化工学报 ›› 2023, Vol. 74 ›› Issue (5): 2057-2066.DOI: 10.11949/0438-1157.20230249
收稿日期:2023-03-17
修回日期:2023-04-14
出版日期:2023-05-05
发布日期:2023-06-29
通讯作者:
潘艳秋
作者简介:李辰鑫(1998—),男,硕士研究生,lichenxin@mail.dlut.edu.cn
基金资助:
Chenxin LI( ), Yanqiu PAN(
), Yanqiu PAN( ), Liu HE, Yabin NIU, Lu YU
), Liu HE, Yabin NIU, Lu YU
Received:2023-03-17
Revised:2023-04-14
Online:2023-05-05
Published:2023-06-29
Contact:
Yanqiu PAN
摘要:
在混合气体的膜分离过程中,组分与膜结构的相互作用直接影响分离效果。采用分子模拟方法,建立基于PEK-C基炭膜结构表征的碳微晶基炭膜模型,探究膜的碳微晶孔径和比表面积对CO2/CH4吸附和扩散的影响。结果表明,气体在炭膜内的吸附位点与其微结构有关,碳微晶孔径与比表面积增加时,吸附位点由碳微晶外部孔隙向碳微晶内部转移;炭膜孔隙体积较小时,分子形状为影响扩散的主要因素,其中直线型的CO2比正四面体型的CH4更易于扩散;30℃、100 kPa下,碳微晶孔径为0.493 nm时炭膜具有最优的气体分离性能,其吸附分离系数为10.49;炭化温度升高,炭膜比表面积增大,不利于气体分离,表明炭化温度不宜过高。研究结果拓展了炭膜气体分离机理,可为高性能炭膜的制备提供依据。
中图分类号:
李辰鑫, 潘艳秋, 何流, 牛亚宾, 俞路. 基于碳微晶结构的炭膜模型及其气体分离模拟[J]. 化工学报, 2023, 74(5): 2057-2066.
Chenxin LI, Yanqiu PAN, Liu HE, Yabin NIU, Lu YU. Carbon membrane model based on carbon microcrystal structure and its gas separation simulation[J]. CIESC Journal, 2023, 74(5): 2057-2066.
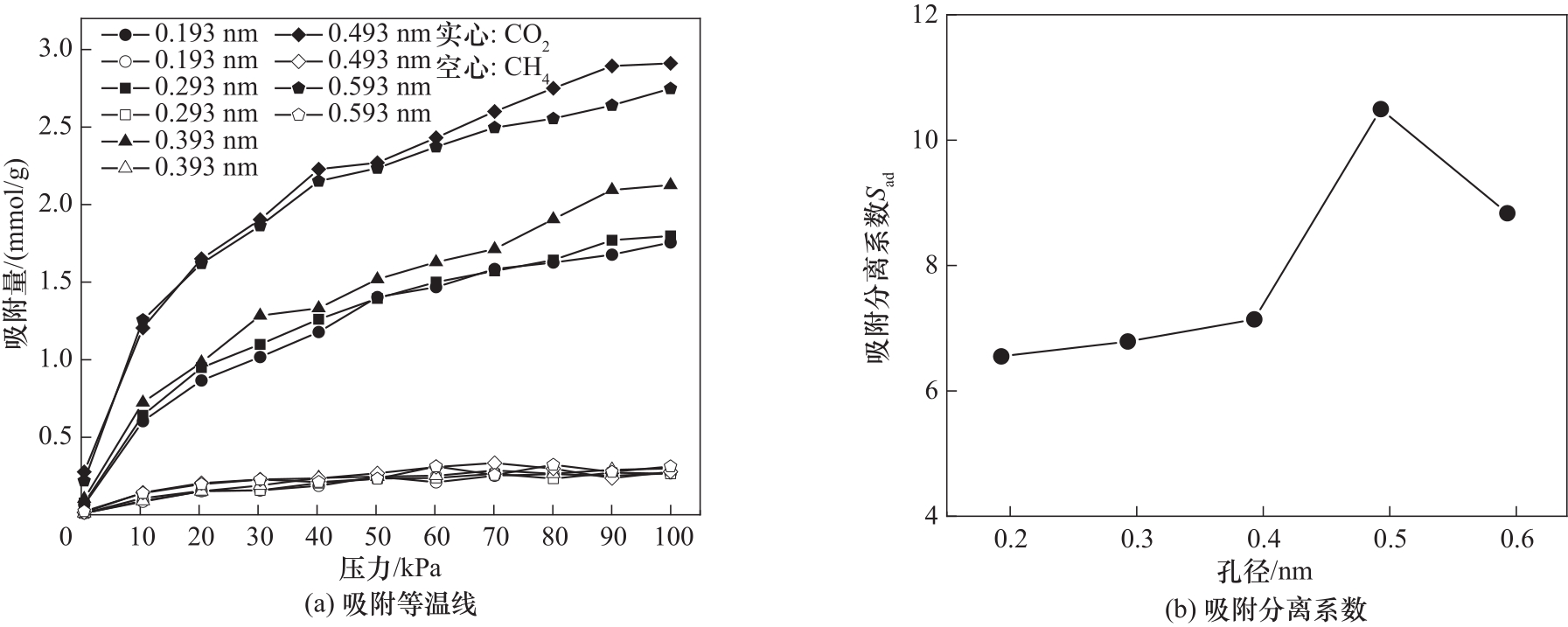
图5 不同碳微晶孔径下CO2/CH4混合气体的吸附等温线与吸附分离系数
Fig.5 Adsorption isotherms and adsorption separation coefficients of CO2/CH4 gas mixtures at different carbon microcrystal pore sizes

图7 不同碳微晶孔径下CO2/CH4混合气体与炭膜间的相互作用能
Fig.7 The interaction energy between CO2/CH4 gas mixture and carbon membrane with different carbon microcrystal pore sizes
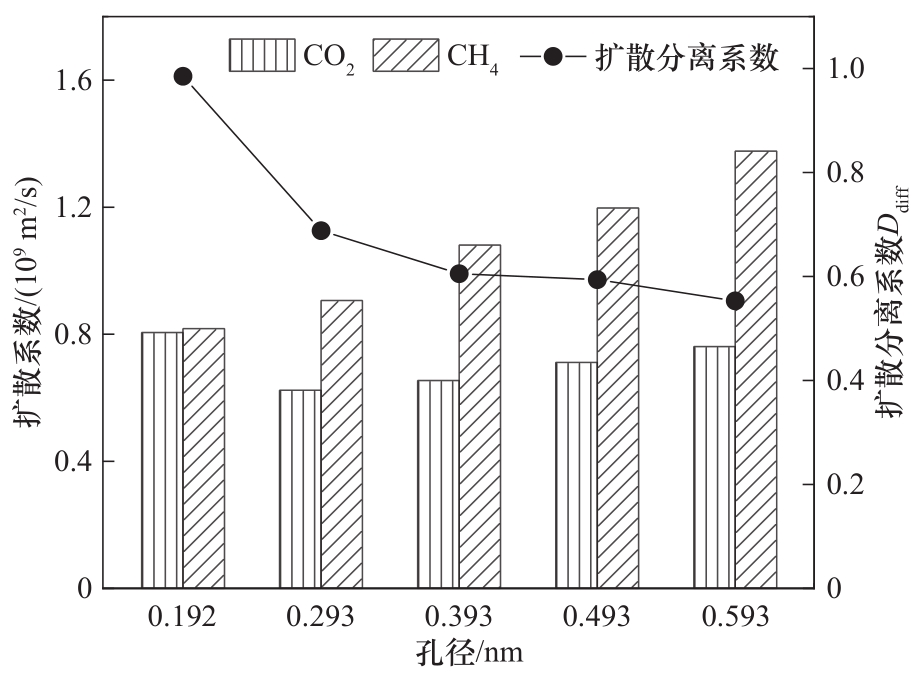
图8 不同碳微晶孔径下CO2/CH4混合气体的扩散系数与扩散分离系数
Fig.8 The diffusion coefficient and diffusion separation coefficient of CO2/CH4 gas mixture with different carbon microcrystal pore sizes
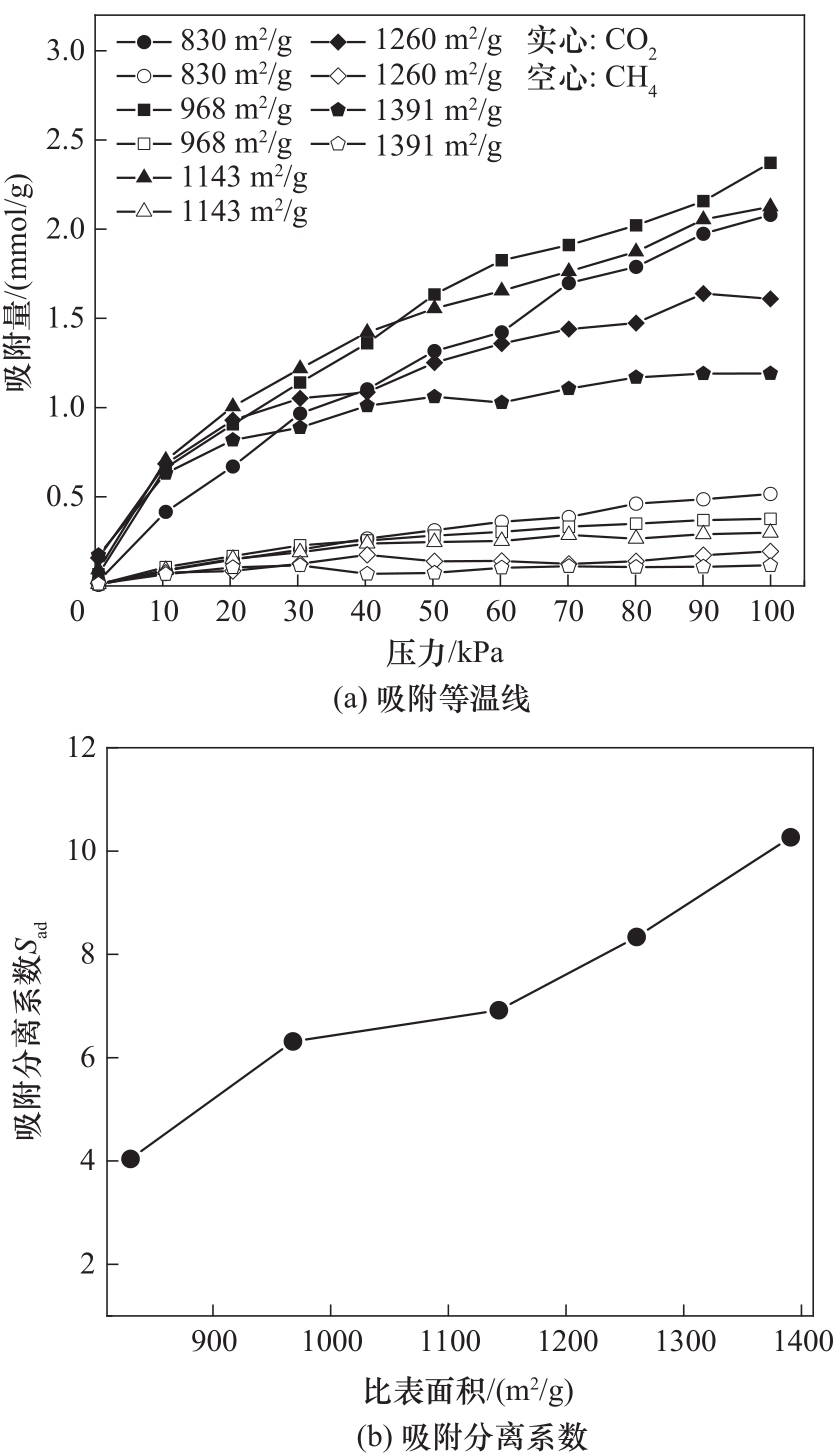
图10 不同比表面积下CO2/CH4混合气体的吸附等温线与吸附分离系数
Fig.10 Adsorption isotherms and adsorption separation coefficients of CO2/CH4 gas mixtures at different specific surface area
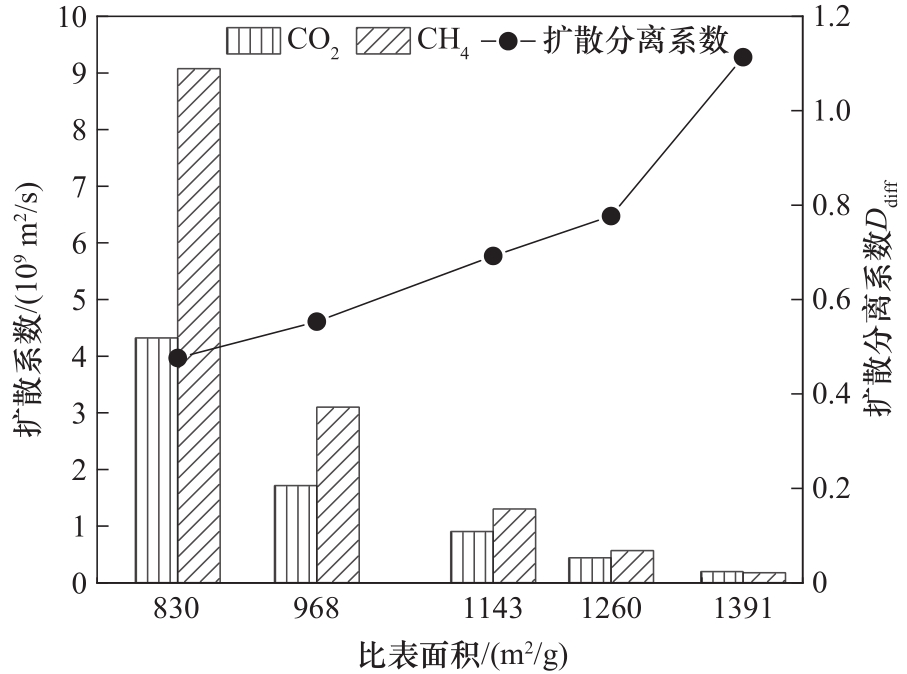
图13 不同比表面积下CO2/CH4混合气体的扩散系数与扩散分离系数
Fig.13 The diffusion coefficients and diffusion separation coefficients of CO2/CH4 gas mixture with different specific surface area
| 1 | Farnam M, bin Mukhtar H, bin Mohd Shariff A. A review on glassy and rubbery polymeric membranes for natural gas purification[J]. ChemBioEng Reviews, 2021, 8(2): 90-109. |
| 2 | Feng S C, Du X B, Luo J Q, et al. A review on facilitated transport membranes based on π-complexation for carbon dioxide separation[J]. Separation and Purification Technology, 2023, 309: 122972 |
| 3 | 徐瑞松,李琳,侯蒙杰,等.新型炭基膜材料前驱体聚合物的研究进展[J].膜科学与技术,2020, 40(1): 250-259. |
| Xu R S, Li L, Hou M J, et al. Development of polymeric precursor for the fabrication of carbon molecular sieve membrane[J]. Membrane Science and Technology, 2020, 40(1): 250-259. | |
| 4 | Salinas O, Ma X H, Litwiller E, et al. High-performance carbon nolecular sieve membranes for ethylene/ethane separation derived from an intrinsically microporous polyimide[J]. Journal of Membrane Science, 2016, 500: 115-123. |
| 5 | Adams J S, Itta A K, Zhang C, et al. New insights into structural evolution in carbon molecular sieve membranes during pyrolysis[J]. Carbon, 2019, 141: 238-246. |
| 6 | Swaidan R, Ma X H, Litwiller E, et al. High pressure pure- and mixed-gas separation of CO2/CH4 by thermally-rearranged and carbon molecular sieve membranes derived from a polyimide of intrinsic microporosity[J]. Journal of Membrane Science, 2013, 447: 387-394. |
| 7 | Zhu Y D, Lu X H, Xie W L, et al. The progress of quantitatively description of membrane process based on the mechanism of nanoconfined mass transfer[J]. Chinese Science Bulletin, 2017, 62(2/3): 223-232. |
| 8 | MacElroy J M D, Friedman S P, Seaton N A. On the origin of transport resistances within carbon molecular sieves[J]. Chemical Engineering Science, 1999, 54(8): 1015-1027. |
| 9 | 曹伟,吕玲红,黄亮亮,等.不同管径碳纳米管中CO2/CH4分离的分子模拟[J].化工学报, 2014, 65(5): 1736-1742. |
| Cao W, Lü L H, Huang L L, et al. Molecular simulations on diameter effect of carbon nanotube for separation of CO2/CH4 [J]. CIESC Journal, 2014, 65(5): 1736-1742. | |
| 10 | Yeganegi S, Gholampour F. Simulation of methane adsorption and diffusion in a carbon nanotube channel[J]. Chemical Engineering Science, 2016, 140: 62-70. |
| 11 | 赵昊瀚,潘艳秋,何流,等.炭膜分离CO2/CH4混合气的分子模拟[J].化工学报, 2016, 67(6): 2393-2400. |
| Zhao H H, Pan Y Q, He L, et al. Molecular simulation on separation of CO2/CH4 gas mixture with carbon membrane[J]. CIESC Journal, 2016, 67(6): 2393-2400. | |
| 12 | Di Biase E, Sarkisov L. Systematic development of predictive molecular models of high surface area activated carbons for adsorption applications[J]. Carbon, 2013, 64: 262-280. |
| 13 | Wang S S, Lu L, Wu D, et al. Molecular simulation study of the adsorption and diffusion of a mixture of CO2/CH4 in activated carbon: effect of textural properties and surface chemistry[J]. Journal of Chemical & Engineering Data, 2016, 61(12): 4139-4147. |
| 14 | Li S, Song K L, Zhao D F, et al. Molecular simulation of benzene adsorption on different activated carbon under different temperatures[J]. Microporous and Mesoporous Materials,2020, 302: 110220. |
| 15 | Widiastuti N, Widyanto A R, Caralin I S, et al. Development of a P84/ZCC composite carbon membrane for gas separation of H2/CO2 and H2/CH4 [J]. ACS Omega, 2021, 6 (24): 15637-15650. |
| 16 | Rezlerová E, Jain S K, Lísal M. Adsorption, diffusion, and transport of C1 to C3 alkanes and carbon dioxide in dual-porosity kerogens: insights from molecular simulations[J]. Energy Fuels, 2023, 37(1): 492-508. |
| 17 | Caro J. Diffusion coefficients in nanoporous solids derived from membrane permeation measurements[J]. Adsorption, 2021, 27(3): 283-293. |
| 18 | Chokbunpiam T, Fritzsche S, Parasuk V, et al. Molecular simulations of a CO2/CO mixture in MIL-127[J]. Chemical Physics Letters, 2018, 696: 86-91. |
| 19 | Sun H. COMPASS: an ab initio force-field optimized for condensed-phase applications overview with details on alkane and benzene compounds[J]. The Journal of Physical Chemistry B, 1998, 102(38): 7338-7364. |
| 20 | 何流. 炭分子筛膜微结构及其对气体分离性能的影响机制研究[D].大连: 大连理工大学, 2021. |
| He L. Microstructure of carbon molecular sieve membrane and its influence mechanism on gas separation performance[D]. Dalian: Dalian University of Technology, 2021. | |
| 21 | Di Biase E, Sarkisov L. Molecular simulation of multi-component adsorption processes related to carbon capture in a high surface area, disordered activated carbon[J]. Carbon,2015, 94: 27-40. |
| 22 | Morris J R, Contescu C I, Chisholm M F, et al. Modern approaches to studying gas adsorption in nanoporous carbons[J], Journal of Materials Chemisty A, 2013, 1(33): 9341-9350. |
| 23 | Xu R S, He L, Li L, et al. Ultraselective carbon molecular sieve membrane for hydrogen purification[J]. Journal of Energy Chemistry, 2020, 50: 16-24. |
| 24 | Suzuki T, Kobori R, Kaneko K. Grand canonical Monte Carlo simulation-assisted pore-width determination of molecular sieve carbons by use of ambient temperature N2 adsorption[J]. Carbon, 2000, 38(4): 630-633. |
| 25 | 李秉繁,刘刚,陈雷.基于分子动力学模拟的CH4溶解对原油分子间作用的影响机制研究[J].化工学报, 2021, 72(3): 1253-1263. |
| Li B F, Liu G, Chen L. Study on the influence mechanism of CH4 dissolution on the intermolecular interaction between crude oil molecules based on molecular dynamics simulation[J]. CIESC Journal, 2021, 72(3): 1253-1263. | |
| 26 | 张兵. 分子筛炭膜的制备、微结构及气体分离性能[D].大连: 大连理工大学,2007. |
| Zhang B. Preparation, microstructure and gas separation performance of molecular sieving carbon membranes[D]. Dalian: Dalian University of Technology, 2007. | |
| 27 | 池君杰,梁晓怿,余青霓,等.分子模拟研究活性炭表面官能团对丙酮吸附的影响[J].航天医学与医学工程, 2015, 28(4): 284-287. |
| Chi J J, Liang X Y, Yu Q N, et al. Molecular simulation study on influence of surface functional groups of activated carbon on acetone adsorption[J]. Space Medicine & Medical Engineering, 2015, 28(4): 284-287. | |
| 28 | 稻垣道夫. 膜技术基本原理[M]. 康飞宇, 译. 2版. 北京: 清华大学出版社, 2014: 93. |
| Michio I. Materials Science and Engineering of Carbon: Fundamentals[M]. Kang F Y, trans. 2nd ed. Beijing: Tsinghua University Press, 2014: 93. | |
| 29 | Sarkisov L, Harrison A. Computational structure characterisation tools in application to ordered and disordered porous materials[J]. Molecular Simulation, 2011, 37 (15): 1248-1257. |
| 30 | Li S, Yu L, Song K L, et al. Study on microscopic mechanism of activated carbon adsorption of benzene by molecular simulation technology[J]. Chemistry Letters, 2020, 49(12): 1452-1455. |
| 31 | Wu Q Y, Liang H Q, Li M, et al. Hierarchically porous carbon membranes derived from PAN and their selective adsorption of organic dyes[J]. Chinese Journal of Polymer Science, 2016, 34(1): 23-33. |
| 32 | Ye Z Y, Zhang Y, Hou L, et al. Preparation of a GO/PB-modified nanofiltration membrane for removal of radioactive cesium and strontium from water[J]. Chemical Engineering Journal, 2022, 446: 137143. |
| 33 | Bai K, Fan S Q, Chen Y, et al. Membrane adsorber with hierarchically porous HKUST-1 immobilized in membrane pores by flowing synthesis[J]. Journal of Membrane Science, 2022, 650: 120424. |
| [1] | 晁京伟, 许嘉兴, 李廷贤. 基于无管束蒸发换热强化策略的吸附热池的供热性能研究[J]. 化工学报, 2023, 74(S1): 302-310. |
| [2] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [5] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [6] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [7] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [8] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [9] | 高燕, 伍鹏, 尚超, 胡泽君, 陈晓东. 基于双流体喷嘴的磁性琼脂糖微球的制备及其蛋白吸附性能探究[J]. 化工学报, 2023, 74(8): 3457-3471. |
| [10] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [11] | 王海, 林宏, 王晨, 许浩洁, 左磊, 王军锋. 高压静电场强化多孔介质表面沸腾传热特性研究[J]. 化工学报, 2023, 74(7): 2869-2879. |
| [12] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [13] | 董明, 徐进良, 刘广林. 超临界水非均质特性分子动力学研究[J]. 化工学报, 2023, 74(7): 2836-2847. |
| [14] | 刘春雨, 周桓宇, 马跃, 岳长涛. CaO调质含油污泥干燥特性及数学模型[J]. 化工学报, 2023, 74(7): 3018-3027. |
| [15] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号