62 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 32.45 | 32.55 | 32.64 | 32.72 | 32.81 | 32.90 | 32.99 |
表4 293.15~323.15 K下[Mmim][DMP] (1) + DMSO (2)和[Mmim][DMP] (1) + 乙腈 (2)体系的偏摩尔体积
Table 4 Partial molar volumes( V ? ) of mixtures of [Mmim][DMP] with DMSO and acetonitrile at temperatures from 293.15 to 323.15 K
| x 1 | | | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | 293.15 K | 298.15 K | 303.15 K | 308.15 K | 313.15 K | 318.15 K | 323.15 K | |
| [Mmim][DMP] (1) + DMSO (2) | ||||||||||||||
| 0 | 201.93 | 202.85 | 203.78 | 204.73 | 205.68 | 206.64 | 207.61 | 70.63 | 70.94 | 71.26 | 71.58 | 71.90 | 72.23 | 72.55 |
| 0.1002 | 195.17 | 195.49 | 196.29 | 197.09 | 197.90 | 198.72 | 199.55 | 68.31 | 68.58 | 68.86 | 69.14 | 69.42 | 69.71 | 69.99 |
| 0.1997 | 190.64 | 190.62 | 191.34 | 192.07 | 192.79 | 193.52 | 194.26 | 66.72 | 66.97 | 67.23 | 67.48 | 67.74 | 67.99 | 68.25 |
| 0.2998 | 187.30 | 187.08 | 187.74 | 188.40 | 189.07 | 189.74 | 190.41 | 65.58 | 65.81 | 66.07 | 66.30 | 66.54 | 66.78 | 67.02 |
| 0.4000 | 184.77 | 184.42 | 185.05 | 185.70 | 186.32 | 186.95 | 187.58 | 64.66 | 64.88 | 65.08 | 65.30 | 65.52 | 65.74 | 65.96 |
| 0.5008 | 182.71 | 182.26 | 182.85 | 183.42 | 184.01 | 184.60 | 185.20 | 63.97 | 64.18 | 64.39 | 64.60 | 64.81 | 65.02 | 65.23 |
| 0.6023 | 181.05 | 180.55 | 181.12 | 181.67 | 182.23 | 182.79 | 183.36 | 63.41 | 63.59 | 63.79 | 63.99 | 64.19 | 64.39 | 64.59 |
| 0.7014 | 179.69 | 179.19 | 179.72 | 180.24 | 180.78 | 181.32 | 181.86 | 62.96 | 63.14 | 63.32 | 63.51 | 63.71 | 63.90 | 64.09 |
| 0.7996 | 178.55 | 178.07 | 178.57 | 179.07 | 179.59 | 180.11 | 180.63 | 62.58 | 62.77 | 62.95 | 63.13 | 63.31 | 63.50 | 63.68 |
| 0.8964 | 177.57 | 177.12 | 177.62 | 178.10 | 178.60 | 179.10 | 179.60 | 62.17 | 62.36 | 62.55 | 62.72 | 62.90 | 63.08 | 63.26 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 61.77 | 61.94 | 62.12 | 62.28 | 62.45 | 62.62 | 62.80 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||||||||||||
| 0 | 283.98 | 285.95 | 287.96 | 290.02 | 292.11 | 294.25 | 296.43 | 51.50 | 51.81 | 52.14 | 52.48 | 52.82 | 53.16 | 53.51 |
| 0.0901 | 243.49 | 243.74 | 245.05 | 246.39 | 247.74 | 249.10 | 250.48 | 44.40 | 44.62 | 44.85 | 45.07 | 45.30 | 45.56 | 45.81 |
| 0.2010 | 219.19 | 218.63 | 219.59 | 220.55 | 221.53 | 222.55 | 223.61 | 40.07 | 40.24 | 40.41 | 40.59 | 40.76 | 40.93 | 41.10 |
| 0.3001 | 206.58 | 205.63 | 206.43 | 207.23 | 208.04 | 208.85 | 209.67 | 37.88 | 38.02 | 38.18 | 38.33 | 38.48 | 38.64 | 38.79 |
| 0.4013 | 198.07 | 196.96 | 197.66 | 198.36 | 199.07 | 199.78 | 200.50 | 36.38 | 36.52 | 36.65 | 36.79 | 36.94 | 37.10 | 37.25 |
| 0.5005 | 192.11 | 190.97 | 191.60 | 192.23 | 192.88 | 193.55 | 194.22 | 35.35 | 35.48 | 35.61 | 35.74 | 35.86 | 35.98 | 36.11 |
| 0.5984 | 187.65 | 186.56 | 187.16 | 187.74 | 188.34 | 188.93 | 189.54 | 34.65 | 34.76 | 34.88 | 35.00 | 35.12 | 35.24 | 35.36 |
| 0.6978 | 184.11 | 183.17 | 183.72 | 184.26 | 184.82 | 185.38 | 185.95 | 34.06 | 34.17 | 34.28 | 34.39 | 34.50 | 34.62 | 34.74 |
| 0.8007 | 181.12 | 180.37 | 180.88 | 181.40 | 181.92 | 182.45 | 182.99 | 33.48 | 33.63 | 33.74 | 33.86 | 33.98 | 34.11 | 34.24 |
| 0.8995 | 178.72 | 178.11 | 178.64 | 179.14 | 179.65 | 180.17 | 180.70 | 33.05 | 33.14 | 33.25 | 33.34 | 33.44 | 33.53 | 33.62 |
| 1.0000 | 176.65 | 176.15 | 176.64 | 177.12 | 177.60 | 178.09 | 178.57 | 32.45 | 32.55 | 32.64 | 32.72 | 32.81 | 32.90 | 32.99 |
| x 1 | A/(mPa·s) | B/K | T 0/K | R 2 |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 0 | 0.074 | 518.8 | 135.9 | 0.99999 |
| 0.1002 | 0.102 | 521.2 | 154.7 | 1.00000 |
| 0.1997 | 0.132 | 589.7 | 157.4 | 0.99992 |
| 0.2998 | 0.246 | 477.2 | 183.1 | 0.99999 |
| 0.4000 | 0.024 | 1264.1 | 115.9 | 0.99970 |
| 0.5008 | 0.324 | 514.3 | 192.9 | 1.00000 |
| 0.6023 | 0.336 | 536.0 | 196.6 | 1.00000 |
| 0.7014 | 0.294 | 594.8 | 195.7 | 1.00000 |
| 0.7996 | 0.418 | 579.2 | 200.0 | 0.99994 |
| 0.8964 | 0.031 | 1295.5 | 150.8 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 0 | 0.069 | 386.2 | 56.85 | 0.99963 |
| 0.0901 | 0.080 | 419.7 | 123.3 | 0.99999 |
| 0.2010 | 0.043 | 889.3 | 79.73 | 0.99992 |
| 0.3001 | 0.032 | 1055.9 | 94.83 | 0.99484 |
| 0.4013 | 0.223 | 515.1 | 172.1 | 0.99999 |
| 0.5005 | 0.032 | 1229.1 | 111.9 | 0.99860 |
| 0.5984 | 0.192 | 633.3 | 180.1 | 0.99998 |
| 0.6978 | 0.341 | 547.0 | 195.7 | 1.00000 |
| 0.8007 | 0.064 | 1134.4 | 144.4 | 0.99878 |
| 0.8995 | 0.038 | 1240.9 | 152.1 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
表5 VFT方程拟合参数A,B和T 0的值
Table 5 Fitted values of empirical parameters, A, B and T 0 for viscosity based on VFT equation
| x 1 | A/(mPa·s) | B/K | T 0/K | R 2 |
|---|---|---|---|---|
| [Mmim][DMP] (1) + DMSO (2) | ||||
| 0 | 0.074 | 518.8 | 135.9 | 0.99999 |
| 0.1002 | 0.102 | 521.2 | 154.7 | 1.00000 |
| 0.1997 | 0.132 | 589.7 | 157.4 | 0.99992 |
| 0.2998 | 0.246 | 477.2 | 183.1 | 0.99999 |
| 0.4000 | 0.024 | 1264.1 | 115.9 | 0.99970 |
| 0.5008 | 0.324 | 514.3 | 192.9 | 1.00000 |
| 0.6023 | 0.336 | 536.0 | 196.6 | 1.00000 |
| 0.7014 | 0.294 | 594.8 | 195.7 | 1.00000 |
| 0.7996 | 0.418 | 579.2 | 200.0 | 0.99994 |
| 0.8964 | 0.031 | 1295.5 | 150.8 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
| [Mmim][DMP] (1) + 乙腈 (2) | ||||
| 0 | 0.069 | 386.2 | 56.85 | 0.99963 |
| 0.0901 | 0.080 | 419.7 | 123.3 | 0.99999 |
| 0.2010 | 0.043 | 889.3 | 79.73 | 0.99992 |
| 0.3001 | 0.032 | 1055.9 | 94.83 | 0.99484 |
| 0.4013 | 0.223 | 515.1 | 172.1 | 0.99999 |
| 0.5005 | 0.032 | 1229.1 | 111.9 | 0.99860 |
| 0.5984 | 0.192 | 633.3 | 180.1 | 0.99998 |
| 0.6978 | 0.341 | 547.0 | 195.7 | 1.00000 |
| 0.8007 | 0.064 | 1134.4 | 144.4 | 0.99878 |
| 0.8995 | 0.038 | 1240.9 | 152.1 | 0.99971 |
| 1.0000 | 0.287 | 701.9 | 196.7 | 0.99999 |
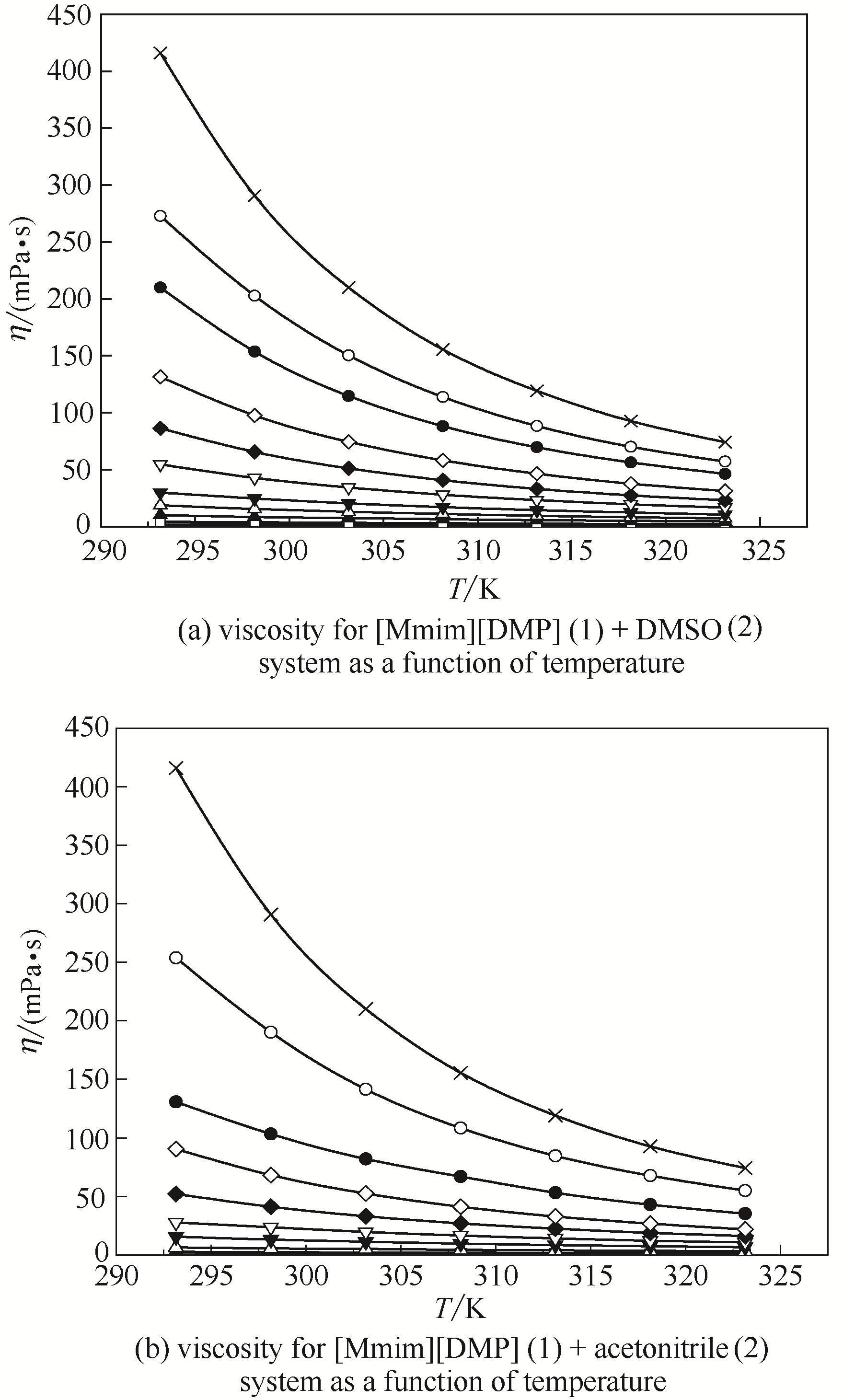
图5 [Mmim][DMP]二元体系黏度在不同组成下随温度的变化
Fig.5 Viscosity for [Mmim][DMP] binary system as a function of temperature at different mole fractions of IL(The composition x 1 was the mole fraction of [Mmim][DMP] in DMSO and acetonitrile, respectively) ■ x 1 = 0; □ x 1 = 0.1002; ▲ x 1 = 0.1997; △ x 1 = 0.2998; ▼ x 1 = 0.4000; ▽ x 1 = 0.5008; ◆ x 1 = 0.6023; ◇ x 1 = 0.7014; ● x 1 = 0.7996; ○ x 1 = 0.8964; × x 1 = 1.0000

图6 [Mmim][DMP]二元体系的黏度不同温度下随组成的变化
Fig.6 Viscosity for [Mmim][DMP] (1) + DMSO binary system as a function of mole fractions of IL at different temperatures ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K

图7 B3LYP/ 6-311++G**基组优化的[Mmim]阳离子与乙腈和DMSO的结构
Fig.7 Optimized structures of [Mmim]-acetonitrile and [Mmim]-DMSO obtained at the B3LYP/6-311++G**level (Hydrogen bonds are indicated by dotted lines, and distances are in angstroms)

图8 B3LYP/ 6-311++G**基组优化的[DMP]阴离子与乙腈和DMSO的结构
Fig.8 Optimized structures of [DMP]- acetonitrile and [DMP]- DMSO obtained at B3LYP/6-311++G**level(Hydrogen bonds are indicated by dotted lines, and distances are in angstroms)
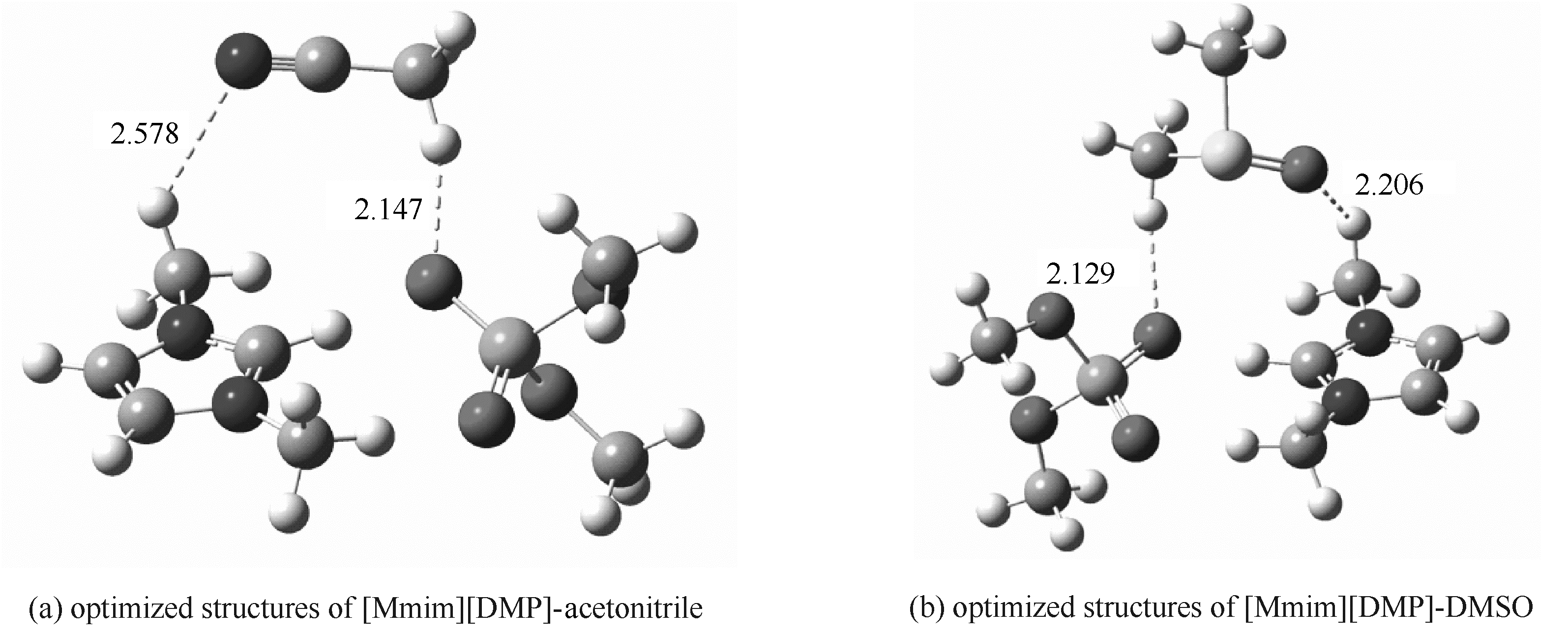
图9 B3LYP/ 6-311++G**基组优化的[Mmim][DMP]与乙腈和DMSO的结构
Fig.9 Optimized structures of [Mmim][DMP]-acetonitrile and [Mmim][DMP]-DMSO obtained at the B3LYP/6-311++G**level(Hydrogen bonds are indicated by dotted lines, and distances are in angstroms)
| T / K | V E/(cm3·mol-1) | Δη/(mPa·s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A 0 | A 1 | A 2 | A 3 | σ(Y) | A 0 | A 1 | A 2 | A 3 | σ(Y) | |
| [Mmim][DMP] + DMSO | ||||||||||
| 293.15 | -2.171 | 1.142 | -0.532 | 1.704 | 0.005 | -605.0 | -214.1 | -193.0 | -277.3 | 6.098 |
| 298.15 | -2.236 | 1.111 | -0.601 | 1.793 | 0.007 | -412.8 | -157.9 | -56.80 | -61.20 | 3.020 |
| 303.15 | -2.262 | 1.098 | -0.807 | 1.752 | 0.011 | -287.2 | -107.1 | -24.24 | -17.72 | 1.937 |
| 308.15 | -2.293 | 1.155 | -0.860 | 1.792 | 0.011 | -203.3 | -69.46 | -10.52 | -6.481 | 1.499 |
| 313.15 | -2.336 | 1.191 | -0.889 | 1.840 | 0.011 | -149.2 | -46.55 | -6.010 | -4.752 | 1.255 |
| 318.15 | -2.381 | 1.229 | -0.947 | 1.894 | 0.012 | -110.8 | -30.66 | 0.984 | 1.179 | 1.116 |
| 323.15 | -2.436 | 1.289 | -0.988 | 1.904 | 0.013 | -85.25 | -22.46 | 4.856 | 7.009 | 0.897 |
| [Mmim][DMP] + 乙腈 | ||||||||||
| 293.15 | -5.157 | 3.292 | -2.771 | 3.492 | 0.032 | -720.3 | -514.3 | -291.2 | -72.87 | 3.540 |
| 298.15 | -5.400 | 3.269 | -2.798 | 4.162 | 0.030 | -499.1 | -371.5 | -114.9 | 93.96 | 4.418 |
| 303.15 | -5.630 | 3.372 | -2.961 | 4.368 | 0.030 | -350.8 | -246.0 | -58.80 | 78.62 | 2.564 |
| 308.15 | -5.837 | 3.527 | -3.051 | 4.620 | 0.029 | -253.2 | -165.1 | -17.75 | 74.31 | 1.552 |
| 313.15 | -6.059 | 3.673 | -3.169 | 4.931 | 0.028 | -189.2 | -121.2 | -7.246 | 64.44 | 1.295 |
| 318.15 | -6.279 | 3.712 | -3.261 | 5.477 | 0.034 | -143.9 | -93.14 | 4.74 | 67.30 | 1.258 |
| 323.15 | -6.529 | 3.778 | -3.255 | 6.099 | 0.040 | -112.4 | -72.45 | 6.374 | 58.34 | 1.098 |
表6 二元混合体系V E和Δη 的回归系数及标准偏差
Table 6 Coefficients of standard deviation and Redlich-Kister equation for V E and Δη for two mixtures of [Mmim][DMP] with DMSO and acetonitrile
| T / K | V E/(cm3·mol-1) | Δη/(mPa·s) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A 0 | A 1 | A 2 | A 3 | σ(Y) | A 0 | A 1 | A 2 | A 3 | σ(Y) | |
| [Mmim][DMP] + DMSO | ||||||||||
| 293.15 | -2.171 | 1.142 | -0.532 | 1.704 | 0.005 | -605.0 | -214.1 | -193.0 | -277.3 | 6.098 |
| 298.15 | -2.236 | 1.111 | -0.601 | 1.793 | 0.007 | -412.8 | -157.9 | -56.80 | -61.20 | 3.020 |
| 303.15 | -2.262 | 1.098 | -0.807 | 1.752 | 0.011 | -287.2 | -107.1 | -24.24 | -17.72 | 1.937 |
| 308.15 | -2.293 | 1.155 | -0.860 | 1.792 | 0.011 | -203.3 | -69.46 | -10.52 | -6.481 | 1.499 |
| 313.15 | -2.336 | 1.191 | -0.889 | 1.840 | 0.011 | -149.2 | -46.55 | -6.010 | -4.752 | 1.255 |
| 318.15 | -2.381 | 1.229 | -0.947 | 1.894 | 0.012 | -110.8 | -30.66 | 0.984 | 1.179 | 1.116 |
| 323.15 | -2.436 | 1.289 | -0.988 | 1.904 | 0.013 | -85.25 | -22.46 | 4.856 | 7.009 | 0.897 |
| [Mmim][DMP] + 乙腈 | ||||||||||
| 293.15 | -5.157 | 3.292 | -2.771 | 3.492 | 0.032 | -720.3 | -514.3 | -291.2 | -72.87 | 3.540 |
| 298.15 | -5.400 | 3.269 | -2.798 | 4.162 | 0.030 | -499.1 | -371.5 | -114.9 | 93.96 | 4.418 |
| 303.15 | -5.630 | 3.372 | -2.961 | 4.368 | 0.030 | -350.8 | -246.0 | -58.80 | 78.62 | 2.564 |
| 308.15 | -5.837 | 3.527 | -3.051 | 4.620 | 0.029 | -253.2 | -165.1 | -17.75 | 74.31 | 1.552 |
| 313.15 | -6.059 | 3.673 | -3.169 | 4.931 | 0.028 | -189.2 | -121.2 | -7.246 | 64.44 | 1.295 |
| 318.15 | -6.279 | 3.712 | -3.261 | 5.477 | 0.034 | -143.9 | -93.14 | 4.74 | 67.30 | 1.258 |
| 323.15 | -6.529 | 3.778 | -3.255 | 6.099 | 0.040 | -112.4 | -72.45 | 6.374 | 58.34 | 1.098 |

图10 [Mmim][DMP]二元体系的超额摩尔体积不同温度下随组成的变化
Fig.10 Excess molar volumes for mixtures of [Mmim][DMP] binary system as a function of mole fractions of IL at different temperatures(The solid curves are calculated with Redlich-Kister equation, and symbols represent experimental values) ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K
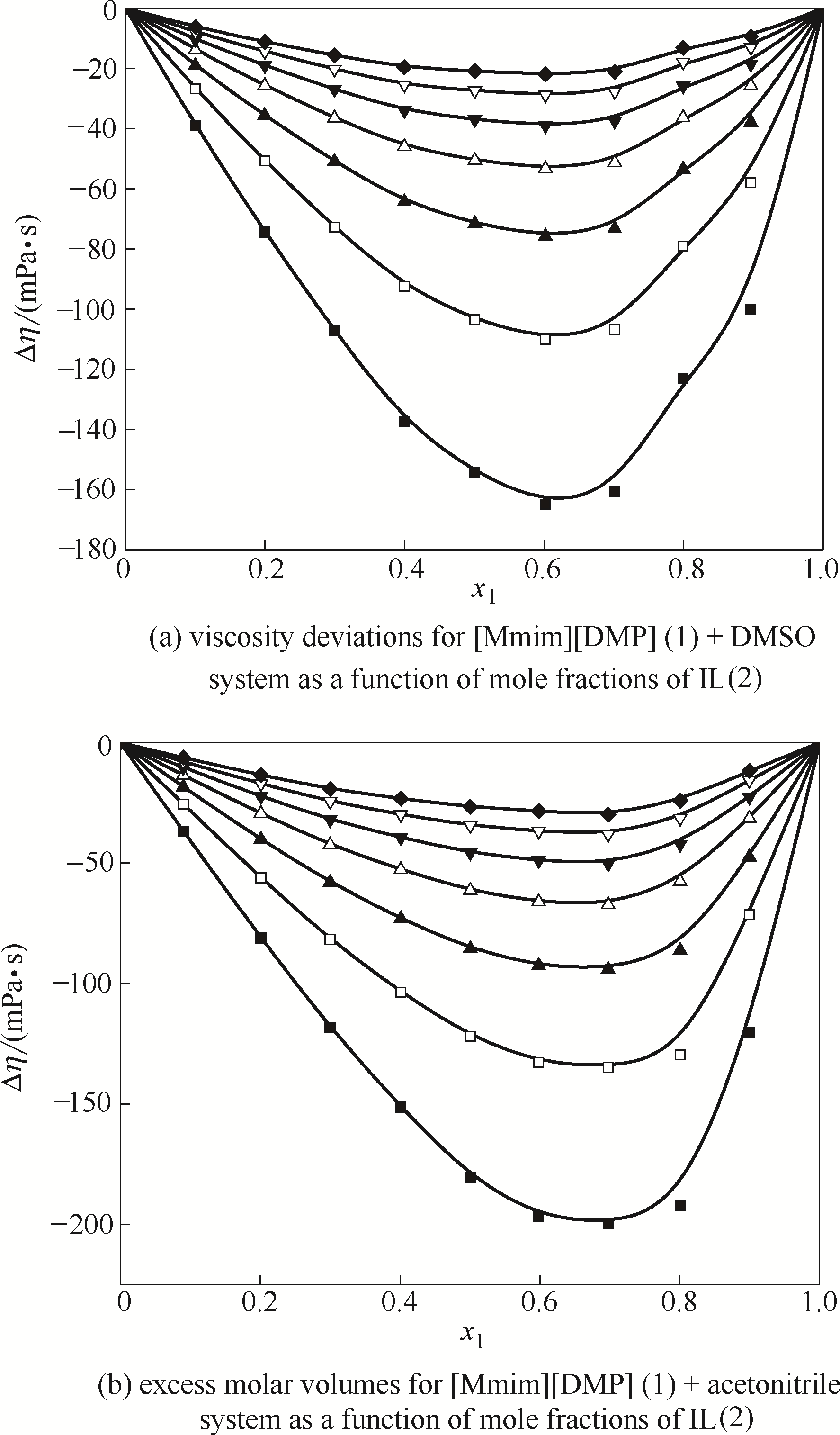
图11 [Mmim][DMP]二元体系的黏度偏差不同温度下随组成的变化
Fig.11 Viscosity deviations for mixtures of [Mmim][DMP]binary system at different temperatures(The solid curves are calculated with Redlich-Kister equation, and symbols represent experimental values) ■ 293.15 K; □ 298.15 K; ▲ 303.15 K; △ 308.15 K; ▼ 313.15 K; ▽ 318.15 K; ◆ 323.15 K
| 1 | Wang H , Gurau G , Rogers R D . Ionic liquid processing of cellulose[J]. Chem. Soc. Rev., 2012, 41(4): 1519-1537. |
| 2 | Zhao Y , Liu X , Wang J , et al . Insight into the cosolvent effect of cellulose dissolution in imidazolium-based ionic liquid systems[J]. J. Phys. Chem. B, 2013, 117(30): 9042-9049. |
| 3 | Xu J , Yao X , Zhou Q , et al . Enhanced delignification of cornstalk by employing superbase TBD in ionic liquids[J]. RSC Adv., 2014, 4(52): 27430-27438. |
| 4 | Xu J , Yao X , Xin J , et al . An effective two-step ionic liquids method for cornstalk pretreatment[J]. J. Chem. Technol. Biotechnol., 2015, 90: 2057-2065. |
| 5 | Chen Z , Zeng J , Di J , et al . Facile microwave-assisted ionic liquid synthesis of sphere-like BiOBr hollow and porous nanostructures with enhanced photocatalytic performance[J]. Green Energy Environ., 2017, 2(2): 124-133. |
| 6 | Vitz J , Erdmenger T , Haensch C , et al . Extended dissolution studies of cellulose in imidazolium based ionic liquids[J]. Green Chem., 2009, 11(3): 417-424. |
| 7 | Abe M , Fukaya Y , Ohno H . Extraction of polysaccharides from bran with phosphonate or phosphinate-derived ionic liquids under short mixing time and low temperature[J]. Green Chem., 2010, 12: 1274-1280. |
| 8 | Fukaya Y , Hayashi K , Wada M , et al . Cellulose dissolution with polar ionic liquids under mild conditions: required factors for anions[J]. Green Chem., 2008, 10(1): 44-46. |
| 9 | Xu A , Wang J , Wang H . Effects of anionic structure and lithium salts addition on the dissolution of cellulose in 1-butyl-3-methylimidazolium-based ionic liquid solvent systems[J]. Green Chem., 2010, 12(2): 268-275. |
| 10 | Swatloski R P , Spear S K , Holbrey J D , et al . Dissolution of cellose with ionic liquids[J]. J. Am. Chem. Soc., 2002, 124(18): 4974-4975. |
| 11 | Zhao Y , Liu X , Wang J , et al . Effects of anionic structure on the dissolution of cellulose in ionic liquids revealed by molecular simulation[J]. Carbohydr Polym., 2013, 94(2): 723-730. |
| 12 | Zhao Y , Liu X , Wang J , et al . Effects of cationic structure on cellulose dissolution in ionic liquids: a molecular dynamics study[J]. ChemPhysChem, 2012, 13(13): 3126-3133. |
| 13 | Rooney D , Jacquemin J , Gardas R . Thermophysical properties of ionic liquids[J]. Top Curr. Chem., 2009, 290: 185-212. |
| 14 | Seddon K R , Stark A , Torres M J . Influence of chloride, water, and organic solvents on the physical properties of ionic liquids[J]. Pure Appl. Chem., 2000, 72(12): 2275-2287. |
| 15 | Wahlström R , Rahikainen J , Kruus K , et al . Cellulose hydrolysis and binding with trihoderma reesei Cel5A and Cel7A and their core domains in ionic liquid solutions[J]. Biotechnol. Bioeng., 2014, 111(4): 726-733. |
| 16 | Qing Q , Hu R , He Y , et al . Investigation of a novel acid-catalyzed ionic liquid pretreatment method to improve biomass enzymatic hydrolysis conversion[J]. Appl. Microbiol. Biotechnol., 2014, 98(11): 5275-5286. |
| 17 | Wahlstrom R , Rovio S , Suurnakki A . Analysis of mono- and oligosaccharides in ionic liquid containing matrices[J]. Carbohydr. Res., 2013, 373: 42-51. |
| 18 | Engel P , Krull S , Seiferheld B , et al . Rational approach to optimize cellulase mixtures for hydrolysis of regenerated cellulose containing residual ionic liquid[J]. Bioresour. Technology., 2012, 115: 27-34. |
| 19 | Abels C , Redepenning C , Moll A , et al . Simple purification of ionic liquid solvents by nanofiltration in biorefining of lignocellulosic substrates[J]. J. Membr. Sci., 2012, 405/406: 1-10. |
| 20 | Engel P , Mladenov R , Wulfhorst H , et al . Point by point analysis: how ionic liquid affects the enzymatic hydrolysis of native and modified cellulose[J]. Green Chem., 2010, 12(11): 1959-1966. |
| 21 | Mazza M , Catana D A , Vaca-Garcia C , et al . Influence of water on the dissolution of cellulose in selected ionic liquids[J]. Cellulose, 2008, 16(2): 207-215. |
| 22 | Zhu S , Wu Y , Chen Q , et al . Dissolution of cellulose with ionic liquids and its application: a mini-review[J]. Green Chem., 2006, 8(4): 325-327. |
| 23 | Cao Y , Zhang R , Cheng T , et al . Imidazolium-based ionic liquids for cellulose pretreatment: recent progresses and future perspectives[J]. Appl. Microbiol. Biotechnol., 2017, 101(2): 521-532. |
| 24 | Zhao D , Li H , Zhang J , et al . Dissolution of cellulose in phosphate-based ionic liquids[J]. Carbohydr. Polym., 2012, 87(2): 1490-1494. |
| 25 | Cai F , Zhu W , Wang Y , et al . Dialkylphosphate-based ionic liquids as solvents to extract toluene from heptane[J]. J. Chem. Eng. Data, 2015, 60(6): 1776-1780. |
| 26 | Cai F , Zhao M , Wang Y , et al . Phosphoric-based ionic liquids as solvents to separate the azeotropic mixture of ethanol and hexane[J]. J. Chem. Thermodyn., 2015, 81: 177-183. |
| 27 | Cai F , Xiao G . Liquid-liquid equilibria for ternary systems ethanol + heptane + phosphoric-based ionic liquids[J]. Fluid Phase Equilib., 2015, 386: 155-161. |
| 28 | Cai F , Xiao G . (Liquid + liquid) extraction of methanol from alkanes using dialkylphosphate-based ionic liquids as solvents[J]. J. Chem. Thermodyn., 2015, 87: 110-116. |
| 29 | Cai F , Ibrahim J J , Niu L , et al . Liquid-liquid equilibrium for ternary system methanol + methyl acetate + 1,3-dimethylimidazolium dimethylphosphate at several temperatures and atmospheric pressure[J]. J. Chem. Eng. Data, 2015, 60(1): 57-64. |
| 30 | Sakal S A , Shen C , Li C X . (Liquid + liquid) equilibria of {benzene + cyclohexane + two ionic liquids} at different temperature and atmospheric pressure[J]. J. Chem. Thermodyn., 2012, 49: 81-86. |
| 31 | Cao J , Yu G , Chen X , et al . Determination of vapor-liquid equilibrium of methyl acetate + methanol + 1-alkyl-3-methylimidazolium dialkylphosphates at 101.3 kPa[J]. J. Chem. Eng. Data, 2017, 62(2): 816-824. |
| 32 | Chen X , Yang B , Abdeltawab A A , et al . Isobaric vapor-liquid equilibrium for acetone + methanol + phosphate ionic liquids[J]. J. Chem. Eng. Data, 2015, 60(3): 612-620. |
| 33 | Li Q , Cao L , Zhang Y , et al . Isobaric vapor-liquid equilibrium for chloroform + methanol + 1,3-dimethylimidazolium dimethylphosphate at 101.3 kPa[J]. J. Chem. Eng. Data, 2014, 59(2): 234-239. |
| 34 | Wang J , Li Z . Measurement and modeling of vapor-liquid equilibria for systems containing alcohols, water, and imidazolium-based phosphate ionic liquids[J]. J. Chem. Eng. Data, 2013, 58(6): 1641-1649. |
| 35 | Dong L , Zheng D , Li J , et al . Suitability prediction and affinity regularity assessment of H2O + imidazolium ionic liquid working pairs of absorption cycle by excess property criteria and UNIFAC model[J]. Fluid Phase Equilib., 2013, 348: 1-8. |
| 36 | Li Q , Zhu W , Wang H , et al . Isobaric vapor-liquid equilibrium for the ethanol + water + 1,3-dimethylimidazolium dimethylphosphate system at 101.3 kPa[J]. J. Chem. Eng. Data, 2012, 57(3): 696-700. |
| 37 | Jia P , Zhao Z , Gao Q , et al . Isobaric vapor-liquid equilibrium of the acetonitrile + 1-propanol + ionic liquids at an atmospheric pressure[J]. J. Chem. Eng. Data, 2019, 64(7): 2963-2972. |
| 38 | Luo C , Wang Y , Li Y , et al . Thermodynamic properties and application of LiNO3-MMIM DMP /H2O ternary working pair[J]. Renewable Energy, 2019, 134: 147-160. |
| 39 | Dong L , Zheng D , Nie N , et al . Performance prediction of absorption refrigeration cycle based on the measurements of vapor pressure and heat capacity of H2O + [DMIM]DMP system[J]. Appl. Energy, 2012, 98: 326-332. |
| 40 | Ge M L , Lu C Y , Liu X Y , et al . Activity coefficients at infinite dilution of alkanes, alkenes, alkyl benzenes in dimethylphosphate based ionic liquids using gas-liquid chromatography[J]. J. Chem. Thermodyn., 2015, 91: 279-285. |
| 41 | Gaciño F M , Regueira T , Bolotov A V , et al . Volumetric behaviour of six ionic liquids from T = (278 to 398) K and up to 120 MPa[J]. J. Chem. Thermodyn., 2016, 93: 24-33. |
| 42 | Ghani N A , Sairi N A , Aroua M K , et al . Density, surface tension, and viscosity of ionic liquids (1-ethyl-3-methylimidazolium diethylphosphate and 1,3-dimethylimidazolium dimethylphosphate) aqueous ternary mixtures with MDEA[J]. J. Chem. Eng. Data, 2014, 59(6): 1737-1746. |
| 43 | He Z , Zhao Z , Zhang X , et al . Thermodynamic properties of new heat pump working pairs: 1,3-dimethylimidazolium dimethylphosphate and water, ethanol and methanol[J]. Fluid Phase Equilib., 2010, 298(1): 83-91. |
| 44 | Zhang Z , Zhou Q , Lu X , et al . Densities and viscosities of binary mixtures containing 1,3-dimethylimidazolium dimethylphosphate and alcohols[J]. J. Chem. Eng. Data, 2014, 59(8): 2377-2388. |
| 45 | Wang J , Zhang Z , Jin S , et al . Efficient conversion of carbohydrates into 5-hydroxylmethylfurfan and 5-ethoxymethylfurfural over sufonic acid-functionalized mesoporous carbon catalyst[J]. Fuel, 2017, 192: 102-107. |
| 46 | Sampath G , Srinivasan K . Remarkable catalytic synergism of alumina, metal salt and solvent for conversion of biomass sugars to furan compounds[J]. Appl. Catal., A, 2017, 533: 75-80. |
| 47 | Morais-de-Carvalho D , Martinez-Abad A , Evtuguin D V , et al . Isolation and characterization of acetylated glucuronoarabinoxylan from sugarcane bagasse and straw[J]. Carbohydr Polym., 2017, 156: 223-234. |
| 48 | Holding A J , Parviainen A , Kilpeläinen I , et al . Efficiency of hydrophobic phosphonium ionic liquids and DMSO as recyclable cellulose dissolution and regeneration media[J]. RSC Adv., 2017, 7(28): 17451-17461. |
| 49 | Gajula S , Inthumathi K , Arumugam S R , et al . Strategic designing on selection of solvent systems for conversion of biomass sugars to furan derivatives and their separation[J]. ACS Sustainable Chem. Eng., 2017, 5(6): 5373-5381. |
| 50 | Chen T Y , Wang B , Shen X J , et al . Assessment of structural characteristics of regenerated cellulolytic enzyme lignin based on a mild DMSO/[Emim]OAc dissolution system from triploid of populus tomentosa carr[J]. RSC Adv., 2017, 7(6): 3376-3387. |
| 51 | Brzonova I , Asina F , Andrianova A A , et al . Fungal biotransformation of insoluble kraft lignin into a water soluble polymer[J]. Ind. Eng. Chem. Res., 2017, 56(21): 6103-6113. |
| 52 | Bhanja P , Modak A , Chatterjee S , et al . Bifunctionalized mesoporous SBA-15: a new heterogeneous catalyst for the facile synthesis of 5-hydroxymethylfurfural[J]. ACS Sustainable Chem. Eng., 2017, 5(3): 2763-2773. |
| 53 | Xue Z , Zhao X , Sun R C , et al . Biomass-derived γ-valerolactone-based solvent systems for highly efficient dissolution of various lignins: dissolution behavior and mechanism study[J]. ACS Sustainable Chem. Eng., 2016, 4(7): 3864-3870. |
| 54 | Zuo M , Le K , Li Z , et al . Green process for production of 5-hydroxymethylfurfural from carbohydrates with high purity in deep eutectic solvents[J]. Ind. Crops Prod., 2017, 99: 1-6. |
| 55 | Hattori K , Arai A . Preparation and hydrolysis of water-stable amorphous cellulose[J]. ACS Sustainable Chem. Eng., 2016, 4(3): 1180-1186. |
| 56 | Chen H , Zhou J , Mao J , et al . Enhancement of mass transfer through bubbling effect during extraction and reaction in biphasic systems containing ionic liquid[J]. RSC Adv., 2016, 6(103): 101485-101491. |
| 57 | Chidambaram M , Bell A T . A two-step approach for the catalytic conversion of glucose to 2,5-dimethylfuran in ionic liquids[J]. Green Chem., 2010, 12(7): 1253-1262. |
| 58 | Tian S , Ren S , Hou Y , et al . Densities, viscosities and excess properties of binary mixtures of 1,1,3,3-tetramethylguanidinium lactate + water at T = (303.15 to 328.15) K[J]. J. Chem. Eng. Data, 2013, 58(7): 1885-1892. |
| 59 | Kermanpour F , Niakan H Z , Sharifi T . Density and viscosity measurements of binary alkanol mixtures from (293.15 to 333.15) K at atmospheric pressure[J]. J. Chem. Eng. Data, 2013, 58(5): 1086-1091. |
| 60 | Ciocirlan O , Croitoru O , Iulian O . Densities and viscosities for binary mixtures of 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid with molecular solvents[J]. J. Chem. Eng. Data, 2011, 56(4): 1526-1534. |
| 61 | Roy M N , Sarkar B K , Chanda R . Viscosity, density, and speed of sound for the binary mixtures of formamide with 2-methoxyethanol, acetophenone, acetonitrile, 1,2-dimethoxyethane, and dimethylsulfoxide at different temperatures[J]. J. Chem. Eng. Data, 2007, 52: 1630-1637. |
| 62 | Yasmeen S , Riyazuddeen, Anwar N . Interaction of 1-butyl-3-methylimidazolium bis(trifluoromethylsulfonyl)-imide with methanol/dimethyl sulfoxide at (298.15, 303.15, 308.15, 313.15, 318.15 and 323.15) K: measurements and correlations of thermophysical properties[J]. J. Mol. Liq., 2016, 221: 1207-1217. |
| 63 | Keshapolla D , Gardas R L . Apparent molar volumes and isentropic compressions of benzylalkylammonium ionic liquids in dimethylsulfoxide from 293.15 K to 328.15 K[J]. Fluid Phase Equilib., 2014, 383: 32-42. |
| 64 | Redlich O , Meyer D M . The molal volumes of electrolytes[J]. Chem Rev., 1964, 24(3): 221-227. |
| 65 | Carmen Grande M D , Juliá J A , García M , et al . On the density and viscosity of (water + dimethylsulphoxide) binary mixtures[J]. J. Chem. Thermodyn., 2007, 39(7): 1049-1056. |
| 66 | Tôrres R B , Marchiore A C M , Volpe P L O . Volumetric properties of binary mixtures of (water + organic solvents) at temperatures between T = 288.15 K and T = 303.15 K at p = 0.1 MPa[J]. J. Chem. Thermodyn., 2006, 38(5): 526-541. |
| 67 | González E J , Alonso L , Á Domínguez . Physical properties of binary mixtures of the ionic liquid 1-methyl-3-octylimidazolium chloride with methanol, ethanol, and 1-propanol at T = (298.15, 313.15, and 328.15) K and at P = 0.1 MPa[J]. J. Chem. Eng. Data, 2006, 51(4): 1446-1452. |
| 68 | González E J , González B , Macedo E A . Thermophysical properties of the pure ionic liquid 1-butyl-1-methylpyrrolidinium dicyanamide and its binary mixtures with alcohols[J]. J. Chem. Eng. Data, 2013, 58(6): 1440-1448. |
| 69 | Zheng Y , Dong K , Wang Q , et al . Density, viscosity, and conductivity of lewis acidic 1-butyl- and 1-hydrogen-3-methylimidazolium chloroaluminate ionic liquids[J]. J. Chem. Eng. Data, 2013, 58(1): 32-42. |
| 70 | Ciocirlan O , Iulian O . Properties of pure 1-butyl-2,3-dimethylimidazolium tetrafluoroborate ionic liquid and its binary mixtures with dimethyl sulfoxide and acetonitrile[J]. J. Chem. Eng. Data, 2012, 57(11): 3142-3148. |
| 71 | Iulian O , Ciocirlan O . Volumetric properties of binary mixtures of two 1-alkyl-3-methylimidazolium tetrafluoroborate ionic liquids with molecular solvents[J]. J. Chem. Eng. Data, 2012, 57(10): 2640-2646. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 张龙, 宋孟杰, 邵苛苛, 张旋, 沈俊, 高润淼, 甄泽康, 江正勇. 管翅式换热器迎风侧翅片末端霜层生长模拟研究[J]. 化工学报, 2023, 74(S1): 179-182. |
| [3] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [4] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [5] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [6] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [7] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [8] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [9] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [10] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [11] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [12] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [13] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [14] | 刘爽, 张霖宙, 许志明, 赵锁奇. 渣油及其组分黏度的分子层次组成关联研究[J]. 化工学报, 2023, 74(8): 3226-3241. |
| [15] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号