化工学报 ›› 2022, Vol. 73 ›› Issue (10): 4268-4284.DOI: 10.11949/0438-1157.20220600
吴建猛1,2,3( ), 郑爽3, 曾少娟1,3(
), 郑爽3, 曾少娟1,3( ), 张香平1,3, 杨灿2,3, 董海峰1,3
), 张香平1,3, 杨灿2,3, 董海峰1,3
收稿日期:2022-04-05
修回日期:2022-06-23
出版日期:2022-10-05
发布日期:2022-11-02
通讯作者:
曾少娟
作者简介:吴建猛(1998—),男,硕士研究生,wujianmeng2021@ipe.ac.cn
基金资助:
Jianmeng WU1,2,3( ), Shuang ZHENG3, Shaojuan ZENG1,3(
), Shuang ZHENG3, Shaojuan ZENG1,3( ), Xiangping ZHANG1,3, Can YANG2,3, Haifeng DONG1,3
), Xiangping ZHANG1,3, Can YANG2,3, Haifeng DONG1,3
Received:2022-04-05
Revised:2022-06-23
Online:2022-10-05
Published:2022-11-02
Contact:
Shaojuan ZENG
摘要:
人口增长与全球工业化的加速发展促使化石能源需求量逐年递增,由此导致大气中二氧化碳(CO2)含量快速上升并引发了全球系列气候问题,“碳达峰·碳中和”背景下的CO2减排刻不容缓。传统工业捕集CO2方法由于能耗高、选择性较差、溶剂损耗大等问题限制了其大规模推广应用,离子液体因其极低挥发性、强的气体亲和性、可调的结构性质等特点在CO2捕集分离领域逐渐显示出独特优势,但离子液体特别是功能化后通常黏度较高或室温呈固态,导致气液传质效果差或无法直接应用于吸收分离过程。负载型离子液体兼具离子液体和多孔材料的共同优势,不仅能提升选择性分离效果,有效避免离子液体直接吸收造成的高黏度,还可拓展离子液体应用范围,具有广阔的发展前景。重点总结了近些年物理和化学负载型离子液体在CO2吸附分离方面的研究现状和进展,并对负载型离子液体捕集分离CO2研究的发展趋势进行了展望。
中图分类号:
吴建猛, 郑爽, 曾少娟, 张香平, 杨灿, 董海峰. 负载型离子液体吸附分离CO2的研究现状及展望[J]. 化工学报, 2022, 73(10): 4268-4284.
Jianmeng WU, Shuang ZHENG, Shaojuan ZENG, Xiangping ZHANG, Can YANG, Haifeng DONG. Status and prospect on CO2 adsorption and separation by supported ionic liquids[J]. CIESC Journal, 2022, 73(10): 4268-4284.
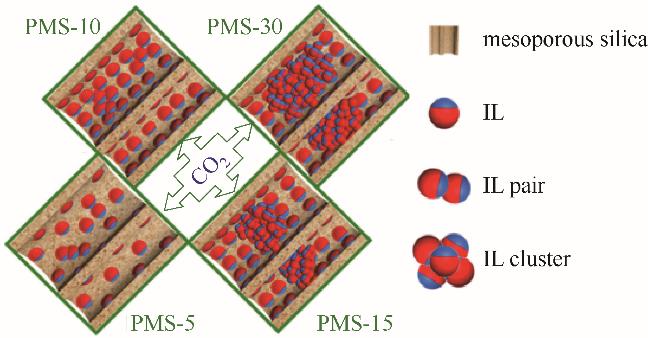
图2 [P4444][2-Op]@介孔SiO2吸附分离CO2机理(PMS-w中的w为离子液体质量分数)[35]
Fig.2 Mechanism of CO2 separation by adsorption of [P4444][2-Op]@ mesoporous SiO2 (w in PMS-w is the mass fraction of ionic liquid) [35]

图3 离子液体[N4444][Ac](a)、纤维素(b)、[N4444][Ac]@75%纤维素(c)、[N4444][Ac]@90%纤维素(d)的SEM图[42]
Fig.3 SEM images of ionic liquid [N4444][Ac] (a), cellulose (b), [N4444][Ac]@75% cellulose (c),[N4444][Ac]@90% cellulose (d)[42]
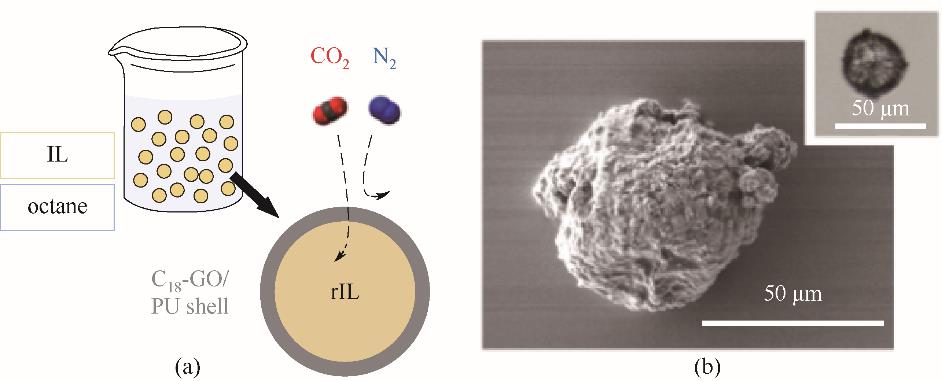
图6 (a)使用模板法包裹离子液体的原理图;(b)离子液体胶囊的SEM和光学显微镜(OM)图像[59]
Fig.6 (a) Schematic of ionic liquid wrapped by template method; (b) SEM and optical microscope (OM) images of ionic liquid capsules [59]
| 吸附剂(离子液体@载体) | IL负载量/%(mass) | 温度/℃ | 压力/MPa | 吸附量/(mmol CO2/g sorbent) | 文献 |
|---|---|---|---|---|---|
| [Bmim][NO3]@SiO2 | 50 | 25 | 0.1 | 0.35 | [ |
| [Bmim][PF6]@硅胶 | 20 | 0 | 0.1 | 0.84 | [ |
| [Emim][Ac]@气相SiO2 | 40 | 40 | 0.1 | 0.82 | [ |
| [Apmim][Br]@硅胶 | 40 | 35 | 0.1 | 0.67 | [ |
| [N1111][Gly]@硅胶 | 22.4 | 30 | 0.1 | 0.93 | [ |
| [P4444][2-Op]@MS | 10 | 50 | 0.1 | 1.68 | [ |
| [N2222][Gly]@PDVB | 51 | 30 | 0.1 | 1.63 | [ |
| [AEmim][Lys]@PMMA | 50 | 30 | 0.1 | 1.50 | [ |
| [EOEOEmim][Gly]@D101 | 38 | 25 | 0.1 | 0.99 | [ |
| [Bmim][NTf2]@PSF | 32 | 45 | 0.4 | 1.30 | [ |
| [N4444][Ac]@纤维素 | 25 | 25 | 3.0 | 0.72 | [ |
| [dmedah][PR]@AC | 10 | 25 | 0.1 | 1.21 | [ |
| [vbtma][Gly]@AC | 20 | 25 | 0.1 | 1.51 | [ |
| [Emim][Gly]@F600-900 | — | 30 | 0.015 | 0.50 | [ |
| [Bmim][Ac]@SBA-15 | 48.5 | 25 | 0.1 | 2.11 | [ |
| [Apmim][Lys]@PE-SBA-15 | 50 | 30 | 0.1 | 0.88 | [ |
| [Teta]L@ZIF-8 | 25 | 25 | 0.1 | 1.53 | [ |
| [C4(Vim)2]Br2@CuBTC | 5 | 20 | 0.1 | 4.30 | [ |
| P[VCIm]Cl@MA | 50 | 40 | 0.1 | 0.56 | [ |
| [Bmim][Gly]@HCM | 60 | 30 | 0.1 | 0.52 | [ |
| [Bmim][PF6]@HMDA-HDI | 70 | 20 | 0.1 | 0.07 | [ |
| [Emim][2-CNpyr]@C18-GO/PU | 60 | 25 | 0.1 | 0.82 | [ |
表1 物理负载型离子液体对CO2的吸附量
Table 1 CO2 adsorption capacity of physically supported ionic liquids
| 吸附剂(离子液体@载体) | IL负载量/%(mass) | 温度/℃ | 压力/MPa | 吸附量/(mmol CO2/g sorbent) | 文献 |
|---|---|---|---|---|---|
| [Bmim][NO3]@SiO2 | 50 | 25 | 0.1 | 0.35 | [ |
| [Bmim][PF6]@硅胶 | 20 | 0 | 0.1 | 0.84 | [ |
| [Emim][Ac]@气相SiO2 | 40 | 40 | 0.1 | 0.82 | [ |
| [Apmim][Br]@硅胶 | 40 | 35 | 0.1 | 0.67 | [ |
| [N1111][Gly]@硅胶 | 22.4 | 30 | 0.1 | 0.93 | [ |
| [P4444][2-Op]@MS | 10 | 50 | 0.1 | 1.68 | [ |
| [N2222][Gly]@PDVB | 51 | 30 | 0.1 | 1.63 | [ |
| [AEmim][Lys]@PMMA | 50 | 30 | 0.1 | 1.50 | [ |
| [EOEOEmim][Gly]@D101 | 38 | 25 | 0.1 | 0.99 | [ |
| [Bmim][NTf2]@PSF | 32 | 45 | 0.4 | 1.30 | [ |
| [N4444][Ac]@纤维素 | 25 | 25 | 3.0 | 0.72 | [ |
| [dmedah][PR]@AC | 10 | 25 | 0.1 | 1.21 | [ |
| [vbtma][Gly]@AC | 20 | 25 | 0.1 | 1.51 | [ |
| [Emim][Gly]@F600-900 | — | 30 | 0.015 | 0.50 | [ |
| [Bmim][Ac]@SBA-15 | 48.5 | 25 | 0.1 | 2.11 | [ |
| [Apmim][Lys]@PE-SBA-15 | 50 | 30 | 0.1 | 0.88 | [ |
| [Teta]L@ZIF-8 | 25 | 25 | 0.1 | 1.53 | [ |
| [C4(Vim)2]Br2@CuBTC | 5 | 20 | 0.1 | 4.30 | [ |
| P[VCIm]Cl@MA | 50 | 40 | 0.1 | 0.56 | [ |
| [Bmim][Gly]@HCM | 60 | 30 | 0.1 | 0.52 | [ |
| [Bmim][PF6]@HMDA-HDI | 70 | 20 | 0.1 | 0.07 | [ |
| [Emim][2-CNpyr]@C18-GO/PU | 60 | 25 | 0.1 | 0.82 | [ |
| 吸附剂(离子液体-载体) | IL负载量/%(mass) | 温度/℃ | 压力/MPa | 吸附量/(mmol CO2/g sorbent) | 文献 |
|---|---|---|---|---|---|
| [i-C5TPIm][Tf2N]-MS | 5 | 45 | 0.4 | 1.81 | [ |
| Si-[P8883][Tf2N]-SiO2 | — | 40 | 0.1 | 0.99 | [ |
| P[VYIM][Bu2PO4]-SiO2 | 8.2 | 0 | 0.1 | 1.03 | [ |
| P[Amim][BF4]-SiO2 | 5.0 | 0 | 0.1 | 0.87 | [ |
| [Bmim][Lys]-OMS | 11.7 | 25 | 0.1 | 0.61 | [ |
| P[(META)+CF3 | — | 25 | 0.078 | 2.00 | [ |
| Si-P(C8H17)3[Tf2N]-AC | 9.9 | 0 | 0.1 | 2.40 | [ |
| Si-P(C8H17)3[Tf2N]-AC | 9.9 | 25 | 0.2 | 1.11 | [ |
| [(MeO)3Sipmim][Cl]-MCM-41 | 25 | 25 | 0.1 | 1.49 | [ |
| [(MeO)3Sipmim][Cl]-MCM-41 | 25 | 25 | 1 | 2.50 | [ |
| [Spmim][PF6]-MCM-41 (2.3nm) | — | 35 | 1 | 0.90 | [ |
| [Spmim][PF6]-MCM-41 (3.3nm) | — | 35 | 1 | 0.55 | [ |
| [C2NH2mim][Br]-MIL(A) | 9.4 | 0 | 0.1 | 1.93 | [ |
| [C2NH2mim][Br]-MIL(B) | 8.1 | 0 | 0.1 | 2.77 | [ |
| [C m MOEim][Br]-MA | 8 | 25 | 0.1 | 2.02 | [ |
| P[ViEtIm]Br-TiNTs | 46 | 25 | 0.02 | 2.43 | [ |
| GO-P[MATMA][BF4] | — | 0 | 0.12 | 0.96 | [ |
| DB10%-Pa-TP | — | 0 | 0.1 | 4.83 | [ |
表2 化学负载型离子液体对CO2的吸附量
Table 2 CO2 adsorption capacity of chemically supported ionic liquids
| 吸附剂(离子液体-载体) | IL负载量/%(mass) | 温度/℃ | 压力/MPa | 吸附量/(mmol CO2/g sorbent) | 文献 |
|---|---|---|---|---|---|
| [i-C5TPIm][Tf2N]-MS | 5 | 45 | 0.4 | 1.81 | [ |
| Si-[P8883][Tf2N]-SiO2 | — | 40 | 0.1 | 0.99 | [ |
| P[VYIM][Bu2PO4]-SiO2 | 8.2 | 0 | 0.1 | 1.03 | [ |
| P[Amim][BF4]-SiO2 | 5.0 | 0 | 0.1 | 0.87 | [ |
| [Bmim][Lys]-OMS | 11.7 | 25 | 0.1 | 0.61 | [ |
| P[(META)+CF3 | — | 25 | 0.078 | 2.00 | [ |
| Si-P(C8H17)3[Tf2N]-AC | 9.9 | 0 | 0.1 | 2.40 | [ |
| Si-P(C8H17)3[Tf2N]-AC | 9.9 | 25 | 0.2 | 1.11 | [ |
| [(MeO)3Sipmim][Cl]-MCM-41 | 25 | 25 | 0.1 | 1.49 | [ |
| [(MeO)3Sipmim][Cl]-MCM-41 | 25 | 25 | 1 | 2.50 | [ |
| [Spmim][PF6]-MCM-41 (2.3nm) | — | 35 | 1 | 0.90 | [ |
| [Spmim][PF6]-MCM-41 (3.3nm) | — | 35 | 1 | 0.55 | [ |
| [C2NH2mim][Br]-MIL(A) | 9.4 | 0 | 0.1 | 1.93 | [ |
| [C2NH2mim][Br]-MIL(B) | 8.1 | 0 | 0.1 | 2.77 | [ |
| [C m MOEim][Br]-MA | 8 | 25 | 0.1 | 2.02 | [ |
| P[ViEtIm]Br-TiNTs | 46 | 25 | 0.02 | 2.43 | [ |
| GO-P[MATMA][BF4] | — | 0 | 0.12 | 0.96 | [ |
| DB10%-Pa-TP | — | 0 | 0.1 | 4.83 | [ |
| 1 | Monastersky R. Global carbon dioxide levels near worrisome milestone[J]. Nature, 2013, 497(7447): 13-14. |
| 2 | 邓旭, 谢俊, 滕飞. 何谓“碳中和”?[J]. 气候变化研究进展, 2021, 17(1): 107-113. |
| Deng X, Xie J, Teng F. What is “carbon neutrality”?[J]. Climate Change Research, 2021, 17(1): 107-113. | |
| 3 | 叶亨利, 余兴光, 张继伟, 等. 我国二氧化碳捕集利用与封存项目环境影响评价对策[J]. 环境与可持续发展, 2017, 42(5): 43-46. |
| Ye H L, Yu X G, Zhang J W, et al. Countermeasures on the environmental impact assessment of carbon dioxide capture and storage project in China[J]. Environment and Sustainable Development, 2017, 42(5): 43-46. | |
| 4 | 张中正. 二氧化碳的吸附分离[D]. 天津:天津大学, 2012. |
| Zhang Z Z. Adsorptive separation of carbon dioxide[D]. Tianjin: Tianjin University, 2012. | |
| 5 | Zhang K, Hou Y C, Wang Y M, et al. Efficient and reversible absorption of CO2 by functional deep eutectic solvents[J]. Energy & Fuels, 2018, 32(7): 7727-7733. |
| 6 | Glasscock D A, Critchfield J E, Rochelle G T. CO2 absorption/desorption in mixtures of methyldiethanolamine with monoethanolamine or diethanolamine[J]. Chemical Engineering Science, 1991, 46(11): 2829-2845. |
| 7 | Li X S, Liu J, Jiang W F, et al. Low energy-consuming CO2 capture by phase change absorbents of amine/alcohol/H2O[J]. Separation and Purification Technology, 2021, 275: 119181. |
| 8 | Heldebrant D J, Yonker C R, Jessop P G, et al. Organic liquid CO2 capture agents with high gravimetric CO2 capacity[J]. Energy & Environmental Science, 2008, 1(4): 487-493. |
| 9 | Cognigni A, Kampichler S, Bica K. Surface-active ionic liquids in catalysis: impact of structure and concentration on the aerobic oxidation of octanol in water[J]. Journal of Colloid and Interface Science, 2017, 492: 136-145. |
| 10 | Wang T T, Hu Z Y, Zhang L, et al. Hydrodynamic characteristics of N2-[Bmim][NO3] two-phase Taylor flow in microchannels[J]. Industrial & Engineering Chemistry Research, 2021, 60(47): 17248-17258. |
| 11 | Li X, Ma N, Zhang L, et al. Applications of choline-based ionic liquids in drug delivery[J]. International Journal of Pharmaceutics, 2022, 612: 121366. |
| 12 | Lin H C, De Oliveira P W, Veith M. Application of ionic liquids in photopolymerizable holographic materials[J]. Optical Materials, 2011, 33(6): 759-762. |
| 13 | Blanchard L A, Dan H C, Beckman E J, et al. Green processing using ionic liquids and CO2 [J]. Nature, 1999, 399(6731): 28-29. |
| 14 | 夏裴文, 丁保宏, 张鹏军, 等. 离子液体及其吸收机理的研究进展[J]. 应用化工, 2019, 48(6): 1469-1473. |
| Xia P W, Ding B H, Zhang P J, et al. Research progress on ionic liquids and their absorption mechanism[J]. Applied Chemical Industry, 2019, 48(6): 1469-1473. | |
| 15 | Wang C M, Luo X Y, Zhu X, et al. The strategies for improving carbon dioxide chemisorption by functionalized ionic liquids[J]. RSC Advances, 2013, 3(36): 15518-15527. |
| 16 | Huang Y J, Cui G K, Zhao Y L, et al. Preorganization and cooperation for highly efficient and reversible capture of low-concentration CO2 by ionic liquids[J]. Angewandte Chemie (International Ed. in English), 2017, 56(43): 13293-13297. |
| 17 | Chen F F, Huang K, Zhou Y, et al. Multi-molar absorption of CO2 by the activation of carboxylate groups in amino acid ionic liquids[J]. Angewandte Chemie (International Ed. in English), 2016, 55(25): 7166-7170. |
| 18 | 王薪薪, 唐少峰, 吕兴梅, 等. 氨基酸季铵离子液体水溶液的密度和黏度[J]. 中国科学:化学, 2014, 44(6): 1050-1057. |
| Wang X X, Tang S F, Lyu X M, et al. The density and viscosity of aqueous solutions of quaternary ammonium-amino acid ionic liquids[J]. Scientia Sinica Chimica, 2014, 44(6): 1050-1057. | |
| 19 | Kim D H, Baek I H, Hong S U, et al. Study on immobilized liquid membrane using ionic liquid and PVDF hollow fiber as a support for CO2/N2 separation[J]. Journal of Membrane Science, 2011, 372(1/2): 346-354. |
| 20 | 张锁江, 刘晓敏, 姚晓倩, 等. 离子液体的前沿、进展及应用[J]. 中国科学(B辑:化学), 2009, 39(10): 1134-1144. |
| Zhang S J, Liu X M, Yao X Q, et al. Frontiers, progresses and applications of ionic liquids[J]. Science in China (Series B: Chemistry), 2009, 39(10): 1134-1144. | |
| 21 | Selvam T, Machoke A, Schwieger W. Supported ionic liquids on non-porous and porous inorganic materials—a topical review[J]. Applied Catalysis A: General, 2012, 445: 92-101. |
| 22 | Tan M, Lu J T, Zhang Y, et al. Ionic liquid confined in mesoporous polymer membrane with improved stability for CO2/N2 separation[J]. Nanomaterials, 2017, 7(10): 299-309. |
| 23 | 张佩文. 多孔材料负载离子液体在吸收SO2、NO2、CO2中的应用[D]. 石家庄:河北科技大学, 2019. |
| Zhang P W. Application of porous material loaded ionic liquids in absorption of SO2, NO2, CO2 [D]. Shijiazhuang: Hebei University of Science and Technology, 2019. | |
| 24 | 郭艳东, 佟佳欢, 刘晓敏, 等. 负载型离子液体的研究进展及发展趋势[J]. 中国科学:化学, 2016, 46(12): 1305-1316. |
| Guo Y D, Tong J H, Liu X M, et al. Recent advances and development of supported ionic liquids[J]. Scientia Sinica Chimica, 2016, 46(12): 1305-1316. | |
| 25 | Zhang Y Q, Zhang S J, Lu X M, et al. Dual amino-functionalised phosphonium ionic liquids for CO2 capture[J]. Chemistry (Weinheim an Der Bergstrasse, Germany), 2009, 15(12): 3003-3011. |
| 26 | Ziobrowski Z, Rotkegel A. Feasibility study of CO2/N2 separation intensification on supported ionic liquid membranes by commonly used impregnation methods[J]. Greenhouse Gases: Science and Technology, 2021, 11(2): 297-312. |
| 27 | 陈传东, 翟尚儒, 翟滨, 等. 功能离子液体/氧化硅基多孔复合材料[J]. 化学进展, 2010, 22(10): 1921-1928. |
| Chen C D, Zhai S R, Zhai B, et al. Functional ionic liquid/porous silica composites[J]. Progress in Chemistry, 2010, 22(10): 1921-1928. | |
| 28 | Lee K M, Lee Y T, Lin I J B. Supramolecular liquid crystals of amide functionalized imidazolium salts[J]. Journal of Materials Chemistry, 2003, 13(5): 1079-1084. |
| 29 | Tarkhanova I G, Anisimov A V, Buryak A K, et al. Immobilized ionic liquids based on molybdenum- and tungsten-containing heteropoly acids: structure and catalytic properties in thiophene oxidation[J]. Petroleum Chemistry, 2017, 57(10): 859-867. |
| 30 | Mirzaei M, Badiei A R, Mokhtarani B, et al. Experimental study on CO2 sorption capacity of the neat and porous silica supported ionic liquids and the effect of water content of flue gas[J]. Journal of Molecular Liquids, 2017, 232: 462-470. |
| 31 | 董晓晨, 朱佳媚, 孙雅钗, 等. 硅胶负载离子液体的CO2吸附性能影响因素研究[J]. 材料导报, 2015, 29(14): 77-81. |
| Dong X C, Zhu J M, Sun Y C, et al. Study on factors influencing CO2 adsorption on ionic liquid-immobilized silica gel[J]. Materials Review, 2015, 29(14): 77-81. | |
| 32 | Pohako-Esko K, Bahlmann M, Schulz P S, et al. Chitosan containing supported ionic liquid phase materials for CO2 absorption[J]. Industrial & Engineering Chemistry Research, 2016, 55(25): 7052-7059. |
| 33 | 陈义峰, 王昌松, 丁键, 等. 负载[APMIm][Br]离子液体吸收CO2的性能[J]. 化工学报, 2014, 65(5): 1716-1720. |
| Chen Y F, Wang C S, Ding J, et al. CO2 absorption properties of supported [APMIm][Br][J]. CIESC Journal, 2014, 65(5): 1716-1720. | |
| 34 | 杨刚胜, 曾淦宁, 赵强, 等. 负载型氨基酸离子液体的制备及其对二氧化碳的吸附性能[J]. 燃料化学学报, 2016, 44(1): 106-112. |
| Yang G S, Zeng G N, Zhao Q, et al. Preparation of silica gel supported amino acid ionic liquids and their performance capacity towards carbon dioxide[J]. Journal of Fuel Chemistry and Technology, 2016, 44(1): 106-112. | |
| 35 | Xue C, Feng L, Zhu H, et al. Pyridine-containing ionic liquids lowly loaded in large mesoporous silica and their rapid CO2 gas adsorption at low partial pressure[J]. Journal of CO2 Utilization, 2019, 34: 282-292. |
| 36 | Mehnert C P, Cook R A, Dispenziere N C, et al. Supported ionic liquid catalysis—a new concept for homogeneous hydroformylation catalysis[J]. Journal of the American Chemical Society, 2002, 124(44): 12932-12933. |
| 37 | Xu W L, Zhang J Y, Cheng N N, et al. Facilely synthesized mesoporous polymer for dispersion of amino acid ionic liquid and effective capture of carbon dioxide from anthropogenic source[J]. Journal of the Taiwan Institute of Chemical Engineers, 2021, 125: 115-121. |
| 38 | Huang Z, Karami D, Mahinpey N. Study on the efficiency of multiple amino groups in ionic liquids on their sorbents performance for low-temperature CO2 capture[J]. Chemical Engineering Research and Design, 2021, 167: 198-206. |
| 39 | 徐玉韬, 徐海涛. 多孔树脂负载离子液体作为固体吸附剂用于CO2的捕获[J]. 合成材料老化与应用, 2016, 45(2): 25-31, 59. |
| Xu Y T, Xu H T. Porous resin supported ionic liquid as solid sorbents for reversible CO2 capture[J]. Syntheic Materials Aging and Application, 2016, 45(2): 25-31, 59. | |
| 40 | Nisar M, Bernard F L, Duarte E, et al. New polysulfone microcapsules containing metal oxides and ([BMIM][NTf2]) ionic liquid for CO2 capture[J]. Journal of Environmental Chemical Engineering, 2021, 9(1): 104781. |
| 41 | Gabrienko A A, Ewing A V, Chibiryaev A M, et al. New insights into the mechanism of interaction between CO2 and polymers from thermodynamic parameters obtained by in situ ATR-FTIR spectroscopy[J]. Physical Chemistry Chemical Physics, 2016, 18(9): 6465-6475. |
| 42 | Reed D G, Dowson G R M, Styring P. Cellulose-supported ionic liquids for low-cost pressure swing CO2 capture[J]. Frontiers in Energy Research, 2017, 5: 13-24. |
| 43 | 叶青. 改性多孔材料常温下吸附分离密闭空间二氧化碳[D]. 杭州:浙江大学, 2012. |
| Ye Q. CO2 adsorption from confined space under ambient temperature by modified porous materials[D]. Hangzhou: Zhejiang University, 2012. | |
| 44 | Wang X H, Cheng H R, Ye G Z, et al. Key factors and primary modification methods of activated carbon and their application in adsorption of carbon-based gases: a review[J]. Chemosphere, 2022, 287: 131995. |
| 45 | Shahrom M S R, Nordin A R, Wilfred C D. The improvement of activated carbon as CO2 adsorbent with supported amine functionalized ionic liquids[J]. Journal of Environmental Chemical Engineering, 2019, 7(5): 103319. |
| 46 | Torralba-Calleja E, Skinner J, Gutiérrez-Tauste D. CO2 capture in ionic liquids: a review of solubilities and experimental methods[J]. Journal of Chemistry, 2013, 2013: 473584. |
| 47 | Erto A, Silvestre-Albero A, Silvestre-Albero J, et al. Carbon-supported ionic liquids as innovative adsorbents for CO2 separation from synthetic flue-gas[J]. Journal of Colloid and Interface Science, 2015, 448: 41-50. |
| 48 | Pevida C, Drage T C, Snape C E. Silica-templated melamine-formaldehyde resin derived adsorbents for CO2 capture[J]. Carbon, 2008, 46(11): 1464-1474. |
| 49 | Bui T X, Kang S Y, Lee S H, et al. Organically functionalized mesoporous SBA-15 as sorbents for removal of selected pharmaceuticals from water[J]. Journal of Hazardous Materials, 2011, 193: 156-163. |
| 50 | Yin J Z, Zhen M Y, Cai P, et al. Supercritical CO2 preparation of SBA-15 supported ionic liquid and its adsorption for CO2 [J]. Materials Research Express, 2018, 5(6): 065060. |
| 51 | Huang Z L, Mohamedali M, Karami D, et al. Evaluation of supported multi-functionalized amino acid ionic liquid-based sorbents for low temperature CO2 capture[J]. Fuel, 2022, 310: 122284. |
| 52 | Li Q Y, Ji S F, Hao Z M. Metal-organic framework materials and their applications in catalysis[J]. Progress in Chemistry, 2012, 24(8): 1506-1518. |
| 53 | Han G, Yu N, Liu D, et al. Stepped enhancement of CO2 adsorption and separation in IL‐ZIF‐IL composites with shell‐interlayer‐core structure[J]. AIChE Journal, 2020, 67(2): 17112-17119. |
| 54 | Pan R, Guo Y N, Tang Y N, et al. Dicationic liquid containing alkenyl modified CuBTC improves the performance of the composites: increasing the CO2 adsorption effect[J]. Chemical Engineering Journal, 2021, 430(6): 132127. |
| 55 | Zhou Y, Chang M, Zang X, et al. The polymeric ionic liquids/mesoporous alumina composites: synthesis, characterization and CO2 capture performance test[J]. Polymer Testing, 2020, 81: 106109. |
| 56 | Romanos G E, Schulz P S, Bahlmann M, et al. CO2 capture by novel supported ionic liquid phase systems consisting of silica nanoparticles encapsulating amine-functionalized ionic liquids[J]. The Journal of Physical Chemistry C, 2014, 118(42): 24437-24451. |
| 57 | Santiago R, Lemus J, Moya C, et al. Encapsulated ionic liquids to enable the practical application of amino acid-based ionic liquids in CO2 capture[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(11): 14178-14187. |
| 58 | Gaur S S, Edgehouse K J, Klemm A, et al. Capsules with polyurea shells and ionic liquid cores for CO2 capture[J]. Journal of Polymer Science, 2021, 59(23): 2980-2989. |
| 59 | Lee Y Y, Edgehouse K, Klemm A, et al. Capsules of reactive ionic liquids for selective capture of carbon dioxide at low concentrations[J]. ACS Applied Materials & Interfaces, 2020, 12(16): 19184-19193. |
| 60 | Jin M J, Taher A, Kang H J, et al. Palladium acetate immobilized in a hierarchical MFI zeolite-supported ionic liquid: a highly active and recyclable catalyst for Suzuki reaction in water[J]. Green Chemistry, 2009, 11(3): 309. |
| 61 | Thi T N, Van H D, Ha C H, et al. Preparation and properties of colloidal silica-filled natural rubber grafted with poly(methyl methacrylate)[J]. Polymer Bulletin, 2022, 79: 6011-6027. |
| 62 | Duczinski R, Polesso B B, Bernard F L, et al. Enhancement of CO2/N2 selectivity and CO2 uptake by tuning concentration and chemical structure of imidazolium-based ILs immobilized in mesoporous silica[J]. Journal of Environmental Chemical Engineering, 2020, 8(3): 103740. |
| 63 | Zhu J M, He B T, Huang J H, et al. Effect of immobilization methods and the pore structure on CO2 separation performance in silica-supported ionic liquids[J]. Microporous and Mesoporous Materials, 2018, 260: 190-200. |
| 64 | Qiu H, Lv L, Pan B C, et al. Critical review in adsorption kinetic models[J]. Journal of Zhejiang University-Science A, 2009, 10(5): 716-724. |
| 65 | Sun Y, Zhu J, Huang J, et al. Polymeric ionic liquid modified silica for CO2 adsorption and diffusivity[J]. Polymer Composites, 2017, 38(4): 759-766. |
| 66 | Hiremath V, Jadhav A H, Lee H, et al. Highly reversible CO2 capture using amino acid functionalized ionic liquids immobilized on mesoporous silica[J]. Chemical Engineering Journal, 2016, 287: 602-617. |
| 67 | Nemani S K, Annavarapu R K, Mohammadian B, et al. Surface modification of polymers: methods and applications[J]. Advanced Materials Interfaces, 2018, 5(24): 1801247. |
| 68 | Samadi A, Kemmerlin R K, Husson S M. Polymerized ionic liquid sorbents for CO2 separation[J]. Energy & Fuels, 2010, 24(10): 5797-5804. |
| 69 | Wang T, Hou C, Ge K, et al. Spontaneous cooling absorption of CO2 by a polymeric ionic liquid for direct air capture[J]. The Journal of Physical Chemistry Letters, 2017, 8(17): 3986-3990. |
| 70 | Guo Y F, Zhao C W, Li C H. CO2 adsorption kinetics of K2CO3/activated carbon for low-concentration CO2 removal from confined spaces[J]. Chemical Engineering & Technology, 2015, 38(5): 891-899. |
| 71 | Liu Y, Hu Y H, Zhou J S, et al. Polystyrene-supported novel imidazolium ionic liquids: highly efficient catalyst for the fixation of carbon dioxide under atmospheric pressure[J]. Fuel, 2021, 305: 121495. |
| 72 | 董晓晨. 活性炭负载离子液体的制备及CO2吸附性能研究[D]. 徐州:中国矿业大学, 2015. |
| Dong X C. Synthesis of activated carbon immobilized ionic liquid and CO2 adsorption properties[D]. Xuzhou: China University of Mining and Technology, 2015. | |
| 73 | He X D, Zhu J M, Wang H M, et al. Surface functionalization of activated carbon with phosphonium ionic liquid for CO2 adsorption[J]. Coatings, 2019, 9(9): 590-601. |
| 74 | Aquino A S, Bernard F L, Borges J V, et al. Rationalizing the role of the anion in CO2 capture and conversion using imidazolium-based ionic liquid modified mesoporous silica[J]. RSC Advances, 2015, 5(79): 64220-64227. |
| 75 | Vangeli O C, Romanos G E, Beltsios K G, et al. Grafting of imidazolium based ionic liquid on the pore surface of nanoporous materials: study of physicochemical and thermodynamic properties[J]. The Journal of Physical Chemistry. B, 2010, 114(19): 6480-6491. |
| 76 | Wilson M, Barrientos-Palomo S N, Stevens P C, et al. Substrate-independent epitaxial growth of the metal-organic framework MOF-508a[J]. ACS Applied Materials & Interfaces, 2018, 10(4): 4057-4065. |
| 77 | Bahadori M, Marandi A, Tangestaninejad S, et al. Ionic liquid-decorated MIL-101(Cr) via covalent and coordination bonds for efficient solvent-free CO2 conversion and CO2 capture at low pressure[J]. The Journal of Physical Chemistry C, 2020, 124(16): 8716-8725. |
| 78 | Sun L W, Yin M L, Tang S K. Bi-functionalized ionic liquid-grafted mesoporous alumina: synthesis, characterization and CO2/N2 selectivity[J]. Journal of Environmental Chemical Engineering, 2021, 9(5): 105829. |
| 79 | Yuan J, Fan M L, Zhang F F, et al. Amine-functionalized poly(ionic liquid) brushes for carbon dioxide adsorption[J]. Chemical Engineering Journal, 2017, 316: 903-910. |
| 80 | Huang L, Jin Y, Sun L, et al. Graphene oxide functionalized by poly(ionic liquid)s for carbon dioxide capture[J]. Journal of Applied Polymer Science, 2017, 134(11): 44592. |
| 81 | Cao J J, Shan W J, Wang Q, et al. Ordered porous poly(ionic liquid) crystallines: spacing confined ionic surface enhancing selective CO2 capture and fixation[J]. ACS Applied Materials & Interfaces, 2019, 11(6): 6031-6041. |
| [1] | 张义飞, 刘舫辰, 张双星, 杜文静. 超临界二氧化碳用印刷电路板式换热器性能分析[J]. 化工学报, 2023, 74(S1): 183-190. |
| [2] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [3] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [4] | 宋瑞涛, 王派, 王云鹏, 李敏霞, 党超镔, 陈振国, 童欢, 周佳琦. 二氧化碳直接蒸发冰场排管内流动沸腾换热数值模拟分析[J]. 化工学报, 2023, 74(S1): 96-103. |
| [5] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [6] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [7] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [8] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [9] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [10] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [11] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [12] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [13] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [14] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [15] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号