化工学报 ›› 2023, Vol. 74 ›› Issue (4): 1703-1711.DOI: 10.11949/0438-1157.20230087
龙臻1,2,3,4( ), 王谨航1,2,3,4,5, 何勇1,2,3,4(
), 王谨航1,2,3,4,5, 何勇1,2,3,4( ), 梁德青1,2,3,4(
), 梁德青1,2,3,4( )
)
收稿日期:2023-02-08
修回日期:2023-03-30
出版日期:2023-04-05
发布日期:2023-06-02
通讯作者:
何勇,梁德青
作者简介:龙臻(1986—),女,博士,副研究员,longzhen@ms.giec.ac.cn
基金资助:
Zhen LONG1,2,3,4( ), Jinhang WANG1,2,3,4,5, Yong HE1,2,3,4(
), Jinhang WANG1,2,3,4,5, Yong HE1,2,3,4( ), Deqing LIANG1,2,3,4(
), Deqing LIANG1,2,3,4( )
)
Received:2023-02-08
Revised:2023-03-30
Online:2023-04-05
Published:2023-06-02
Contact:
Yong HE, Deqing LIANG
摘要:
利用等温恒容法实验考察了一种高性能离子液体N-丁基-N-甲基吡咯烷四氟硼酸盐([BMP][BF4])、两种动力学抑制剂(KHIs)[聚乙烯基吡咯烷酮(PVP)和聚乙烯己内酰胺(PVCap)]及[BMP][BF4]与KHI的二元混合物对甲烷/乙烷/丙烷三元混合气体水合物生成动力学过程的影响规律。通过分析压力和气相组分变化规律,发现混合气体水合物呈现两步骤生长模式。在高过冷度(>10℃)和高搅拌速率(1000 r/min)条件下,单一添加剂基本失效,而[BMP][BF4]可较好协助增强PVCap的抑制性能。粉末X射线衍射和激光拉曼光谱测试结果均显示,所有体系中形成的水合物样品结构同时存在sⅠ型和sⅡ型,抑制剂的添加主要影响两种晶体结构的相对含量和各客体分子的笼子占有率。最后探讨了协同抑制机理。
中图分类号:
龙臻, 王谨航, 何勇, 梁德青. 离子液体与动力学抑制剂作用下混合气体水合物生成特性研究[J]. 化工学报, 2023, 74(4): 1703-1711.
Zhen LONG, Jinhang WANG, Yong HE, Deqing LIANG. Characteristics study on hydrates formation from gas mixture under ionic liquid together with kinetic hydrate inhibitors[J]. CIESC Journal, 2023, 74(4): 1703-1711.
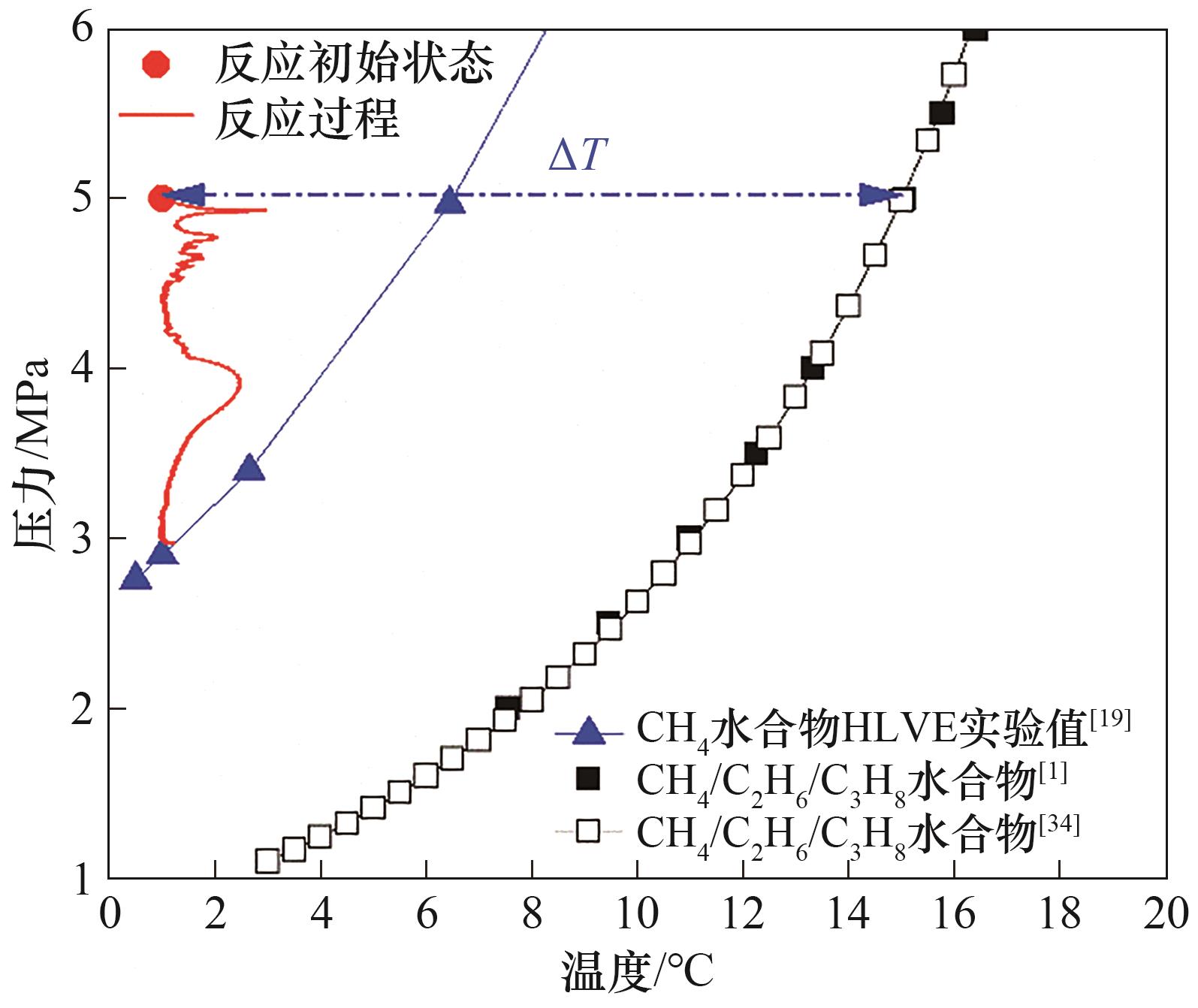
图2 1.0%(质量) PVPK90体系中混合气体水合物生成过程压力-温度变化曲线
Fig.2 Pressure versus temperature curve during CH4/C2H6/C3H8 hydrate formation process with 1.0%(mass) PVPK90
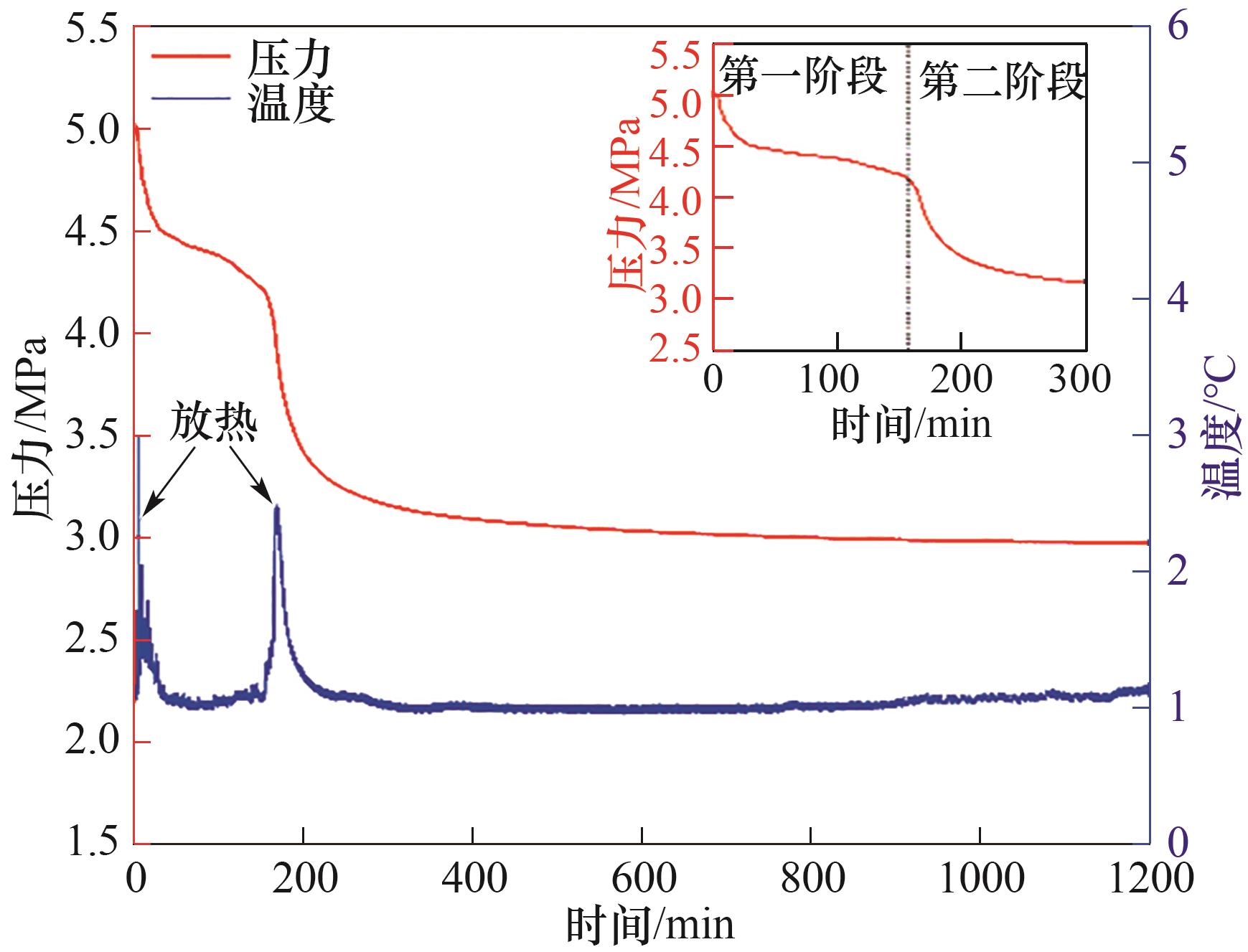
图3 1.0%(质量) PVPK90体系中CH4/C2H6/C3H8水合物生成过程压力和温度随时间变化曲线
Fig.3 Typical changes of pressure and temperature versus time during CH4/C2H6/C3H8 hydrate formed with 1.0%(mass) PVPK90 aqueous solution
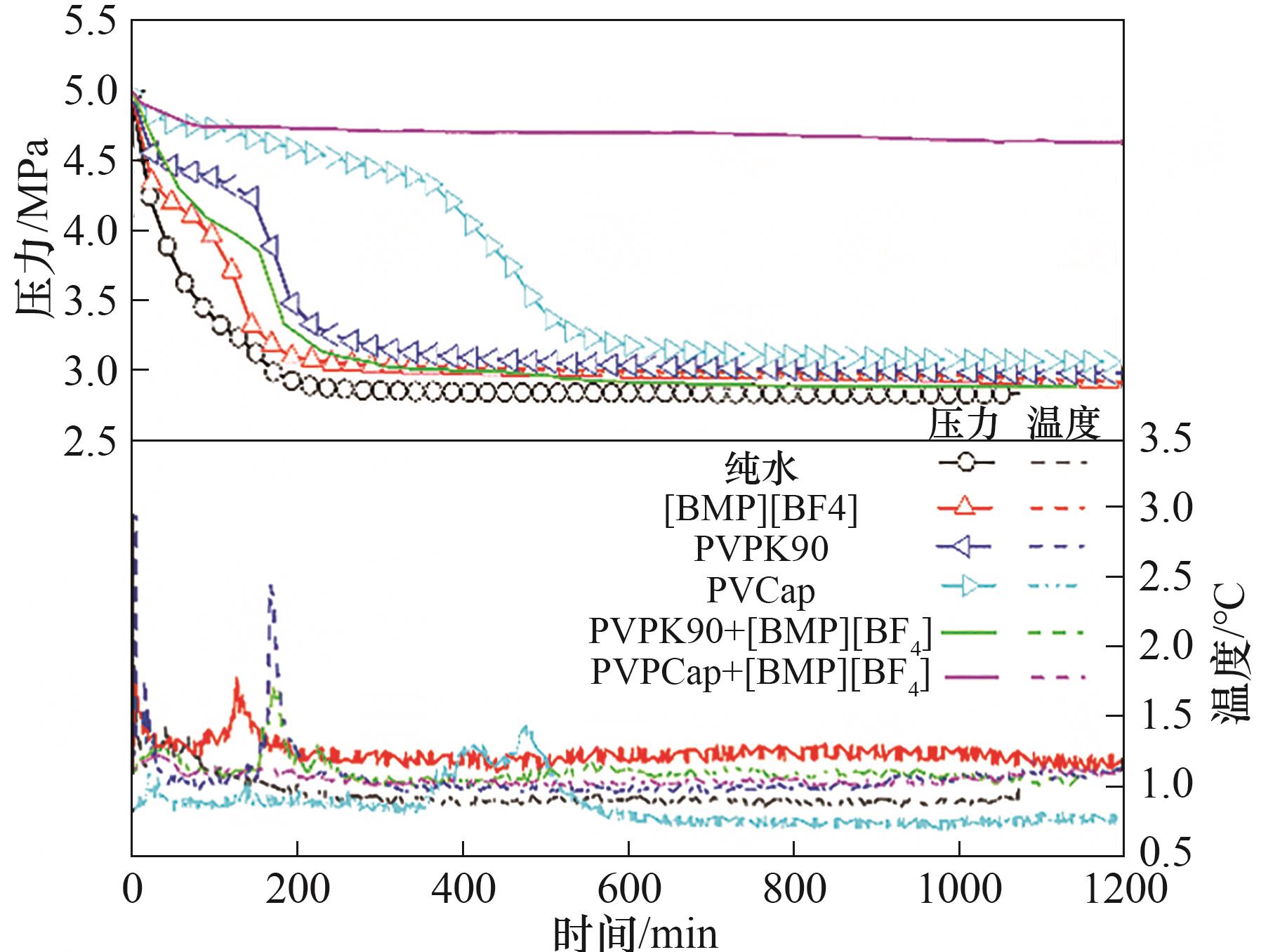
图4 1.0%(质量)抑制剂体系中的CH4/C2H6/C3H8水合物的生成过程压力和温度随时间变化曲线
Fig.4 Changes of pressure and temperature versus time during CH4/C2H6/C3H8 hydrate formed with pure water and 1.0%(mass) different inhibitors
| 体系 | 水合物相组分浓度/%(mol) | 耗气量/mmol | 气相组分浓度/%(mol) | ||||
|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | CH4 | C2H6 | C3H8 | ||
| 纯水 | 83.77 | 10.13 | 6.1 | 86.5 | 99.41 | 0.54 | 0.05 |
| [BMP][BF4] | 85.07 | 8.92 | 6.01 | 86.4 | 98.23 | 1.65 | 0.12 |
| PVPK90 | 83.05 | 10.48 | 6.47 | 81.2 | 99.16 | 0.77 | 0.07 |
| PVCap | 82.80 | 10.57 | 6.63 | 78.4 | 98.91 | 0.97 | 0.12 |
| PVPK90+[BMP][BF4] | 83.77 | 9.99 | 6.24 | 84.4 | 99.06 | 0.88 | 0.06 |
| PVPCap+[BMP][BF4] | 52.78 | 23.37 | 23.85 | 20.2 | 96.82 | 2.83 | 0.32 |
表1 反应结束水合物相和气相组分分布及耗气量
Table 1 Gas compositions in vapor phase and hydrate phase at the end of reaction and total gas consumption in the presence of water or various inhibitors
| 体系 | 水合物相组分浓度/%(mol) | 耗气量/mmol | 气相组分浓度/%(mol) | ||||
|---|---|---|---|---|---|---|---|
| CH4 | C2H6 | C3H8 | CH4 | C2H6 | C3H8 | ||
| 纯水 | 83.77 | 10.13 | 6.1 | 86.5 | 99.41 | 0.54 | 0.05 |
| [BMP][BF4] | 85.07 | 8.92 | 6.01 | 86.4 | 98.23 | 1.65 | 0.12 |
| PVPK90 | 83.05 | 10.48 | 6.47 | 81.2 | 99.16 | 0.77 | 0.07 |
| PVCap | 82.80 | 10.57 | 6.63 | 78.4 | 98.91 | 0.97 | 0.12 |
| PVPK90+[BMP][BF4] | 83.77 | 9.99 | 6.24 | 84.4 | 99.06 | 0.88 | 0.06 |
| PVPCap+[BMP][BF4] | 52.78 | 23.37 | 23.85 | 20.2 | 96.82 | 2.83 | 0.32 |
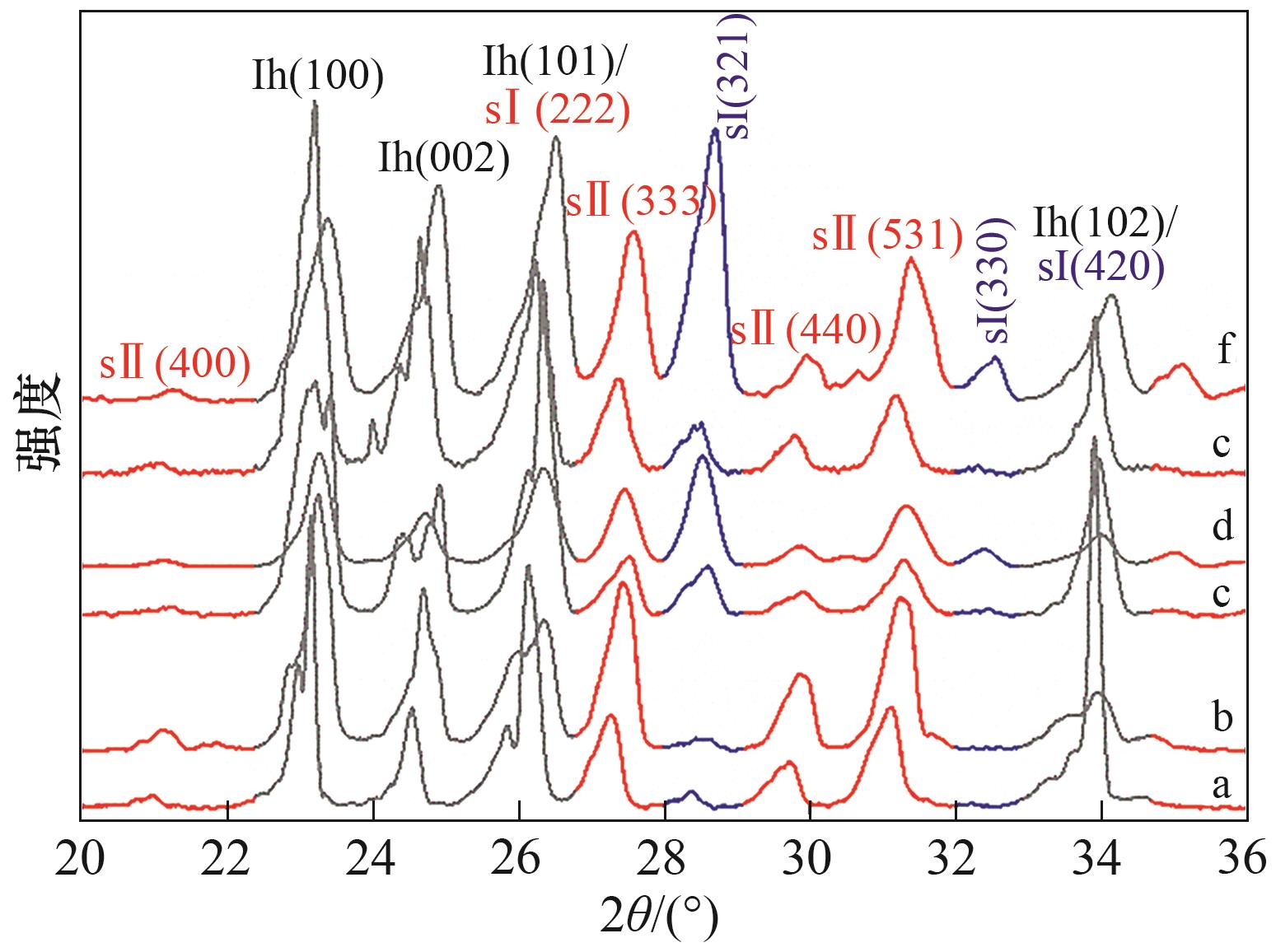
图5 CH4/C2H6/C3H8混合气在不同添加剂体系中形成的水合物晶体PXRD衍射谱图a—纯水;b—1.0%(质量) [BMP][BF4]; c—1.0%(质量) PVPK90; d—1.0%(质量)PVCap; e—0.5%(质量) PVPK90+ 0.5%(质量) [BMP][BF4]; f—0.5%(质量) PVCap + 0.5%(质量) [BMP][BF4]
Fig.5 PXRD patterns of CH4/C2H6/C3H8 hydrate formed in the presence of different additives
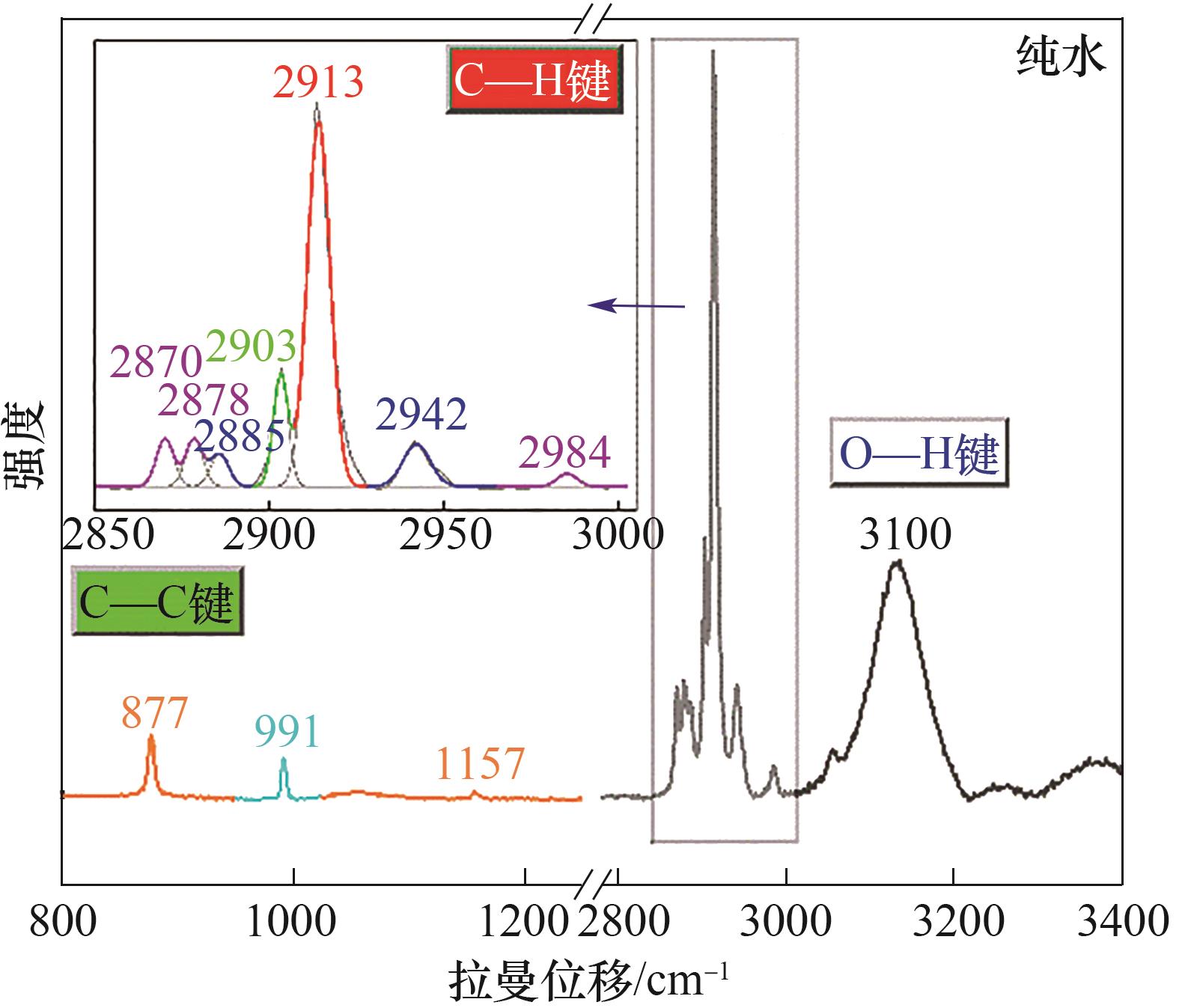
图6 纯水体系中形成的CH4/C2H6/C3H8混合气体水合物晶体拉曼光谱图(800~1200 cm-1:C—C区域;2800~3000 cm-1:C—H区域;3000~3400 cm-1:O—H区域)
Fig.6 Typical Raman spectrum of CH4/C2H6/C3H8 hydrate formed with pure water (800—1200 cm-1: C—C region; 2800—3000 cm-1: C—H region; 3000—3400 cm-1: O—H region)
| 客体 分子 | 客体分子直径/Å | sⅠ型水合物 | sⅡ型水合物 | ||
|---|---|---|---|---|---|
| 512 | 51262 | 512 | 51264 | ||
| CH4 | 4.36 | 0.855 | 0.744 | 0.868 | 0.655 |
| C2H6 | 5.5 | 1.08 | 0.939 | 1.1 | 0.826 |
| C3H8 | 6.28 | 1.23 | 1.07 | 1.25 | 0.943 |
表2 客体分子直径与水合物笼子直径比率[1]
Table 2 Ratios of molecular diameters to cage diameters for some guest molecules[1]
| 客体 分子 | 客体分子直径/Å | sⅠ型水合物 | sⅡ型水合物 | ||
|---|---|---|---|---|---|
| 512 | 51262 | 512 | 51264 | ||
| CH4 | 4.36 | 0.855 | 0.744 | 0.868 | 0.655 |
| C2H6 | 5.5 | 1.08 | 0.939 | 1.1 | 0.826 |
| C3H8 | 6.28 | 1.23 | 1.07 | 1.25 | 0.943 |

图7 不同抑制剂体系中生成的CH4/C2H6/C3H8混合气体水合物拉曼光谱图a—纯水; b—1.0%(质量) [BMP][BF4]; c—1.0%(质量) PVPK90;d—1.0%(质量) PVCap; e—0.5%(质量) PVP + 0.5%(质量) [BMP][BF4]; f—0.5%(质量) PVCap + 0.5%(质量) [BMP][BF4]
Fig.7 Raman spectrum of CH4/C2H6/C3H8 hydrate formed in the presence of different inhibitors
| 1 | Sloan E D, Koh C A. Clathrate Hydrates of Natural Gases[M]. 3rd ed. Boca Raton, FL: CRC Press/Taylor & Francis, 2008. |
| 2 | Song Y C, Yang L, Zhao J F, et al. The status of natural gas hydrate research in China: a review[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 778-791. |
| 3 | Boswell R, Collett T S. Current perspectives on gas hydrate resources[J]. Energy & Environmental Science, 2011, 4(4): 1206-1215. |
| 4 | Hammerschmidt E G. Formation of gas hydrates in natural gas transmission lines[J]. Industrial & Engineering Chemistry, 1934, 26(8): 851-855. |
| 5 | 张剑波, 王志远, 刘书杰, 等. 深水气井测试过程中水合物流动障碍防治方法[J]. 石油勘探与开发, 2020, 47(6): 1256-1264. |
| Zhang J B, Wang Z Y, Liu S J, et al. A method for preventing hydrates from blocking flow during deep-water gas well testing[J]. Petroleum Exploration and Development, 2020, 47(6): 1256-1264. | |
| 6 | Wang Y H, Fan S S, Lang X M. Reviews of gas hydrate inhibitors in gas-dominant pipelines and application of kinetic hydrate inhibitors in China[J]. Chinese Journal of Chemical Engineering, 2019, 27(9): 2118-2132. |
| 7 | 李锐, 宁伏龙, 张凌, 等. 低剂量水合物抑制剂的研究进展[J]. 石油化工, 2018, 47(2): 203-210. |
| Li R, Ning F L, Zhang L, et al. Progress in the research of the low dosage hydrate inhibitors[J]. Petrochemical Technology, 2018, 47(2): 203-210. | |
| 8 | 陈玉川, 史博会, 李文庆, 等. 水合物动力学抑制剂的作用机理研究进展[J]. 化工进展, 2018, 37(5): 1726-1743. |
| Chen Y C, Shi B H, Li W Q, et al. Progress of influence mechanism of kinetic hydrate inhibitors[J]. Chemical Industry and Engineering Progress, 2018, 37(5): 1726-1743. | |
| 9 | 董三宝, 田茂琳, 徐遥远, 等. 天然气水合物防聚剂研究进展[J]. 广东化工, 2021, 48(10): 82-85. |
| Dong S B, Tian M L, Xu Y Y, et al. Progress in the investigation of natural gas hydrate anti-agglomerants[J]. Guangdong Chemical Industry, 2021, 48(10): 82-85. | |
| 10 | Salmin D C, Estanga D, Koh C A. Review of gas hydrate anti-agglomerant screening techniques[J]. Fuel, 2022, 319: 122862. |
| 11 | Kelland M A, Dirdal E G, Ree L H S. Solvent synergists for improved kinetic hydrate inhibitor performance of poly(N-vinylcaprolactam)[J]. Energy & Fuels, 2020, 34(2): 1653-1663. |
| 12 | Xu S R, Fan S S, Fang S T, et al. Excellent synergy effect on preventing CH4 hydrate formation when glycine meets polyvinylcaprolactam[J]. Fuel, 2017, 206: 19-26. |
| 13 | Lee W, Shin J Y, Cha J H, et al. Inhibition effect of ionic liquids and their mixtures with poly(N-vinylcaprolactam) on methane hydrate formation[J]. Journal of Industrial and Engineering Chemistry, 2016, 38: 211-216. |
| 14 | Rogers R D, Seddon K R. Ionic liquids: solvents of the future?[J]. Science, 2003, 302(5646): 792-793. |
| 15 | Xiao C W, Adidharma H. Dual function inhibitors for methane hydrate[J]. Chemical Engineering Science, 2009, 64(7): 1522-1527. |
| 16 | Xiao C W, Wibisono N, Adidharma H. Dialkylimidazolium halide ionic liquids as dual function inhibitors for methane hydrate[J]. Chemical Engineering Science, 2010, 65(10): 3080-3087. |
| 17 | Zare M, Kondori J, Zendehboudi S, et al. PC-SAFT/UNIQUAC model assesses formation condition of methane hydrate in the presence of imidazolium-based ionic liquid systems[J]. Fuel, 2020, 266: 116757. |
| 18 | Tariq M, Connor E, Thompson J, et al. Doubly dual nature of ammonium-based ionic liquids for methane hydrates probed by rocking-rig assembly[J]. RSC Advances, 2016, 6(28): 23827-23836. |
| 19 | Cha J H, Ha C, Kang S P, et al. Thermodynamic inhibition of CO2 hydrate in the presence of morpholinium and piperidinium ionic liquids[J]. Fluid Phase Equilibria, 2016, 413: 75-79. |
| 20 | Long Z, Zhou X B, Shen X D, et al. Phase equilibria and dissociation enthalpies of methane hydrate in imidazolium ionic liquid aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2015, 54(46): 11701-11708. |
| 21 | Nasir Q, Suleman H, Elsheikh Y A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation[J]. Journal of Natural Gas Science and Engineering, 2020, 76: 103211. |
| 22 | Farhadian A, Shadloo A, Zhao X, et al. Challenges and advantages of using environmentally friendly kinetic gas hydrate inhibitors for flow assurance application: a comprehensive review[J]. Fuel, 2023, 336: 127055. |
| 23 | Kim K S, Kang J W, Kang S P. Tuning ionic liquids for hydrate inhibition[J]. Chemical Communications, 2011, 47(22): 6341-6343. |
| 24 | Kang S P, Kim E S, Shin J Y, et al. Unusual synergy effect on methane hydrate inhibition when ionic liquid meets polymer[J]. RSC Advances, 2013, 3(43): 19920-19923. |
| 25 | Shen X D, Zhou X B, Liang D Q. Kinetic effects of ionic liquids on methane hydrate[J]. Energy & Fuels, 2019, 33(2): 1422-1432. |
| 26 | Shen X D, Shi L L, Long Z, et al. Experimental study on the kinetic effect of N-butyl-N-methylpyrrolidinium bromide on CO2 hydrate[J]. Journal of Molecular Liquids, 2016, 223: 672-677. |
| 27 | 任俊杰, 龙臻, 梁德青. 离子液体与PVP K90复合抑制剂对甲烷水合物的生成影响[J]. 化工学报, 2020, 71(11): 5256-5264. |
| Ren J J, Long Z, Liang D Q. Effect of complex inhibitors containing ionic liquids and PVP K90 on formation of methane hydrate[J]. CIESC Journal, 2020, 71(11): 5256-5264. | |
| 28 | Ren J J, Lu Z L, Long Z, et al. Experimental study on the kinetic effect of N-butyl-N-methylpyrrolidinium tetrafluoroborate and poly(N-vinyl-caprolactam) on CH4 hydrate formation[J]. RSC Advances, 2020, 10(26): 15320-15327. |
| 29 | Lee D, Go W, Seo Y. Experimental and computational investigation of methane hydrate inhibition in the presence of amino acids and ionic liquids[J]. Energy, 2019, 182: 632-640. |
| 30 | Del Villano L, Kelland M A. An investigation into the kinetic hydrate inhibitor properties of two imidazolium-based ionic liquids on Structure Ⅱ gas hydrate[J]. Chemical Engineering Science, 2010, 65(19): 5366-5372. |
| 31 | Kang S P, Jung T, Lee J W. Macroscopic and spectroscopic identifications of the synergetic inhibition of an ionic liquid on hydrate formations[J]. Chemical Engineering Science, 2016, 143: 270-275. |
| 32 | Qureshi M F, Atilhan M, Altamash T, et al. Gas hydrate prevention and flow assurance by using mixtures of ionic liquids and synergent compounds: combined kinetics and thermodynamic approach[J]. Energy & Fuels, 2016, 30(4): 3541-3548. |
| 33 | Long Z, Zhou X B, Lu Z L, et al. Kinetic inhibition performance of N-vinyl caprolactam/isopropylacrylamide copolymers on methane hydrate formation[J]. Energy, 2022, 242: 123056. |
| 34 | Redlich O, Kwong J N S. On the thermodynamics of solutions; an equation of state; fugacities of gaseous solutions[J]. Chemical Reviews, 1949, 44(1): 233-244. |
| 35 | Chen G J, Guo T M. A new approach to gas hydrate modelling[J]. Chemical Engineering Journal, 1998, 71(2): 145-151. |
| 36 | Uchida T, Moriwaki M, Takeya S, et al. Two-step formation of methane-propane mixed gas hydrates in a batch-type reactor[J]. AIChE Journal, 2004, 50(2): 518-523. |
| 37 | Uchida T, Takeya S, Kamata Y, et al. Spectroscopic measurements on binary, ternary, and quaternary mixed-gas molecules in clathrate structures[J]. Industrial & Engineering Chemistry Research, 2007, 46(14): 5080-5087. |
| 38 | Kumar R, Linga P, Moudrakovski I, et al. Structure and kinetics of gas hydrates from methane/ethane/propane mixtures relevant to the design of natural gas hydrate storage and transport facilities[J]. AIChE Journal, 2008, 54(8): 2132-2144. |
| 39 | Daraboina N, Ripmeester J, Walker V K, et al. Natural gas hydrate formation and decomposition in the presence of kinetic inhibitors (3): Structural and compositional changes[J]. Energy & Fuels, 2011, 25(10): 4398-4404. |
| 40 | Cha M J, Shin K, Seo Y, et al. Catastrophic growth of gas hydrates in the presence of kinetic hydrate inhibitors[J]. The Journal of Physical Chemistry A, 2013, 117(51): 13988-13995. |
| 41 | Sharifi H, Englezos P. Accelerated hydrate crystal growth in the presence of low dosage additives known as kinetic hydrate inhibitors[J]. Journal of Chemical & Engineering Data, 2015, 60(2): 336-342. |
| 42 | Takeya S, Ripmeester J A. Anomalous preservation of CH4 hydrate and its dependence on the morphology of hexagonal ice[J]. ChemPhysChem, 2010, 11(1): 70-73. |
| 43 | Subramanian S, Sloan E D. Trends in vibrational frequencies of guests trapped in clathrate hydrate cages[J]. The Journal of Physical Chemistry B, 2002, 106(17): 4348-4355. |
| 44 | Yagasaki T, Matsumoto M, Tanaka H. Adsorption mechanism of inhibitor and guest molecules on the surface of gas hydrates[J]. Journal of the American Chemical Society, 2015, 137(37): 12079-12085. |
| 45 | Xu J F, Li L W, Liu J X, et al. The molecular mechanism of the inhibition effects of PVCaps on the growth of sI hydrate: an unstable adsorption mechanism[J]. Physical Chemistry Chemical Physics, 2018, 20(12): 8326-8332. |
| 46 | Castillo-Borja F, Bravo-Sánchez U I. Molecular dynamics simulation study of the performance of different inhibitors for methane hydrate growth[J]. Journal of Molecular Liquids, 2021, 337: 116510. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [6] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [7] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [8] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [9] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [10] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [11] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [12] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [13] | 陈吉, 洪泽, 雷昭, 凌强, 赵志刚, 彭陈辉, 崔平. 基于分子动力学的焦炭溶损反应及其机理研究[J]. 化工学报, 2023, 74(7): 2935-2946. |
| [14] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [15] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号